2. Institute of Materials and Environmental Chemistry, Hungarian Academy of Sciences, Department of Physics, Budapest University of Technology and Economics, Budapest H-1117, Hungary
2. 匈牙利科学院 材料与环境化学研究所, 布达佩斯技术与经济大学物理系, 布达佩斯 H-1117
Noninvasive measurement of local pH is of important significance since abnormal changes of pH are closely associated with cellular dysfunctions and pathologies such as ischemia, oxidative stress[1], apoptosis[2] and tumor size progression[3]. Electron paramagnetic resonance (EPR) coupled with the use of exogenous spin probes has been an indispensable technique to measure pH both in vitro and in vivo due to its noninvasiveness, high sensitivity and appropriate magnetic penetration depth[4-6]. Nitroxide radicals initially reported in the early seventies[7, 8] are the most commonly used EPR probes for pH measurement. Typically, pH-induced changes in EPR spectra are assessed by measuring the isotropic hyperfine splitting (hfs) constant for nitrogen (αN) or, sometimes, for phosphorus (αP), although the electronic g-factor is also affected by proton exchange reaction. The reliability of method can be enhanced by simultaneous analysis of EPR spectra recorded at different pH environments, when the formation constants of mass-balance equation and the EPR parameters are revealed by a two-dimensional (2D) analysis[9]. The method was originally developed for studying copper coordination but proved to be also useful for the pH sensitive nitroxides[10]. The pH-sensitive nitroxides have found numerous applications in in vivo measurement of pH inside the gut[11], release processes in biodegradable polymers[12], drug-induced changes in the stomach acidity of living rats[13], and tumor extracellular pH[14]. However, rapid bioreduction of nitroxides with loss of signal and the relatively broad multiple signals from nitrogen and proton hyperfine splittings greatly limit their applications in in vivo systems due to low EPR sensitivity.
In the past two decades, fully substituted tetrathiatriarylmethyl (TAM) radicals such as OX063 and CT-03 (Fig. 1) have accepted intense attention in the EPR field owing to their sharp EPR single lines, good water solubility and high stability[15-18]. TAM radicals are a class of air-stable carbon-centered radicals in which the unpaired electron is predominantly located at the central carbon and further stabilized by delocalization into three aromatic ring systems. Full substitution of each phenyl ring by alkylthio and carboxylate groups not only eliminates the hyperfine splitting from protons in TAM radicals, but also enhances their stability by electronic and steric effects. In 2007, Khramstov et al.[19] pioneered the use of TAM radicals for measurement of pH based on their pH sensitivity to the magnetic parameters (e.g., αH and g factor). One of the authors in this study also reported a complex of dendritic TAM radical with copper (Ⅱ) as pH probe[20]. Thereafter, the amino and triphosphonate derivatives of TAM radicals were also synthesized[21-23] and their spectral patterns exhibited high sensitivity to pH in the physiological range due to nearly neutral pKa values. However, these probes had much complicated EPR signals due to hyperfine splittings from various paramagnetic nuclei (e.g., 14N, 1H and 31P) in the molecules. Despite this, the triphosphonated TAM probe was used to measure the extracellular pH in tumors[24]. Recently, in order to simplify the EPR signals of pH probes, Khramstov and coauthors[25] have reported a monophosphonated TAM probe (p1TAM-H) and its deuterated derivative (p1TAM-D) (Fig. 1) which is so far the most ideal pH and O2 dual probe owing to its simple doublet EPR spectral pattern and improved water solubility required for in vivo applications. However, the synthetic efficiency of these probes was rather low with a yield of < 2 % for p1TAM-H/p1TAM-D (about 10 mg) from the triarylmethanol (530 mg). As such, it is challenging to obtain sufficient amounts for their applications in in vivo EPR imaging. Moreover, due to the relatively hydrophobic nature, these pH probes may potentially bind with albumins which are present at high concentrations in blood[26], resulting in a drastic decrease of the EPR signal intensity and preventing their application as pH and oxygen probes[27].
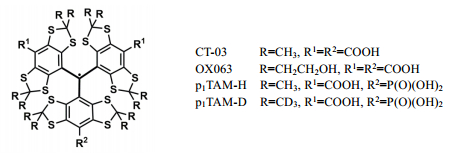
|
Fig. 1 Molecular structure of TAM radicals |
In the present work, we developed an efficient procedure for the synthesis of p1TAM-H and p1TAM-D with a yield of 25% from the triarylmethanol, allowing us to obtain sufficient amounts for their further derivatization. Thereafter, two PEGylated derivatives (i.e., POP and dPOP) of the phosphonated TAM radicals were also synthesized. Effects of PEGylation on pKa, albumin binding, and stability as well as O2 sensitivity of these two probes were explored.
1 Experimental section 1.1 Instruments and reagentsAll manipulations involving air- and moisture-sensitive compounds were carried out under an atmosphere of purified argon by using standard Schlenk-line techniques. Solvents were dried with appropriate drying agents and degassed before use. All commercially available reagents were used as received without further purification. NMR spectra were recorded on a BRUKER Avance Ⅱ 400 MHz NMR spectrometer. Chemical shifts (δ) were reported and referenced to tetramethylsilane (TMS) or NMR solvents used (δH 7.27 for CDCl3). The following abbreviations were used: s=singlet, d=doublet, t=triplet, m=multiplet. Coupling constants (J) were reported in Hz. High resolution mass spectra (HRMS) were obtained on an MALDI-TOF spectrometer (Bruker Autoflex Ⅲ TOF/TOF).
Xanthine (X), xanthine oxidase (XO), glutathione (GSH), ascorbic acid (Asc), hydrogen peroxide (H2O2), ammonium iron (Ⅲ) sulfate hexahydrate [(NH4)2Fe(SO4)2·6H2O], diethylenetriaminepentaacetic acid (DTPA), nitrilotriacetic acid disodium salt (NTA), bovine serum albumin (fatty acid-free, BSA), tetramethylethylenediamine (TMEDA), butyl lithium (1.6 mol/L in hexanes), dimethyl carbonate, diethyl chlorophosphate, trifluoromethanesulfonic acid, trimethylsilyl bromide (TMSBr) and cesium carbonate were commercially available. All other chemicals used throughout the experiments were of analytical grade. The dendrons D1 and dD1were synthesized as previously described[28]. Phosphate buffer (PBS, 1 mmol/L, pH 7.4) was prepared from sodium dihydrogen phosphate and disodium hydrogen phosphate in the presence of DTPA (100 μmol/L).
1.2 EPR spectroscopyEPR spectra were recorded on a Bruker EMX-plus X-band spectrometer at room temperature. General instrumental settings were as follows: microwave power, 40 μW; time constant, 20.48 ms; conversion time, 10 ms; sweep time, 81.92 s; modulation frequency, 10 kHz; modulation amplitude, 2×10-6 T; sweep width, 5×10-4 T; number of points, 8 192. EPR measurements were performed in 50 μL capillary tubes. In addition, EPR measurements under anaerobic conditions were carried out using a gas-permeable Teflon tube (i.d. = 0.8 mm). Briefly, the experimental solution was transferred to the tube which was then sealed at both ends. The sealed sample was placed inside a quartz EPR tube with open ends. Argon gas was allowed to bleed into the EPR tube and then EPR spectrum was recorded.
1.3 EPR simulationSpectral simulation was carried out using the simulation program written by Professor Rockenbauer[9, 10, 29]. As part of the EPR simulation program, the 2D model "EPR pH titration" was employed to analyze the dependence of pH on the fraction of two ionization states of the pH probes in our work. Different EPR parameters including αP, g tensor, resolved and non-resolved proton or deuterium splitting, line width and pKa value were adjusted by combining iteration and least squares procedures, with a search for the minimum square deviation between experimental and computed spectra. It was worth noting that 2D EPR analysis and superposition analysis of EPR spectra originates from the same simulation program just with different modes.
1.4 pH titrationThe aqueous solution of the TAM probe was titrated by addition of a small volume of NaOH or HCl with the final dilution of sample less than 1%. pH was controlled by electrode which was calibrated at 25 ℃ using pH values for reference solution. Temperature of reference and titrated solutions during pH measurements was keep the same data using type of integral temp sensor attached to Mettler-Toledo Lab pH electrode FE20. Anoxic conditions were maintained using a gas-permeable Teflon tube (i.d. = 0.8 mm) which was placed inside a quartz EPR tube with open ends and argon gas was allowed to bleed into the EPR tube.
1.5 pKa value calculationThe values of fraction, f, of each ionization state of the TAM probe were obtained by computer simulation of experimental spectra. The fraction values were then fitted by a standard titration equation to yield the corresponding value of pKa. For example, titration of dPOP is described by the equation:
| $ f({\rm{dPO}}{{\rm{P}}^{2 - }}) = 1 - f({\rm{dPO}}{{\rm{P}}^{1 - }}) = \frac{1}{{1 + {{10}^{ - {\rm{pH}} + {\rm{p}}K{\rm{a}}}}}} $ | (1) |
The synthesis of monophosphonated TAM radicals (i.e., p1TAM-H and p1TAM-D) was originally reported by Khramtsov et al.[25]. As shown in Fig. 2(a), the key intermediate 2 was synthesized previously by complete deprotonation of the triarylmethanol 1 using a large excess of n-BuLi (10 Equiv.) and TMEDA (10 Equiv.), followed by treatment with the mixture of diethyl carbonate (DMC) and diethyl chlorophosphate. Due to the harsh deprotonation condition and subsequent nonselective reaction of the resulting aryl anion with the two electrophiles, the total yield of p1TAM-H/p1TAM-D was very low (1.6%, from the triarylmethanol 1) and the isolation was troublesome due to the presence of multiple byproducts.
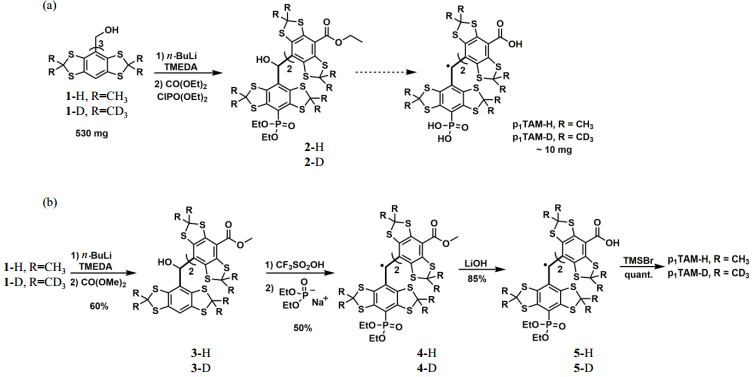
|
Fig. 2 Synthesis of the monophosphonated TAM radicals p1TAM-H and p1TAM-D using the (a) previous[25] and (b) present procedures |
Li et al.[30] reported the seminal work for one-step synthesis of asymmetric TAM radicals by nucleophilic substitution of the TAM cations with various nucleophiles in aqueous solution. This approach was further extended to the synthesis of the monosubstituted TAM radicals in organic solvents[31, 32]. In the present work, we attempted to synthesize p1TAM-H and p1TAM-D using this novel approach with moderate modifications [Fig. 2(b)]. Firstly, the triarylmethanol 1[33-35] in tetrahydrofuran (THF) was partially deprotonated using n-BuLi (3 Equiv.) and TMEDA (3 Equiv.), followed by addition of DMC (30 Equiv.) to afford the diester 3 in a yield of 60%. Then, the diethyl phosphonated TAM radical 4 was obtained in a yield of 50% over two steps by CF3SO2OH-induced conversion of the diester 3 into the corresponding TAM cation, followed by selective nucleophilic reaction at the site of the aromatic H with sodium diethyl phosphite which was prepared by reaction of diethyl phosphite with NaH. It was worth noting that the reaction mixture mainly contained two compounds (i.e., the compound 4 and the radical form of the diester 3)[31, 32], thus enabling facile purification of the compound 4 by column chromatography on silica gel. Finally, the compound 4 underwent two continuous hydrolytic reactions using LiOH and TMSBr to give the water-soluble p1TAM-H and p1TAM-D in a yield of 85% over two steps, which were conveniently purified by column chromatography on reversed-phase C-18. The use of the relatively weak base LiOH instead of KOH improved the yield from 65% to 85%. Compared to the previously reported procedure[25], our new approach exhibits the advantages of nearly 16-fold higher total yield (25% vs. 1.6% from the triarylmethanol 1) and more convenient purifications.
With p1TAM-H and p1TAM-D in hand, we started to synthesize their PEGylated analogues in order to improve their biocompatibility and eliminate their potential binding with albumins[28, 36]. As shown in Fig. 3, The PEGylated TAM probes POP and dPOP were conveniently obtained in almost quantitative yields by SN2 reaction of the dicarboxylates 5-D/5-H with dD1/D1 in the presence of Cs2CO3 and catalytic amount of KI, followed by TMSBr-induced hydrolysis. The probes POP and dPOP were characterized by EPR, 1H NMR and high resolution mass spectroscopy. POP: HRMS (MALDI-TOF), a broad peak from 3 100 to 4 500 (average, n∼7). 1H NMR (400 MHz, CD3OD), δ 4.24 (m, 12H), 3.64 ~ 3.74 (m, 149H), 3.53~3.55 (m, 12H), 3.36 (m, 18H), 2.59 (d, J=2.56 Hz, 12H). dPOP: HRMS (MALDI-TOF), a broad peak from 3 300 to 4 200 (average n∼7). 1H NMR (400 MHz, CD3OD) δ 4.25 (m, 12H), 3.61~3.73 (m, 149H), 3.53~3.56 (m, 18H), 3.36 (m, 18H), 2.59 (d, J=3.4 Hz, 12H).
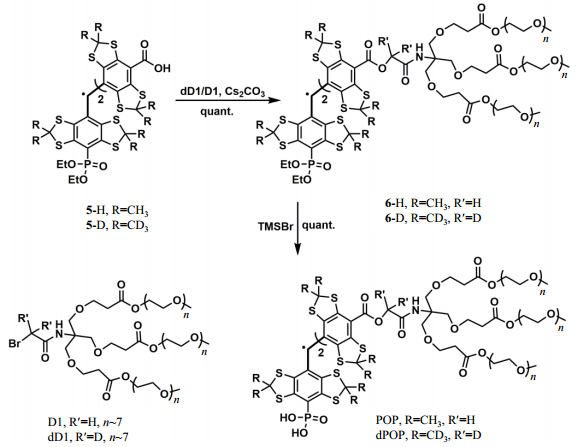
|
Fig. 3 Synthesis of PEGylated TAM analogues POP and dPOP |
Fig. 4 shows EPR spectra of the probes POP and dPOP recorded in phosphate buffer (PB, 1 mol/L) at different pH values (8.6, 6.8 and 4.9) under anaerobic conditions. At pH 8.6, POP existed as a form of POP2- with the phosphonate group being completely deprotonated and exhibits a doublet of quintet (2×5 lines) spectral pattern due to hyperfine splittings from phosphorus (αP) in the phosphonate and four equivalent protons (αH) in two ester linkers. EPR spectral simulation showed that POP2- has the αP and αH values of 3.370×10-4 T and 1.02×10-5 T (Table 1), respectively. Similar spectral pattern was also observed for the partially deprotonated form POP1- at pH 4.9 which has a larger αP value (3.565×10-4 T) but almost same αH value (1.02×10-5 T) compared to POP2-. At nearly neutral pH (6.8), both of two ionization states are present in aqueous solution and their EPR signals are overlapped to a great extent due to relatively small difference of their αP values (ΔαP=1.95×10-5 T) and the presence of multiple proton hyperfine splittings. The 2D EPR analysis also revealed slightly different g factors as 2.003 53 for POP2- and 2.003 55 for POP1-. Comparatively, EPR spectral pattern of dPOP is much simple with a doublet signal from the phosphorus hyperfine splitting either at low or high pH [Fig. 4(b)], because the deuteration eliminates proton hyperfine splittings in the ester linkers. At neutral pH, the low field signals from dPOP2- and dPOP1- were distinguishable. However, the high field peaks of dPOP2- and dPOP1- were merged into one peak with slight line broadening most likely because the field difference (ΔαP) due to αP values of two ionization states is nearly equal to the shift caused by Δg = 0.000 02.
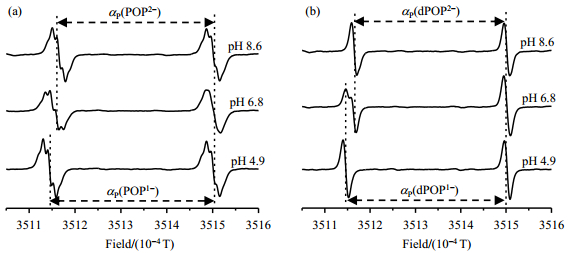
|
Fig. 4 EPR spectra of (a) POP (100 µmol/L) and (b) dPOP (100 µmol/L) in phosphate buffer (1 mmol/L) containing NaCl (150 mmol/L), measured at pH 8.6, 6.8 and 4.9 under argon atmosphere and at room temperature |
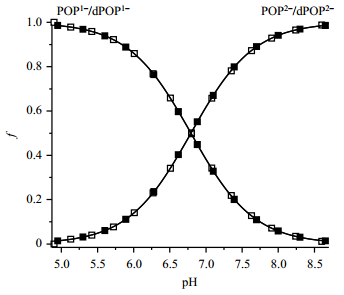
In order to obtain the reliable pKa values, pH titrations of POP and dPOP in phosphate buffer (1 mmol/L) containing NaCl (150 mmol/L) were carried out using low modulation amplitude (2×10-6 T) and modulation frequency (10 kHz) under anaerobic conditions. The pH-dependent fractions of POP2-/POP1- or dPOP2-/dPOP1- were obtained by simulating the individual EPR spectra. These fractions were then fitted by standard titration equation [Equ. (1)] to afford the corresponding pKa values of 6.82 for POP and 6.75 for dPOP (Table 1). In addition, the EPR spectra obtained were also simulated by the 2D EPR approach. This approach enables efficient and accurate determination of pKa by simultaneous fitting of all of spectra in each titration using a whole set of parameters (hfs, g-value, linewidth and pKa). The quality of fit can be significantly improved by taking into account the broadening caused by non-resolved hyperfine splitting of protons or deuteriums. As Fig. 5 shows, smooth fitting of the titration curves provides pKa values of 6.80 for POP and 6.79 for dPOP, which are slightly different from the values obtained by the traditional method. Comparison of pKa of p1TAM-D (about 6.90)[25] with that of dPOP (about 6.79) indicates that the esterification using the PEGylated dendron only slightly decreases pKa of the monophosphonated TAM radical.
| Table 1 pKa values of POP and dPOP and EPR spectral parameters of their different ionization states |
|
Fig. 5 Plot of f of the ionization states of POP (unfilled square) and dPOP (filled square) as a function of pH. The fractions were obtained by simulating the corresponding EPR spectra using the 2D mode. Solid lines represent the best fits using standard titration equation yielding pKa of 6.80±0.05 for POP and 6.79±0.04 for dPOP, for the second deprotonation of phosphonic acid group in the probes |
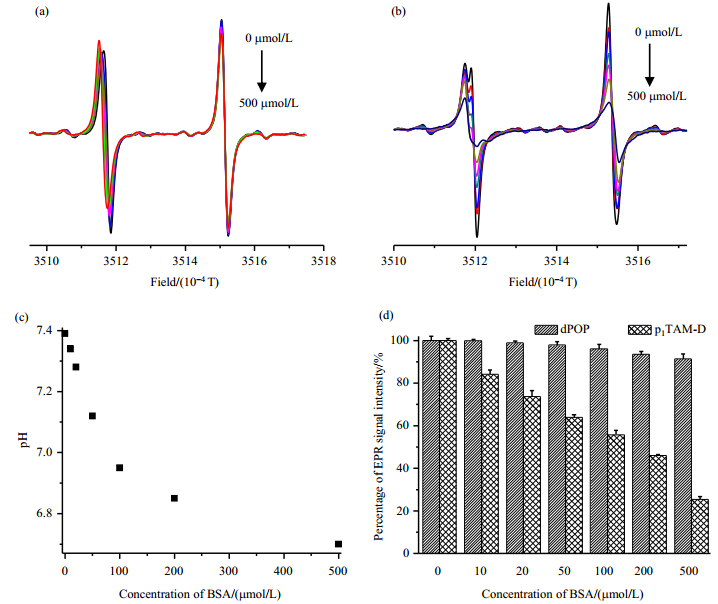
Our previous studies showed that dendritic PEGylation endows TAM radicals with multiple advantages including negligible albumin binding, enhanced biostability and prolonged pharmacological kinetics in mice[28]. To check if the PEGylated probes in this study have similar properties, we firstly investigated and compared the concentration-dependent effect of bovine serum albumin (BSA) on EPR spectra of dPOP and p1TAM-D in PB (1 mmol/L, pH 7.4). As shown in Fig. 6(a), the EPR doublet signal of dPOP in ambient air seems to be almost symmetric due to moderate O2-induced line broadening. Upon addition of BSA (0~500 μmol/L), EPR spectral profile of dPOP do not show any change, indicating no marked binding between them [Fig. 6(a)]. Comparatively, p1TAM-D, like the TAM radical CT-03[27], binds strongly with BSA as verified by severe distortion of its EPR signal due to the line broadening [Fig. 6(b)]. Interestingly, the low field peaks of both pH probes are gradually shifted to low field possibly because the addition of BSA led to the decrease of pH [see Fig. 6(a) and Fig. 6(b)]. This pH decrease induced by addition of BSA is further confirmed by pH electrode [Fig. 6(c)]. The high field peak intensity of dPOP only slightly decreases with the concentration of BSA and 92% of the peak intensity remained in the presence of 500 μmol/L of BSA while only 25% of the peak intensity can be identified for p1TAM-D [Fig. 6(d)].
|
Fig. 6 EPR spectra of dPOP (a) and p1TAM-D (b) in the presence of BSA (0, 10, 20, 50, 100, 200 and 500 μmol/L); (c) Effect of BSA on the pH in PB (1 mmol/L, 150 mmol/L NaCl, pH 7.4) that was measured by pH electrode; (d) Effects of BSA concentration on the EPR peak-to-trough height of dPOP and p1TAM-D |
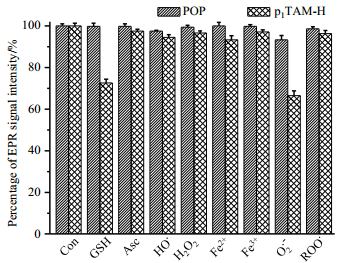
As shown in Fig. 7, the PEGylation modification also enhances the stability of POP toward various reactive species. Almost no signal decay was observed for POP after 30 min incubation with oxidants (e.g., superoxide radical, hydroxyl radical, peroxyl radical, hydrogen peroxide) and reductants (e.g., ascorbate and glutathione). On the contrary, the paramagnetism of p1TAM-H was quenched to different degrees by these oxidoreductants, especially by superoxide radical and glutathione. Similar reactivity with these reactive species was also observed for other TAM radicals[20, 37-39].
|
Fig. 7 Effects of various reactive species on POP and p1TAM-H expressed as a percentage of TAM radicals remaining after exposure to reactive species for 30 min. The percentage was obtained by comparing the signal intensity of the EPR signal in each system with that of TAM radicals (20 µmol/L). Con: control, GSH: glutathione, Asc: ascorbate, HO•: hydroxyl radical, H2O2: hydrogen peroxide, O2•-: superoxide radical, ROO•: peroxyl radical. Each experiment was carried out at least three times. See details in our previous study[38] |
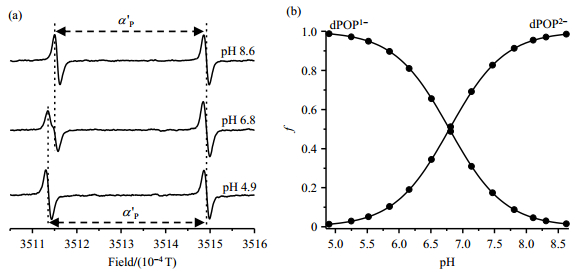
To confirm the accurate measurement of pH using dPOP as the probe in the presence of BSA which may show great interference in applied system, its EPR spectra were recorded in PB (1 mmol/L) containing 500 μmol/L BSA. As shown in Fig. 8(a), EPR spectral profile of dPOP did not show any distorance compared with the according EPR spectra in the absence of BSA [Fig. 4(b)]. Plotting of fractions of the ionization states of dPOP as a function of pH using equation (1) provides a pKa of 6.79 for dPOP in the presence of BSA [Fig. 8(b)], which is same as the value that obtained in PB alone. Therefore, the PEGylation solves the albumin binding issue of p1TAM-H/p1TAM-D and guarantees the application of dPOP in albumin-rich biological systems such as blood. Moreover, EPR signal of dPOP also exhibits O2-sensitive line broadening which is usually used to measure O2 concentration. The ratio of the line widths under aerobic and anaerobic conditions is used to indicate the O2 sensitivity of various probes as did in the previous study[28]. As shown in Table 1, two ionization states of dPOP have similar O2 sensitivity with the ratio value of about 1.5, which is higher than the corresponding value of POP (about 1.3). Interestingly, the O2 sensitivity of dPOP (1.57 for dPOP1- and 1.62 for dPOP2-) slightly increases in the presence of BSA (500 μmol/L) possibly due to higher concentration of dissolved O2 in the solution containing BSA.
|
Fig. 8 (a) EPR spectra of dPOP (20 µmol/L) in phosphate buffer (1 mmol/L) containing NaCl (150 mmol/L) and 500 μmol/L BSA measured at various pH conditions under argon atmosphere and room temperature. a'P: hyperfine splittings from phosphorus in the presence of BSA. (b) Plot of fractions of the ionization states of dPOP as a function of pH, using Equ. (1) that provides a pKa of 6.79±0.03 |
In summary, p1TAM-H and p1TAM-D have been efficiently synthesized and gram-scale synthesis could be conveniently achieved, thus enabling their further derivatization. Unlike p1TAM-D, its PEGylated derivative dPOP still exhibits good pH and O2 sensitivity in the presence of high concentration of BSA (500 μmol/L). The PEGylation could also improve the biocompatibility and extend the blood circulation time of dPOP as observed in our previous study[28]. Thus, dPOP could be administrated by intravenous injection and overcome the limitation of p1TAM-D and the triphosphonated TAM probe which were injected intratissually[25, 40]. Therefore, our present study provides a novel strategy for the design and efficient synthesis of new pH and O2 dual EPR probes with improved properties which could significantly advance the application of in vivo EPR spectroscopy and the imaging in biomedical field.
| [1] | MULKEY D K, RD H R, RITUCCI N A, et al. Oxidative stress decreases pHi and Na(+)/H(+) exchange and increases excitability of solitary complex neurons from rat brain slices[J]. Am J Physiol Cell Physiol, 2004, 286(4): C940-C951. |
| [2] | LAGADIC-GOSSMANN D, HUC L, LECUREUR V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles[J]. Cell Death Differ, 2004, 11(9): 953-961. |
| [3] | PEPPICELLI S, BIANCHINI F, CALORINI L. Extracellular acidity, a " reappreciated" trait of tumor environment driving malignancy: perspectives in diagnosis and therapy[J]. Cancer Metast Rev, 2014, 33(2/3): 823-832. |
| [4] | KHRAMTSOV V V. Biological imaging and spectroscopy of pH[J]. Curr Org Chem, 2005, 9(9): 909-923. DOI: 10.2174/1385272054038309. |
| [5] | HICKS R G. Functional in vivo EPR spectroscopy and imaging using nitroxide and trityl radicals[M]. Wiltshire: Antony Rowe Ltd, 2010. |
| [6] |
FAN K, GUO J W, ZOU J R, et al. An EPR modulation magnetic field driving device for in vivo tooth dosimetry[J].
Chinese J Magn Reson, 2017, 34(3): 365-371.
范凯, 郭俊旺, 邹洁芮, 等. EPR在体测量专用调制磁场驱动装置[J]. 波谱学杂志, 2017, 34(3): 365-371. |
| [7] | ULLMAN E F, CALL L, OSIECKI J H. Stable free radicals. Ⅷ. New imino, amidino, and carbamoyl nitroxides[J]. J Org Chem, 1970: 3623-3631. |
| [8] | KHRAMTSOV V V, WEINER L M, EREMENKO S I, et al. Proton exchange in stable nitroxyl radicals of the imidazoline and imidazolidine series[J]. J Magn Reson, 1985, 61(3): 397-408. |
| [9] | ROCKENBAUER A, SZABO-PLANKA T, ARKOSI Z, et al. A two-dimensional (magnetic field and concentration) electron paramagnetic resonance method for analysis of multispecies complex equilibrium systems. Information content of EPR spectra[J]. J Am Chem Soc, 2001, 123(31): 7646-7654. |
| [10] | CLEMENT J L, BARBATI S, FREJAVILLE C, et al. Synthesis and use as spin-trap of 5-methyl-5-phosphono-1-pyrroline N-oxide (DHPMPO). pH dependence of the EPR parameters of the spin adducts[J]. J Chem Soc Perkins Trans2, 2001, 59(9): 1471-1475. |
| [11] | GALLEZ B, M DER K, SWARTZ H M. Noninvasive measurement of the pH inside the gut by using pH-sensitive nitroxides. An in vivo EPR study[J]. Magn Reson Med, 1996, 36(5): 694-697. |
| [12] | MADER K, GALLEZ B, LIU K J, et al. Non-invasive in vivo characterization of release processes in biodegradable polymers by low-frequency electron paramagnetic resonance spectroscopy[J]. Biomaterials, 1996, 17(4): 457-461. |
| [13] | POTAPENKO D I, FOSTER M A, LURIE D J, et al. Real-time monitoring of drug-induced changes in the stomach acidity of living rats using improved pH-sensitive nitroxides and low-field EPR techniques[J]. J Magn Reson, 2006, 182(1): 1-11. |
| [14] | GOODWIN J, YACHI K, NAGANE M, et al. In vivo tumour extracellular pH monitoring using electron paramagnetic resonance: the effect of X- ray irradiation[J]. NMR Biomed, 2014, 27(4): 453-458. |
| [15] | ARDENKJAER-LARSEN J H, LAURSEN I, LEUNBACH I, et al. EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging[J]. J Magn Reson, 1998, 133(1): 1-12. |
| [16] | REDDY T J, IWAMA T, HALPERN H J, et al. General synthesis of persistent trityl radicals for EPR imaging of biological systems[J]. J Org Chem, 2002, 67(14): 4635-4639. |
| [17] | LIU Y, VILLAMENA F A, SUN J, et al. Synthesis and characterization of ester-derivatized tetrathiatriarylmethyl radicals as intracellular oxygen probes[J]. J Org Chem, 2008, 73(4): 1490-1497. |
| [18] |
LIU Y P, ZWEIER J L. Synthesis and EPR characterization of a novel functional biradical[J].
Chinese J Magn Reson, 2010, 27(1): 95-102.
刘阳平, ZWEIERJ L. 一种新型功能化双自由基的合成及EPR表征[J]. 波谱学杂志, 2010, 27(1): 95-102. |
| [19] | BOBKO A A, DHIMITRUKA I, ZWEIER J L, et al. Trityl radicals as persistent dual function pH and oxygen probes for in vivo electron paramagnetic resonance spectroscopy and imaging: Concept and experiment[J]. J Am Chem Soc, 2007, 129(23): 7240-7241. |
| [20] | LIU Y P, VILLAMENA F A, ZWEIER J L. Highly stable dendritic trityl radicals as oxygen and pH probe[J]. Chem Commun, 2008(36): 4336-4338. |
| [21] | DHIMITRUKA I, BOBKO A A, HADAD C M, et al. Synthesis and characterization of amino derivatives of persistent trityl radicals as dual function pH and oxygen paramagnetic probes[J]. J Am Chem Soc, 2008, 130(32): 10780-10787. DOI: 10.1021/ja803083z. |
| [22] | DRIESSCHAERT B, MARCHAND V, LEVEQUE P, et al. A phosphonated triarylmethyl radical as a probe for measurement of pH by EPR[J]. Chem Commun, 2012, 48(34): 4049-4051. |
| [23] | BOBKO A A, DHIMITRUKA I, KOMAROV D A, et al. Dual-function ph and oxygen phosphonated trityl probe[J]. Anal Chem, 2012, 84(14): 6054-6060. |
| [24] | MARCHAND V, LEVEQUE P, DRIESSCHAERT B, et al. In vivo EPR extracellular pH-metry in tumors using a triphosphonated trityl radical[J]. Magn Reson Med, 2017, 77(6): 2438-2443. |
| [25] | DHIMITRUKA I, BOBKO A A, EUBANK T D, et al. Phosphonated trityl probes for concurrent in vivo tissue oxygen and pH monitoring using electron paramagnetic resonance-based techniques[J]. J Am Chem Soc, 2013, 135(15): 5904-5910. |
| [26] |
CHEN Y, SUN P, LIU M L, et al. Effects of metal ions on human serum albumin studied by radiation damping water-ligand observed via gradient spectroscopy[J].
Chinese J Magn Reson, 2017, 34(3): 266-274.
陈瑶, 孙鹏, 刘买利, 等. 离子对人血清白蛋白影响的1H NMR研究[J]. 波谱学杂志, 2017, 34(3): 266-274. |
| [27] | SONG Y G, LIU Y P, LIU W B, et al. Characterization of the binding of the Finland trityl radical with bovine serum albumin[J]. RSC Adv, 2014, 4(88): 47649-47656. |
| [28] | LIU W B, NIE J P, TAN X L, et al. Synthesis and characterization of PEGylated trityl radicals: Effect of PEGylation on physicochemical properties[J]. J Org Chem, 2017, 82(1): 588-596. |
| [29] | ROCKENBAUER A, KORECZ L. Automatic computer simulations of ESR spectra[J]. Appl Magn Reson, 1996, 10(1): 29-43. |
| [30] | DECROOS C, PRANGE T, MANSUY D, et al. Unprecedented ipso aromatic nucleophilic substitution upon oxidative decarboxylation of tris(p-carboxyltetrathiaaryl)methyl (TAM) radicals: a new access to diversely substituted TAM radicals[J]. Chem Commun, 2011, 47(16): 4805-4807. |
| [31] | ROGOZHNIKOVA O Y, VASILIEV V G, TROITSKAYA T I, et al. Generation of trityl radicals by nucleophilic quenching of tris (2, 3, 5, 6-tetrathiaaryl)methyl cations and practical and convenient large-scale synthesis of persistent tris(4-carboxy-2, 3, 5, 6-tetrathiaaryl) methyl radical[J]. Eur J Org Chem, 2013, 2013(16): 3347-3355. DOI: 10.1002/ejoc.201300176. |
| [32] | TORMYSHEV V M, ROGOZHNIKOVA O Y, BOWMAN M K, et al. Preparation of diversely substituted triarylmethyl radicals by the quenching of tris(2, 3, 5, 6-tetrathiaaryl)methyl cations with C-, N-, P-, and S-nucleophiles[J]. Eur J Org Chem, 2014, 2014(2): 371-380. |
| [33] | XIA S J, VILLAMENA F A, HADAD C M, et al. Reactivity of molecular oxygen with ethoxycarbonyl derivatives of tetrathiatriarylmethyl radicals[J]. J Org Chem, 2006, 71(19): 7268-7279. |
| [34] | DHIMITRUKA I, VELAYUTHAM M, BOBKO A A, et al. Large-scale synthesis of a persistent trityl radical for use in biomedical EPR applications and imaging[J]. Bioorg Med Chem Lett, 2007, 17(24): 6801-6805. |
| [35] | DHIMITRUKA I, GRIGORIEVA O, ZWEIER J L, et al. Synthesis, structure, and EPR characterization of deuterated derivatives of Finland trityl radical[J]. Bioorg Med Chem Lett, 2010, 20(13): 3946-3949. |
| [36] | SONG Y G, LIU Y P, HEMANN C, et al. Esterified dendritic TAM radicals with very high stability and enhanced oxygen sensitivity[J]. J Org Chem, 2013, 78(4): 1371-1376. |
| [37] | RIZZI C, SAMOUILOV A, KUTALA V K, et al. Application of a trityl-based radical probe for measuring superoxide[J]. Free Radical Bio Med, 2003, 35(12): 1608-1618. |
| [38] | LIU Y, SONG Y, DE PASCALI F, et al. Tetrathiatriarylmethyl radical with a single aromatic hydrogen as a highly sensitive and specific superoxide probe[J]. Free Radical Bio Med, 2012, 53(11): 2081-2091. |
| [39] | TAN X, CHEN L, SONG Y, et al. Thiol-dependent reduction of the triester and triamide derivatives of finland trityl radical triggers O2-dependent superoxide production[J]. Chem Res Toxicol, 2017, 30(9): 1664-1672. |
| [40] | BOBKO A A, EUBANK T D, DRIESSCHAERT B, et al. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression[J]. Sci Rep, 2017, 7: 41233-41244. |
 2019, Vol. 36
2019, Vol. 36 
