2. Department of Traditional Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou 510006, China;
3. Engineering & Technology Research Center for Chinese Materia Medica Quality of the Universities of Guangdong Province, Guangzhou 510006, China
2. 广东药科大学 中药学院, 广东 广州 510006;
3. 广东高校中药质量工程技术研究中心, 广东 广州 510006
Insulin resistance (IR), defined as the decreased response of peripheral tissue to normal insulin levels, is a known pathogenesis basis of type 2 diabetes mellitus (T2DM). IR is also correlated with an increased risk of complications including cardiovascular diseases and metabolic syndrome (MS)[1]. The morbidity and prevalence of these IR-associated diseases are increasing dramatically worldwide[2], and the influence of these metabolic diseases is likely to be worsen as the global urbanization level is predicted to reach up to 60% in 2025[3]. With the changes in modern lifestyle, it is increasingly common for people to eat food containing high fructose contents. Fructose is the sweetest natural sugar, which widely exists in honey, fruits and vegetables. Studies have shown that high fructose intake is significantly correlated with IR, cardiovascular disease, kidney disease and T2DM[4-6]. In our previous study, it was found that high fructose level could induce IR and the corresponding metabolism disorders[7].
Currently, many western medicines such as metformin, captopril and gemfibrozil are available for the treatment of IR. These drugs primarily act as hypoglycemic drugs, lipid regulators and hypotensive drugs[8, 9]. However, they have several disadvantages such as toxic reactions, drug tolerance and high costs. Traditional Chinese medicines (TCMs) have been effectively used for the treatment of T2DM for thousands of years, and their therapeutic effect is multi-targeted and multi-faceted. Wang[10] demonstrated the effect of Huanglian Jiedu Decoction (HJD) on improving IR. It was reported that the treatment with Sanhuang Jiangtang (SHJT) recipe decreased insulin peripheral resistance (partial reversal) by reducing hyperinsulinemia and improving insulin sensitivity[11]. Zou et al.[12] showed that Radix Puerariae Lobatae has the ability to improve the insulin resistance of fat cells. Chinese complex prescription Gegen Qinlian Decoction (GQD), involving four kinds of Chinese herbs: Ge Gen(Radix Puerariae Lobatae), Huang Lian (Rhizoma Coptidis), Huang Qin (Radix Scutellariae), Gan Cao (Radix Glycyrrhizae), is commonly used in the treatment of IR-related diseases. Clinical studies have reported that GQD could significantly reduce blood glucose in patients with diabetes[13]. Li et al.[14] reported that GQD could increase the sensitivity of tissues to insulin and improve the antioxidative ability of living systems. It has also been reported that the hypoglycemic effect of GQD was similar to those of metformin and gliclazide[15, 16].
Gut microbiota is one of the largest and most populous microbial ecosystems on earth, and its symbiotic and mutualistic relationship with the host determines a complex and dynamic ecosystem. As is known, gut microbiota is extensively involved in human health and disease, and unbalanced microbial colonies may disturb the physiological homeostasis leading to various diseases such as IR, MS and cardiovascular diseases[17-20]. Studies have reported that alterations in the gut microbiota composition may contribute to the pathophysiology of obesity and its related comorbidities[21, 22]. It has been reported that GQD had an effect on the structural alterations of gut microbiota in IR and T2DM patients[23-25]. Additionally, studies have shown that berberine, puerarin, baicalin and glycyrrhizin, the active components of GQD, could regulate the metabolism of intestinal flora[26-28]. So far, there is limited research regarding the metabolic regulation effect of GQD on fecal metabolism in fructose-induced IR.
Metabonomics is a new technique to explore the metabolic responses throughout entire organisms induced by external and intrinsic factors. As an effective and noninvasive method, it has been extensively used to research the treatment effect of TCMs[29, 30]. Zhang et al.[31] and Wang et al.[32] summarized the potential applications of robust metabonomics approaches in the area of TCMs and highlighted the key role of metabonomics on metabolic biomarker discovery.
In this study, we established the IR model by feeding rats with 10% fructose drinking water as described in previous reports[7, 33]. Then we studied the effects of GQD on fecal metabolism by proton nuclear magnetic resonance (1H NMR)-based metabonomics in order to elucidate the metabolic regulation effect of GQD on gut microbiota disorders in IR rats.
1 Materials and methods 1.1 Preparation of GQDGQD contains four herbal materials, Ge Gen(Radix Puerariae Lobatae) (batch number: 20150501), Huang Lian (Rhizoma Coptidis) (batch number: 20150701), Huang Qin (Radix Scutellariae) (batch number: 20150101) and Gan Cao (Radix Glycyrrhizae) (batch number: 20150801), which were purchased from Kang St. Pharmaceutical Co., Ltd. (Guangzhou, China) and identified by Associate Prof. Suying Tian (Experiment Centre of Guangdong Pharmaceutical University). The ratio of the four herbal materials in GQD is 8:3:3:2, and all the herbal materials are officially listed in the Chinese Pharmacopoeia 2015. GQD was prepared according to the previous reports[34]. Radix Puerariae lobatae was boiled for 20 min before mixing with the other crude drugs. The mixture was then decocted twice with a 10-fold excess mass of water for the first time of 1.0 h and for the second time of 0.5 h. Finally, the extracted solutions were mixed, filtered and concentrated to achieve the final concentration of 1 g/mL and then stored at 4 ℃.
1.2 Animal experiments and sample collection32 male Wistar rats (150~180 g) were obtained from the Medical Laboratory Animal Center of Southern Medical University. All animal experiments were reviewed and approved by the Ethics Committee of Guangdong Pharmaceutical University. The 32 rats were kept under well-ventilated animal lab conditions with a 12 h/12 h light-dark cycle, ambient temperature of (25±1) ℃ and relative humidity of (50%±10%). The animals were provided a certified standard food. After adaptation for one week, the rats were randomly assigned to four groups: a control group (n=8), an IR model group (n=8), a GQD treatment group (n=8), and a rosiglitazone treatment group (n=8, as positive control drug). The IR modeling method was in accordance with the previously reported method[7, 33]. The control group was given distilled water for 8 weeks, and the other three groups were fed with 10% fructose water for 8 weeks. Simultaneously, the rosiglitazone-treated group was gavaged with rosiglitazone (3.0 mg/kg/day)[35] and the GQD-treated rats were gavaged with the extract of GQD (18.2 g/kg/day)[36, 37] from the 5th to 8th week. The weights of the rats were measured every week and the dose was adjusted according to the weight.
For each group, fecal samples were collected at the end of the 4th, 6th and 8th weeks. Fecal samples were taken immediately from the metabolic cage before the animals were removed. All the samples for NMR analysis were stored in a -80 ℃ freezer.
1.3 Fecal sample preparation250 mg fecal samples were weighed after removing excess water with absorbent paper. Then, the samples were mixed with 1 mL of phosphate buffer solution (PBS, 0.2 mol/L Na2HPO4/NaH2PO4, pH 7.4), and subjected to 10 cycles of ultrasonic-vortex-stationary operation (ultrasonic 20 s-vortex 10 s-stationary 30 s). After the subsequent centrifugation (14 000 rpm, 10 min, 4 ℃), 400 μL of the supernatant was pipetted into a 5 mm NMR tube, and 100 μL D2O containing 0.05% 3-trimethylsilyl-(2, 2, 3, 3-d4)-1- propionate (TSP, as the internal standard) was added.
1.4 1H NMR experiment1H NMR spectra of all samples were collected at 298 K on a Bruker spectrometer (Avance III, 500 MHz). In order to represent the total metabolite compositions, the 1H NMR spectra of the fecal extract samples were recorded using the water-presaturated standard one-dimensional NOESYPR1D pulse sequence (recycle delay-90˚-t1-90˚-tm-90˚-acquisition). 128 transients were collected with 32 k data points. The spectral width was 10 kHz with a relaxation delay of 3 s and a mixing time (tm) of 100 ms, and t1 was set to 3 μs. Before Fourier transformation, the free induction decays (FIDs) were multiplied by an exponential function with a line broadening factor of 0.3 Hz. To assist metabolite assignment, two-dimensional NMR (2D NMR) spectra such as 1H-1H correlation spectroscopy (COSY) and 1H-13C heteronuclear single quantum coherence spectroscopy (HSQC) were carried out on the selected samples. COSY experiment was performed with a total of 128 increment and 80 transients accumulated into 2 048 data points with spectral width of 5.25 kHz for both dimensions. For HSQC, 2 048 data points with 240 scans per increment and 120 increments were acquired with the spectral widths of 5 kHz and 18.75 kHz for 1H and 13C respectively.
1.5 Pattern recognition and statistical analysisThe phase and baseline of all the spectra were corrected manually using Topspin software (Version 2.1, Bruker Biospin, Germany), and referenced to the chemical shift of TSP (δH 0.00). Then, the spectra were bucketed and automatically integrated with an automation routine in AMIX (Bruker Biospin, Germany). The spectra in the region of δH 0.50~9.00 were segmented into integral regions with equal widths of 10 Hz. In order to reduce the effects of water suppression, the region of δH 4.70~5.20 was discarded. The integrals of these buckets were normalized to the total sum of the spectral integrals.
All the normalized integral values were submitted for principal component analysis (PCA) and orthogonal partial least squares projection on discriminant analysis (OPLS-DA) using Simca-P+12.0 software (Umetrics, Sweden). The results were presented as scores and loadings plots. The significance of the contributions of different metabolites was indicated by color-coded variables. The significance was reduced gradually from a "hot" color (e.g., red) to a "cold" color (e.g., blue). In this study, statistical analysis was performed for the metabolites screened in multivariate analysis using SPSS software (Version 19.0, SPSS Inc., USA). A value of p < 0.05 was considered significant.
1.6 Body weight measurements, fasting plasma glucose and IR index assessmentWe measured the body weight of each animal every week. Following the method of Barham and Trinder[38], fasting plasma glucose (FPG) levels were assayed colorimetrically by using a Randox reagent kit. Blood insulin levels were determined with a sandwich ELISA (Millipore) using a microtiter plate coated with mouse monoclonal anti-rat insulin antibody. The homeostasis model assessment for the insulin resistance index (IR index) was calculated according to the following equation[39]: HOMA-IR= fasting blood glucose levels (FPG, mmol/L)× fasting insulin levels (FINS, mU/L)/22.5. An independent sample t test was used for the comparison between the control, IR model and GQD-treated rats.
2 Results 2.1 Analysis of biochemical indexesThe average water and food intake of IR model rats were higher than those of the control group rats (Table 1). It was found that high fructose intake significantly increased the weight and IR index of the IR model rats from the 4th to 8th week (p < 0.05) (Table 2), while the FPG increased only at the end of the 8th week, similar to the previously reported results[7]. These data showed the successful establishment of IR model rats. In the GQD-treated group, the water and food intake decreased in comparison with the IR model group. Moreover, GQD treatment significantly reduced the body weight and IR index to the level of control group, which suggested that GQD had an effect on improving insulin resistance.
| Table 1 The water and food intake at the 4th and 8th weeks of rats in each group |
| Table 2 Body weight, FPG and IR index of rats in each group at the end of the 4th and 8th weeks |
Fig. 1 shows the representative fecal 1H NMR spectra from each group at the end of the 8th week. Endogenous metabolites were assigned based on literature[40-42] and two-dimensional spectra (Table S1, available on the CJMR website at doi: 10.11938/cjmr20182643). We observed that the main metabolites in the fecal extract spectra were short chain fatty acids (SCFAs, such as butyrate, propionate and acetate), amino acids (leucine, isoleucine, valine, alanine and lysine), uracil, lactate, glucose and so on. Their characteristic 1H NMR signals were listed in Table S1. Visually, there were no obvious differences among these four groups in the fecal metabolic profiles because of the individual variability. Therefore, we analyzed the fecal metabolic changes by using multivariate data analysis.
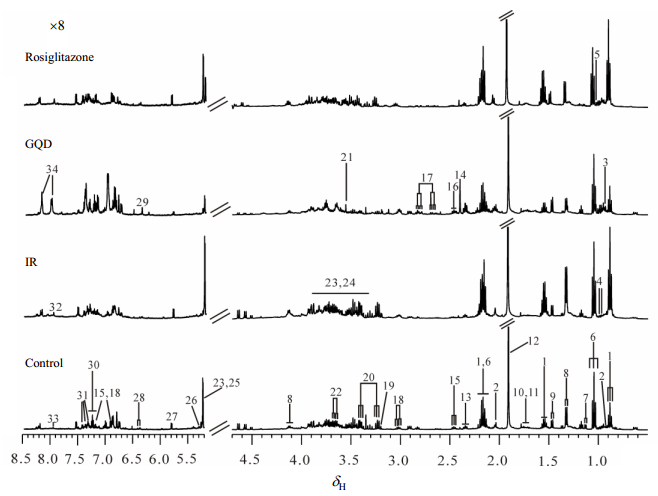
|
Fig. 1 Representative 1H NMR spectra of fecal extracts from each group at the end of the 8th week (500 MHz). Keys: 1. n-butyrate; 2. isovalerate; 3. isoleucine; 4. leucine; 5. valine; 6. propionate; 7. α-ketoisovalerate; 8. lactate; 9. alanine; 10. lysine; 11. cadaverine; 12. acetate; 13. glutamate; 14. succinate; 15. 3-(4'-hydroxyphenyl) propionate 16. glutamine; 17. aspartate; 18. tyrosine; 19. choline; 20. taurine; 21. glycine; 22. glycerol; 23. α-glucose; 24. β-glucose; 25. α-arabinose; 26. α-galactose; 27. uracil; 28. urocannate; 29. fumarate; 30. tryptophan; 31. phenylalanine; 32. histidine; 33. xanthine; 34. adenine |
Fig. 2 presents the three-dimensional diagrams of score plots in the PCA analysis at the end of 4th, 6th and 8th weeks. At the end of 4th week [Fig. 2(a)], the controls were separated from the other three groups, indicating the metabolic changes induced by fructose intake. The GQD- and rosiglitazone-treated groups were located in the middle of the control and the IR model groups at the end of 6th week, which demonstrated the therapeutic effect of these two drugs [Fig. 2(b)]. At the end of the 8th week, the differentiations between the IR model group and the two treatment groups were more obvious, and the two treatment groups were closer to the control group [Fig. 2(c)]. These results revealed that the metabolic characteristics of the fecal extracts in the GQD-treated group were similar to those in the control group, and the metabolic regulation effect of GQD was the same as that of rosiglitazone.
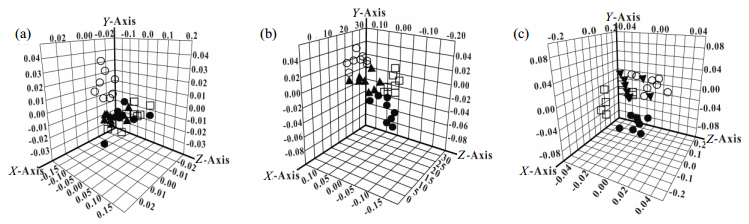
|
Fig. 2 PCA score plots based on 1H NMR data from control group (○), IR model group (●), GQD-treated group (▲) and rosiglitazone-treated group (□) at the end of the 4th (a), 6th (b) and 8th (c) weeks |
In this study, OPLS-DA models were further established to investigate potential biomarkers and elucidate the metabolic regulation effect of GQD (Fig. 3). At the end of the 6th and 8th weeks, the levels of propionate, acetate, succinate, taurine and glycerol decreased, while the levels of n-butyrate, alanine and glutamate increased in IR rats compared to the control group [Fig. 3(a) and 3(d)]. The distinct fecal metabolic differences between the control and the IR model rats were in accordance with our previous published results[7]. At the end of the 6th week, the samples in the GQD-treated group were differentiated from the IR model group [Fig. 3(b)]. The loadings plot showed that the levels of propionate, acetate, succinate, taurine and glycerol increased, while the levels of n-butyrate, alanine and glutamate decreased in GQD-treated rats compared with IR model rats, which indicated the metabolic regulation of the GQD treatment. Compared to the controls, the levels of propionate, acetate and taurine in the GQD-treated group were lower [Fig. 3(c)]. With the prolongation of GQD administration, the metabolic changes in GQD-treated group were accumulated at the end of the 8th week in comparison with the IR model group [Fig. 3(e)]. At the end of the 8th week, the metabolic profile in GQD-treated group was similar with that in controls, only with a mild decrease in acetate level, which suggested the regulation effect of GQD [Fig. 3(f)]. All the models were validated by permutation test (Fig. S1, available on the CJMR website at doi:10.11938/cjmr20182643).

|
Fig. 3 Multivariate data analyses of fecal 1H NMR spectra at the end of the 6th (a~c) and 8th (d~f) weeks. (a) R2X=98.4%, Q2Y=82.2%; (b) R2X =66.3%, Q2Y =85.5%; (c) R2X =55.6%, Q2Y =67.7%; (d) R2X =99.4%, Q2Y =88.9%; (e) R2X =60.8%, Q2Y =89.3%; (f) R2X =45.4%, Q2Y =64.6% |
The statistical analysis results of the selected metabolites screened out in Fig. 3 are summarized in Table 3. The results clearly indicate the metabolic differentiation among the three groups at the end of the 6th and 8th weeks.
| Table 3 The metabolites variations in fecal extracts of control, IR model and GQD-treated groups at the end of the 6th and 8th weeks using multivariate data analysis |
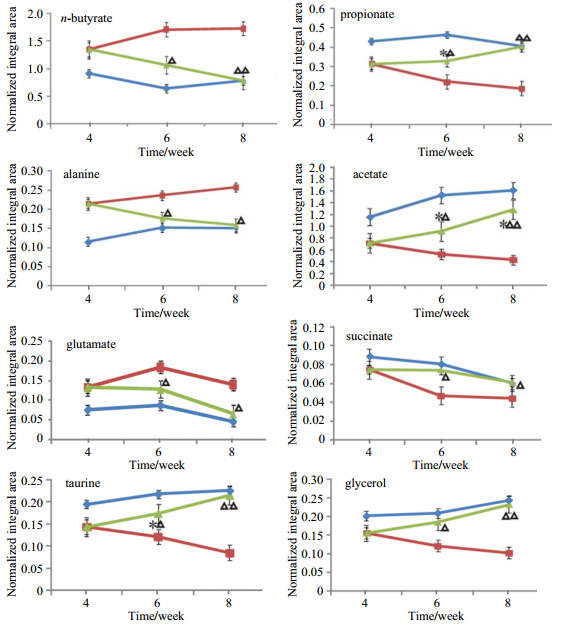
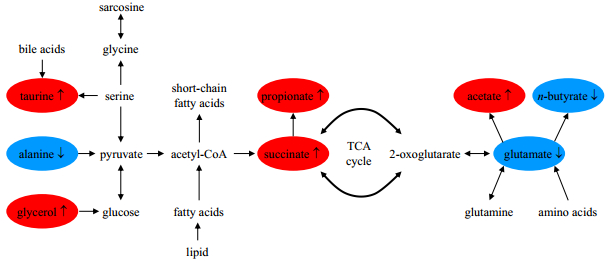
The normalized integral changes of the endogenous fecal metabolites are shown in Fig. 4. It is obvious that the metabolic changes caused by GQD increase with the administration time, which reveale the time-dependent positive metabolic regulation and recovery induced by GQD treatment. The corresponding regulation pathway is given in Fig. 5, accounting for the metabolite changes in fecal extract.
|
Fig. 4 The changes of the normalized integrals from the relevant endogenous metabolites in the control (   |
|
Fig. 5 Effects of GQD treatment on the fecal metabolic pathways involved in IR model rats. Metabolites in red or blue represent a higher or lower level in GQD-treated rats compared with the IR model rats, and metabolites in black are unchanged or undetected |
The results in Table 1 and Table 2 indicate that excessive consumption of fructose induced insulin resistance, which is in accordance with previous study[7]. This confirms that the IR model was successfully established in this study. The metabonomics study evidence that the high fructose intake interfere with the metabolic pathways involving amino acid metabolism, fatty acid oxidation and gut microbiota metabolism, which is consistent with our previous study[7]. The results showed that GQD had significant therapeutic effects on improving IR and could reverse some of the metabolic changes, including restoring the original levels of propionate, acetate, succinate, taurine, glycerol, n-butyrate, alanine and glutamate. Metabolic profiles in the control and GQD-treated groups shared similar characteristics at the end of the 8th week.
As shown in Fig. 2, the GQD-treated group was separated from the IR model group, and it was also slightly different from the control group at the end of the 6th week. At the end of the 8th week, the GQD-treated group was far away from the IR model group and closer to the control group. It indicated that GQD could significantly regulate the metabolic disorder in IR, and the characteristics of GQD treated group were similar to the control group after 4 weeks of GQD treatment. These results indicated that GQD was effective for metabolic regulation of intestinal flora and presented the accumulation of therapeutic effect.
In this study, an increased level of alanine was observed in the IR model group compared with the control group. It has been reported that elevated alanine levels are due to hypoxia and glycolysis[43]. The level of alanine was reversed partially at the end of the 6th week and returned to its normal level at the end of the 8th week after treatment with GQD. This result showed that GQD may improve hypoxia and glycolysis. Decreased level of succinate was observed in the IR model rats compared with the control rats. Microbiota-produced succinate improves glucose through activation of intestinal gluconeogenesis (IGN)[44]. In the GQD-treated group, the level of succinate increased, which indicated that GQD could decrease blood glucose and improve IR.
It was also found that taurine decreased in IR model rats, consistent with previous results[7]. Taurine, one of the most abundant free amino acids, is involved in many life activities in living organisms. In addition, it has been identified as a specific marker of liver toxicity and damage[45, 46], including necrosis and steatosis. Ribeiro et al.[47] demonstrated that taurine supplementation improved glucose tolerance and insulin sensitivity in mice, and also increased insulin secretion from isolated islets. In our study, the level of taurine was increased after administration of GQD. This result suggested that GQD may regulate the metabolism of taurine to improve IR, liver damage or/and dysfunction.
Glycerol is an important precursor for the synthesis of glucose by gluconeogenesis and is also a product of lipolysis[48]. In our study, decreased level of glycerol was observed in the IR model group, which indicated that gluconeogenesis may be inhibited and lipolysis may be enhanced. We found that GQD treatment increased glycerol to a normal level. Therefore, this result suggested that GQD may up-regulate gluconeogenesis and inhibit lipolysis to alleviate the metabolic disorder induced by high fructose intake.
The close association between fecal metabolites and intestinal microbiota is undeniable. The fermentation process by intestinal bacteria mainly produces SCFAs, such as acetate, propionate and n-butyrate, which can indirectly affect the metabolism and activity of intestinal anaerobes, and play an important role in maintaining the normal function of the large intestine. Dolara et al.[49] demonstrated that lower concentrations of SCFAs in feces are associated with higher rates of colonic mucosal proliferation, which is directly related to increased risk of colon cancer. The increase in acetate and propionate levels, induced by the GQD treatment, indicated a possibly decreased risk of colorectal cancer. It has been reported that n-butyrate could prevent colon cancer development by inducing the apoptosis of cancer cells and controlling proto-oncogene expression[50]. However, the level of n-butyrate was found to be up-regulated in IR model rats and decreased in GQD-treated rats in our study. This inconsistency was possibly caused by the difference in the model. It is known that SCFAs are mostly produced by carbohydrates and soluble dietary fiber. Moreover, they are also major products of protein degradation and amino acid fermentation[51]. In our study, the levels of carbohydrates and glucose showed no significant changes in the GQD-treated group. However, the levels of glutamate and alanine decreased significantly, which possibly induced the change in levels of SCFAs. The reversal of changes in SCFAs contents in the treatment group indicated that GQD promotes the gut microbiota fermentation process and improves the disorder of intestinal flora to the control degree.
Glutamate is considered to be an important part of human and animal nutrition[52]. Many factors affect the content of glutamate in fecal extracts, including the absorption by the gut epithelium and the metabolism of the gut microbiota. A previous study suggested that glutamate is a major oxidative fuel for the gut and free glutamate is absorbed by the stomach as well as the small intestine, thus implicating the gastric mucosa in the metabolism of dietary glutamate[53]. As seen from the data, the content of glutamate obviously increased in the fecal extracts of IR model rats, while it decreased in GQD-treated rats. This result indicates that GQD may inhibit the imbalance of intestinal flora and protect the intestinal tract of the IR model rats.
4 ConclusionThis study has demonstrated that high fructose intake induces metabolic disorders in fecal metabolism. The results demonstrated that GQD could regulate many metabolic disorders in IR model rats involving amino acid metabolism, fatty acid oxidation and gut microbiota disorders. It is concluded that the metabonomic approach based on 1H NMR is a useful method for elucidating the metabolic regulation effect of GQD from a systematic and holistic viewpoint.
| [1] | ISEZUO S A. The metabolic syndrome:Review of current concepts[J]. Niger Postgrad Med J, 2006, 13(3): 247-255. |
| [2] | ASTRUP A, DYERBERG J, SELLECK M, et al. Nutrition transition and its relationship to the development of obesity and related chronic diseases[J]. Obes Rev, 2008, 9(suppl 1): 48-52. |
| [3] | MISRA A, KHURANA L. Obesity and the metabolic syndrome in developing countries[J]. J Clin Endocrinol Metab, 2008, 93(Suppl 1): S9-S30. |
| [4] |
GAO Y, SONG G Y, ZHOU Y, et al. Vasodilation reduction and insulin resistance in rats induced by high sucrose, high saturated fatty acid and high unsaturated fatty acid diets[J].
Basic & Clinical Medicine, 2006, 26(3): 275-279.
高宇, 宋光耀, 周宇, 等. 高糖、高脂饮食诱导大鼠胰岛素抵抗和血管舒张功能减弱[J]. 基础医学与临床, 2006, 26(3): 275-279. DOI: 10.3969/j.issn.1001-6325.2006.03.011. |
| [5] | HWANG I S, HO H, HOFFMAN B B, et al. Fructose-induced insulin resistance and hypertension in rats[J]. Hypertension, 1987, 10(5): 512-516. DOI: 10.1161/01.HYP.10.5.512. |
| [6] | DEKKER M J, SU Q Z, BAKER C, et al. Fructose:a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome[J]. Am J Physiol Endocrinol Metab, 2010, 299(5): E685-E694. DOI: 10.1152/ajpendo.00283.2010. |
| [7] | YANG Y X, WANG L L, WANG S M, et al. An integrated metabonomic approach to studing metabolic profiles in rat models with insulin resistance induced by high fructose[J]. Mol Biosyst, 2015, 10(7): 1803-1811. |
| [8] |
SI D H, CHEN L Y. Drug treatment of insulin resistance[J].
China Pharmacist, 2005, 8(7): 70-72.
史道华, 陈鹭颖. 胰岛素抵抗的药物治疗[J]. 中国药师, 2005, 8(7): 70-72. |
| [9] |
LIU H Q, ZHONG L J, JI B P, et al. Drug treatment of insulin resistance[J].
China Pharmaceuticals, 2005, 14(1): 77-78.
刘洪庆, 仲丽军, 纪宝平, 等. 胰岛素抵抗的药物治疗[J]. 中国药业, 2005, 14(1): 77-78. DOI: 10.3969/j.issn.1006-4931.2005.01.065. |
| [10] | 王琳琳.黄连解毒汤改善胰岛素抵抗的代谢组学研究[D].广州: 广东药学院, 2015. |
| [11] | ZHUZ Z, XIONGM Q, LINA Z, 等. Effect of Sanhuang Jiangtang (三黄降糖) recipe on insulin peripheral resistance in type Ⅱ diabetes mellitus[J]. Chinese Journal of Integrated Traditional and Western Medicine, 1999, 5(1): 36-40. DOI: 10.3321/j.issn:1008-9691.1999.01.019. |
| [12] |
ZOU W J, BAI H Y, GAO X P. The influence of P thomsonii Benth on insulin resistance induced by dexamethasone[J].
Chinese Pharmacological Bulletin, 2004, 20(6): 715-716.
邹文俊, 白红艳, 高小平. 中药葛根改善胰岛素抵抗作用的实验研究[J]. 中国药理学通报, 2004, 20(6): 715-716. DOI: 10.3321/j.issn:1001-1978.2004.06.030. |
| [13] |
JIN L, AN W C, AN W D. Treatment of 120 cases of diabetes with Gegen Qinlian Decoction[J].
Journal of Changchun University of Traditional Chinese Medicine, 2012, 28(2): 315.
金莉, 安文灿, 安文铎. 葛根芩连汤治疗糖尿病120例[J]. 长春中医药大学学报, 2012, 28(2): 315. DOI: 10.3969/j.issn.1007-4813.2012.02.067. |
| [14] |
LI Y M, FAN X M, WANG Y M, et al. Therapeutic effects of Gegen Qinlian decoction and its mechanism of action on type 2 diabetic rats[J].
Acta Pharmaceutica Sinica, 2013, 48(9): 1415-1421.
李颖萌, 范雪梅, 王义明, 等. 葛根芩连汤对2型糖尿病大鼠的治疗作用及其机制探讨[J]. 药学学报, 2013, 48(9): 1415-1421. |
| [15] |
PAN J Q, XIAO L Y, HAN C, et al. Effects of Gegenqinlian Decoction on antagonizing impaired glucose tolerance in D-galactose-induced rats[J].
Guangdong Pharmaceutical Journal, 2004, 14(1): 31-33.
潘竞锵, 肖柳英, 韩超, 等. 葛根芩连汤拮抗D-半乳糖诱导大鼠糖耐量减退作用[J]. 广东药学, 2004, 14(1): 31-33. |
| [16] |
PAN J Q, HAN C, LIU H C, et al. Experimental study on Hypoglycemic effects of Gegenqinliantang (GGQLT)[J].
Chinese New Drugs Journal, 2000, 9(3): 167-170.
潘竞锵, 韩超, 刘惠纯, 等. 葛根芩连汤降血糖作用的实验研究[J]. 中国新药杂志, 2000, 9(3): 167-170. DOI: 10.3321/j.issn:1003-3734.2000.03.007. |
| [17] | LPCI K, ALTINTOPRAK N, MULUK N B, et al. The possible mechanisms of the human microbiome in allergic diseases[J]. Eur Arch Otorhinolaryngol, 2012, 274(2): 617-626. |
| [18] | WANG Z N, KLIPFELL E, BENNETT B J, et al. Gut flora metabolism of phosphatidylcholing promotes cardiovascular disease[J]. Nat ure, 2011, 472(7341): 57-63. |
| [19] | CLEMENTE J C, URSELL L K, PARFREY L W, et al. The impact of the gut microbiota on human health:an integrative view[J]. Cell, 2012, 148(6): 1258-70. DOI: 10.1016/j.cell.2012.01.035. |
| [20] | CANI P D, AMAR J, IGLESIAS M A, et al. Metabolic endotoxemia initiates obesity and insulin resistance[J]. Diabetes, 2007, 56(7): 1761-72. DOI: 10.2337/db06-1491. |
| [21] | TILG H, KASER A. Gut microbiome, obesity, and metabolic dysfunction[J]. J Clin Invest, 2011, 121(6): 2126-2132. DOI: 10.1172/JCI58109. |
| [22] | CARICILLI A M, SAAD M J. Gut microbiota composition and its effects on obesity and insulin resistance[J]. Curr Opin Clin Nutr Metab Care, 2014, 17(4): 312-318. DOI: 10.1097/MCO.0000000000000067. |
| [23] |
FENG X G, YAN Y Z, ZENG Y P, et al. The effect of Gegen Qinlian Decoction on intestinal flora in damp-heat syndrome of type 2 diabetes[J].
World Journal of Integrated Traditional and Western Medicine, 2016, 11(8): 1110-1112.
冯新格, 严育忠, 曾艺鹏, 等. 葛根芩连汤对2型糖尿病湿热证肠道菌群的影响[J]. 世界中西医结合杂志, 2016, 11(8): 1110-1112. |
| [24] |
ZENG Y P, FENG X G, GU C Y, et al. Effect of intestinal flora of type 2 diabetes patients with dampness-heat syndrome treated with Gegen Qinlian Decoction[J].
Hebei Medicine, 2016, 22(10): 1731-1734.
曾艺鹏, 冯新格, 谷成英, 等. 葛根芩连汤治疗对2型糖尿病湿热证肠道菌群影响[J]. 河北医学, 2016, 22(10): 1731-1734. DOI: 10.3969/j.issn.1006-6233.2016.10.064. |
| [25] | XU J, LIAN F M, ZHAO L H, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula[J]. ISME J, 2015, 9(3): 552-562. DOI: 10.1038/ismej.2014.177. |
| [26] | GUO Y, ZHANG Y C, HUANG W H, et al. Dose-response effect of berberine on bile acid profile and gut microbiota in mice[J]. BMC Complement Altern Med, 2016, 16(1): 394. DOI: 10.1186/s12906-016-1367-7. |
| [27] | NOH K, KANG Y, NEPAL M R, et al. Role of intestinal microbiota in baicalin-induced drug interaction and its Pharmacokinetics[J]. Molecules, 2016, 21(3): 337. DOI: 10.3390/molecules21030337. |
| [28] | AKAO T. Differences in the metabolism of glycyrrhizin, glycyrrhetic acid and glycyrrhetic acid monoglucuronide by human intes tinal flora[J]. Biol Pharm Bull, 2000, 23(12): 1418-1423. DOI: 10.1248/bpb.23.1418. |
| [29] | CHAO J, HUO T I, CHENG H Y, et al. Gallic acid ameliorated impaired giucose and lipid homeostasis in high fat diet -induced NAFLD mice[J]. PloS One, 2014, 9(2): e96969. |
| [30] | PAN S N, CHEN A L, HAN Z H, et al. 1H NMR-based metabonomic study on the effects of Epimedium on glucocorticoid-induced osteoporosis[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2016, 1038: 118-126. DOI: 10.1016/j.jchromb.2016.10.015. |
| [31] | ZHANG A H, SUN H, WANG Z G, et al. Metabolomics:towards understanding traditional Chinese medicine[J]. Planta Med, 2010, 76(17): 2026-2035. DOI: 10.1055/s-0030-1250542. |
| [32] | WANG X J, SUN H, ZHANG A H, et al. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine:as pillars of the bridge between Chinese and western medicine[J]. J Pharm Biomed Anal, 2011, 55(5): 859-868. DOI: 10.1016/j.jpba.2011.01.042. |
| [33] | MAHMOUD M F, EI-NAGAR M, EI-BASSOSSY H M. Anti-inflammatory effect of atorvastatin on vascular reactivity and insulin resistance in fructose fed rats[J]. Arch Pharm Res, 2012, 35(1): 155-162. DOI: 10.1007/s12272-012-0117-8. |
| [34] | ZHANG Y F, YUAN J, ZHANG Y Z, et al. LC-MS/MS analysis of Gegen Qinlian Decoction and its pharmacokinetics after oral administration to rats[J]. Biomed Chromatogr, 2015, 29(4): 485-495. DOI: 10.1002/bmc.v29.4. |
| [35] |
HAN Z H, WANG Y L, WANG S M, et al. Effects of Gegen Qinlian decoction on plasma metabonomic profile in insulin resistance rats based on1H NMR metabonomics[J].
Journal of Guangdong Pharmaceutical University, 2015, 31(6): 786-790.
韩智慧, 王亚玲, 王淑美, 等. 基于1H NMR代谢组学方法研究葛根芩连汤对IR大鼠模型血浆代谢组的影响[J]. 广东药学院学报, 2015, 31(6): 786-790. DOI: 10.3969/j.issn.1006-8783.2015.06.020. |
| [36] | ZHANG C H, XU G L, LIU Y H, et al. Anti-diabetic activities of Gegen Qinlian Decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes[J]. Phytomedicine, 2013, 20(3/4): 221-229. |
| [37] | WANG Z L, XIAO P Y, QI T Z. Hypoglycemic effect of Gegen Qinlian Decoction on type 2 diabetes rats[J]. China Pharmacy, 2014, 25(23): 2131-2133. |
| [38] | BARHAM D, TRINDER P. An improved colour reagent for the determination of blood glucose by the oxidase system[J]. Analyst, 1972, 97(151): 142-145. |
| [39] | MATTHEWS D R, HOSKER J P, RUDENSKI A S, et al. Homeostasis model assessment:insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man[J]. Diabetologia, 1985, 28(7): 412-419. DOI: 10.1007/BF00280883. |
| [40] | LE GALL G, NOOR S O, RIDGWAY K, et al. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome[J]. J Proteome Res, 2011, 10(9): 4208-4218. DOI: 10.1021/pr2003598. |
| [41] | SHI X H, XIAO C N, WANG Y L, et al. Gallic acid intake induced alterations to systems metabolism in rats[J]. J Proteome Res, 2013, 12(2): 991-1006. DOI: 10.1021/pr301041k. |
| [42] | ZHAO Y, WU J F, LI J V, et al. Gut microbiota composition modifies fecal metabolic profiles in mice[J]. J Proteome Res, 2013, 12(6): 2987-2999. DOI: 10.1021/pr400263n. |
| [43] | MANSOR L S, MEHTA K, AKSENTIJEVIC D, et al. Increased oxidative metabolism following hypoxia in the type 2 diabetic heart, despite normal hypoxia signalling and metabolic adaptation[J]. J Physiol, 2016, 594(2): 307-320. DOI: 10.1113/JP271242. |
| [44] | DE VADDER F, KOVATCHEVA-DATCHARY P, ZITOUN C, et al. Microbiota-Produced succinate improves glucose homeostasis via intestinal gluconeogenesis[J]. Cell Metab, 2012, 24(1): 151-157. |
| [45] | CLAYTON T A, LINDON J C, EVERETT J R, et al. An hypothesis for a mechanism underlying hepatotoxin-induced hypercreatinuria[J]. Arch Toxicol, 2003, 77(4): 208-217. DOI: 10.1007/s00204-002-0431-x. |
| [46] | WATERFIELD C J, TURTON J A, SCALES M D, et al. Investigations into the effects of various hepatotoxic compounds on urinary and liver taurine levels in rats[J]. Arch Toxicol, 1993, 67(4): 244-254. DOI: 10.1007/BF01974343. |
| [47] | RIBEIRO R A, BONFLEUR M L, AMARAL A G, et al. Taurine supplementation enhances nutrient-induced insulin secretion in pancreatic mice islets[J]. Diabetes Metab Res Rev, 2009, 25(4): 370-379. DOI: 10.1002/dmrr.v25:4. |
| [48] | CHUNG S T, HSIA D S, CHACKO S K, et al. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes[J]. Diabetologia, 2015, 58(3): 596-603. DOI: 10.1007/s00125-014-3455-x. |
| [49] | DOLARA P, CADERNI G, SALVADORI M, et al. Fecal levels of short-chain fatty acids and bile acids as determinants of colonic mucosal cell proliferation in humans[J]. Nutr Cancer, 2002, 42(2): 186-190. DOI: 10.1207/S15327914NC422_6. |
| [50] | ANDOH A, TSUJIKAWA T, FUJIYAMA Y. Role of dietary fiber and short -chain fatty acids in the colon[J]. Current Pharmaceutical Design, 2003, 9(4): 347-358. DOI: 10.2174/1381612033391973. |
| [51] |
CHEN Y, CAO Y S, LIU X H. Short chain fatty acids and intestinal microflora[J].
Jiangxi Science, 2006, 24(1): 38-39.
陈燕, 曹郁生, 刘晓华. 短链脂肪酸与肠道菌群[J]. 江西科学, 2006, 24(1): 38-39. DOI: 10.3969/j.issn.1001-3679.2006.01.010. |
| [52] | WU M M, XIAO H, REN W K, et al. An NMR-based metabolomic approach to investigate the effects of supplementation with glutamic acid in piglets challenged with deoxynivalenol[J]. PLoS One, 2014, 9(12): e113687. DOI: 10.1371/journal.pone.0113687. |
| [53] | BURRIN D G, STOLL B. Metabolic fate and function of dietary glutamate in the gut[J]. Am J Clin Nutr, 2009, 90(3): 850S-856S. DOI: 10.3945/ajcn.2009.27462Y. |
 2019, Vol. 36
2019, Vol. 36 
