2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Pacific Northwest National Laboratory, Richland, WA 99352, USA
2. 中国科学院大学, 北京 100049;
3. Pacific Northwest National Laboratory, Richland, WA 99352
The effects of ionizing radiation to organisms have been focused in many studies due to its wide-spread existence in radiotherapy, nuclear accident and outer-space exploration, etc. Whole body acute ionizing radiation could induce oxidative stress, energy and amino acid metabolism disturbance[1]. However, the association between these underlying pathological mechanisms with the perturbations in important metabolic pathways of kidney tissues is still not well elucidated.
Metabolites are the end products or intermediates in metabolic pathways, which can reflect the functional status of cells as they are downstream to DNA, RNA and protein. Metabolomics allows the detection of subtle changes in metabolic pathways and deviation from homoeostasis before phenotypic changes, and allows a snapshot of the metabolic state under a particular physically condition[2]. Nuclear magnetic resonance (NMR)-based metabolomics has a number of advantages, such as unbiased, quantitative, reproducible, non-destructive, etc.[3, 4] One-dimensional (1D) 1H NMR spectral analysis of bio-fluids and tissues extraction has been the cornerstone of NMR based metabolomics for many years[5-7].
Multivariate data statistical methods have become an essential part of the metabolomics field[8, 9]. These methods are quite useful to reduce the dimensionality and extract the maximum information from of the metadata. Principal component analysis (PCA) is the most commonly used unsupervised method in multivariate data analysis, which generates orthogonal and ranked principal components (PCs) that explain the variance in the data structure, and each sample represented by a spectrum can be treated as a point in a multi-dimensional space. The goal of PCA is to obtain an overview of the metadata and to detect potential outliers. In the supervised analysis, like orthogonal projection to latent structure (OPLS) takes advantage of prior class information to make two groups of variables, which techniques can be appropriate for biomarkers discovery[10, 11].
In the present study, we demonstrated an application of 1H NMR-based metabolomics approach to investigate the metabolic profiling difference of hydrophilic tissue extracts from the excised kidney between control mice and whole-body irradiated mice (3.0 or 7.8 Gy gamma, or 3.0 Gy proton) at days 4 and 11 post-irradiation. The spectral deconvolution technique was employed to identify and quantify all metabolites from NMR spectra. Then the multivariate data analysis methods (including PCA and OPLS) were used to search for meaningful differences among them, and performed for pattern recognition and identification of metabolites in which concentrations were statistically significantly changed as the results of ionizing radiation exposure.
1 Materials and methods 1.1 Animal and sample preparationA total of 27 seven weeks old female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). After acclimation for one week at the animal facility of Pacific Northwest National Laboratory (PNNL) or Brookhaven National Laboratory (BNL), they were randomly divided into six groups and then exposed to whole-body gamma or proton irradiation. The individually housed mice were fed a standard rodent diet and maintained in a controlled environment facility with an ambient temperature of 22~25 ℃ and 45% relative humidity on 12 h light-dark cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at PNNL or BNL.
The detailed recipes for radiation exposure can be found in our previous publication[12]. The 6 groups of mice (n=27) were exposed to gamma radiation with dose of 0 Gy (n=4), 3.0 Gy (n=10, 2 groups), 7.8 Gy (n=4), and proton radiation with dose of 0 Gy (n=4), 3.0 Gy (n=5), respectively. At day 4 post-irradiation, the mice exposed to gamma radiation [0 Gy (control, n=4), 3.0 Gy (n=5) and 7.8 Gy (n=4)] were sacrificed with CO2/O2 at ratio of 7/3. At day 11 post-irradiation, the remaining mice exposed to gamma radiation [3.0 Gy (n =5)], and all mice exposed to proton radiation [0 Gy (control, n=4), 3.0 Gy (n=5)] were sacrificed with CO2/O2 at the same ratio (Table 1). And the kidney from each mouse were immediately collected and snap-frozen in liquid nitrogen, then weighed and stored at -80 ℃ freezer until subsequent NMR analysis.
| Table 1 Number of mice in each group (at each time point with ionizing radiation) |
Polar metabolites were extracted using a modified Folch method by following the established protocol[13]. Kidney tissue was extracted employing the MeOH/H2O/CHCl3 system as previously report described[14]. And the extracts were stored at −80 ℃ freezer before performing NMR measurements.
1.2 1H NMR SpectroscopyFor NMR measurements, the extractions were redissolved in 600 μL D2O containing 0.1 mmol/L sodium 3-(trimethylsilyl) propionate-2, 2, 3, 3-d4 (TSP) (Sigma-Aldrich, Missouri, USA) as chemical shift reference (δ 0.00) and internal concentration standard, and 0.2% sodium azide (w/v) as bacteriostatic agent to prevent biodegradation. About 550 μL prepared sample was transferred into the standard 5 mm NMR tube (Wilmad, Buena, NJ). NMR spectra were recorded on a Varian 800 MHz NMR spectrometer (Varian, Inc, USA) operating at 799.39 MHz for 1H equipped with a Z axis-gradient 5 mm HCN probe at 293 K. 1D 1H NMR spectra were acquired from each sample employing the standard Varian presaturation pulse sequence with a single pulse excitation and 2.0 s low power pre-saturation at the water peak position to suppress the residual water signal. 1H NMR spectra were recorded with 4 k transients and spectral width of 9 600 Hz to ensure a high quality 1H NMR spectrum was obtained with sufficient signal-to-noise ratio for metabolites in the NMR tube. For metabolite signal assignment and confirmation purposes, two-dimensional (2D) NMR spectra, including 1H-1H correlation spectroscopy (COSY) and 1H J-resolved spectroscopy (J-RES) were acquired at 293 K for selected samples.
1.3 NMR data processing and multivariate data analysisAll the spectra were Fourier transformed by multiplication of the free induction decays (FIDs) with an exponential weighting function corresponding to a Lorentz line broadening factor of 0.5 Hz. And the 1H NMR spectra of kidney extracts were manually corrected for phase and baseline distortions using the processor module of Chenomx (NMR suite 8.1, Professional). The high-throughput data produced by 1H NMR-based metabolomics needs to be tabulated prior to perform multivariate statistical analysis. For peak assignment purposes, targeted profiling of hydrophilic extracts using spectral deconvolution method was performed to get the absolute metabolite concentrations with the help of the profiler module of Chenomx, which contains a database consisted of more than 330 common metabolites associated with mammals and bacteria. The concentration of each metabolite in the NMR tube was calculated by the well-established method in Chenomx with TSP as internal concentration standard. The metabolite concentrations were then normalized to per milligram of tissue before importing into SIMCA13 (Umetrics, Sweden) for multivariate data analysis, i.e., PCA and OPLS. Taking every variable having equal importance to the statistical results into consideration in this study, PCA and OPLS were performed using the mean centered and unit-variance scaled NMR data to obtain an overview and detect potential outliers in the samples[15]. And to distinguish the variables with a high contribution between the treated and control groups, the class information as Y-matrix. The quality of these models was evaluated with R2 values representing the fitness and interpretability of the models and Q2 values indicating the predictability of the models. And the validities of all these OPLS models were further assessed for their robustness with the well-known CV-ANOVA approach to ensure the significance of intergroup differentiations[16].
The S-plot was used to interpret the multivariate models from OPLS, which enabling direct inspection and extraction of statistically significant and potentially biochemically important metabolites[17]. The cutoff value of 0.878 (the critical values for Pearson correlation coefficient) was chosen for the current study based on the number of samples in each group (n=4) and the discrimination significance of p < 0.05.
2 Results and discussion 2.1 1H NMR assignment of kidneyExamples of typical 1H NMR spectra of hydrophilic extracts obtained from a control mouse and a whole body gamma irradiation exposed mouse are shown in Fig. 1. The peak intensities are divided to unit weight of kidney tissue before extraction, so that the concentrations of a given metabolite can be directly compared visually according to the peak intensities in the corresponding NMR spectrum.

|
Fig. 1 Examples of typical 1H NMR spectra of hydrophilic extracts obtained from a control mouse and a whole body gamma irradiation exposed mouse. In the plot, spectral region at δ 5.15~9.5 of both groups are vertically expanded by 40 times compared to δ 0.5~4.85. A total of 75 metabolites have been identified with metabolites numbers in Table S1. 1. 2-Hydroxyvalerate; 2. Isoleucine; 3. Leucine; 4. 2-Aminobutyrate; 5. Valine; 6. Isobutyrate; 7. 3-Hydroxybutyrate; 8. Threonine; 9. Lactate; 10. Lysine; 11. Alanine; 12. Arginine; 13. Glutarate; 14. Thymidine; 15. Glutamate; 16. Homoserine; 17. Uridine diphosphate (UDP)-N-acetylglucosamine; 18. 1, 3-Diaminopropane; 19. S-Adenosylhomocysteine; 20. Glutamine; 21. Glutathione; 22. Succinate; 23. Carnitine; 24. 2-Oxobutyrate; 25. Citrate; 26. β-Alanine; 27. Aspartate; 28. Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH); 29. Nicotinamide adenine dinucleotide hydride (NADH); 30. 3-Hydromuconate; 31. Creatine phosphate; 32. Creatine; 33 Creatinine; 34. Tyrosine; 35. Phenylalanine; 36. Histidine; 37. Ethanolamine; 38. π-Methylhistidine; 39. O-Phosphoethanolamine; 40. Choline; 41. O-Phosphocholine; 42. τ-Methylhistidine; 43. Glucose; 44. sn-Glycero-3-phosphocholine; 45. Taurine; 46. Glucose-6-phosphate; 47. myo-Inositol; 48. Tryptophan; 49. Theophylline; 50. UDP-glucose; 51. UDP-glucuronate; 52. Glycine; 53. Mannose; 54. Mannitol; 55. UDP-galactose; 56. Ascorbate; 57. Adenosine; 58. Serine; 59. Glycolate; 60. Uridine monophosphate (UMP); 61. Adenosine monophosphate (AMP); 62. Inosine 5-monophosphate (IMP); 63. Uridine; 64. Nicotinamide adenine dinucleotide phosphate (NADP+); 65. Adenosine diphosphate (ADP); 66. Nicotinic acid adenine dinucleotide; 67. Adenosine triphosphate (ATP); 68. Guanosine-5'-triphosphate (GTP); 69. Nicotinamide adenine dinucleotide (NAD+); 70. Allantoin; 71. Uracil; 72. Fumarate; 73. Niacinamide; 74. Oxypurinol; 75. Hypoxanthine |
A total of 75 metabolites were identified with good confidence based on spectral deconvolution using Chenomx, including a number of amino acids, glycolysis products, membrane component metabolites, TCA cycle intermediates and choline metabolites. The resonances were assigned to specific metabolites according to both literatures[18-20] and the metabolites library of Chenomx, and finally confirmed by COSY and J-RES spectra. The detailed peak assignments are listed in Table S1 (available on the CJMR website at doi:10.11938/cjmr20182622). And the average concentrations are listed in Table S2 (available on the CJMR website at doi:10.11938/cjmr20182622), the data were all expressed as mean ± standard deviation (SD).
Visual inspection of the 1H NMR spectra of mouse kidney tissue extracts (Fig. 1) revealed metabolic changes induced by high dose radiation. For example, the exposed mice had higher isoleucine, valine, isobutyrate, threonine, lactate, alanine, glutarate, glutamate, succinate, NADH, glycine, serine, uridine, NADP+, ADP, uracil, niacinamide and oxypurinol level, while lower 2-aminobutyrate, homoserine, aspartate, creatine, glucose, UDP-glucose, ascorbate, adenosine, glycolate and AMP level in the kidney extracts. To obtain more details about the metabolite changes induced by radiation, both PCA and OPLS modeling were conducted on entire NMR spectra sets.
2.2 Multivariate analysis of metabolic profiles from kidney tissuesInitially, unsupervised data analysis, i.e. PCA (Fig. 2) in this case, was performed on the normalized kidney metabolic profiles datasets to visualize general clustering trends among the samples, and 4 principal components were calculated for the extracts obtained from six groups with a total of 72.9% of variables being explained. The statistical results indicate the metadata with good dataset quality for further analysis.
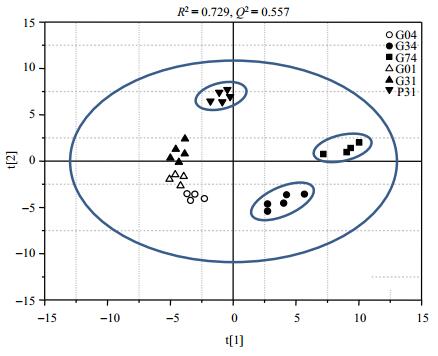
|
Fig. 2 PCA score plot of kidney tissue extracts from all the control and exposed groups based on 75 metabolites. And the model statistical parameters are R2=0.729, Q2=0.557, respectively. G04: 4 days, control; G34: 4 days post exposure to 3.0 Gy gamma radiation; G74: 4 days post exposure to 7.8 Gy gamma radiation; G01: 11 days, control; G31: 11 days post exposure to 3.0 Gy gamma radiation; P31: 11 days post exposure to 3.0 Gy proton radiation |
To understand the significance of metabolites contributing to classification, the metabolic profiles datasets were further subjected to OPLS. The statistical parameters, like R2 and Q2 showed good quality of the generated OPLS models, and p values from CV-ANOVA further confirm the validity of these models (Table 2).
| Table 2 The multivariate statistical parameters R2 and Q2 based on OPLS |
The coefficient plots show the metabolites having contributions to the class difference, and the correlation coefficients for NMR signals indicated the significance of the metabolites contribution are summarized in Tables S3 (available on the CJMR website at doi:10.11938/cjmr20182622).
As shown in Tables S3, the statistically significant metabolites that are responsible for separating the exposed groups from the control groups depend on radiation dose and radiation source. The difference between exposed groups and control group indicate both gamma and proton radiation disturbed the metabolism in kidney tissue. Although the p value from all the OPLS models indicate these exposed and control groups have significant statistical difference, a more dramatic metabolic disturbance in 7.8 Gy gamma-exposed group than 3.0 Gy gamma-exposed group (Table 3) is observed. The same conclusion can be drawn from the fold changes plot (Fig. 3). The difference between the two treatment groups is due to the dose of gamma radiation.
| Table 3 Radiation induced metabolic changes in kidney tissue extracts after 4 days exposure between 3.0 Gy or 7.8 Gy gamma-exposed mice and control group |

|
Fig. 3 The absolute concentrations of statistically significantly changed metabolites captured by OPLS models derived from the control and exposed groups. The absolute concentration of each metabolite was obtained by normalizing the corresponding metabolite concentration obtained from 1H NMR measurements to the unit weight (mg) of liver tissue before extraction. Statistical significance for each was calculated using Welch's t test between control (sham irradiated) and treated groups. *p < 0.05, **p < 0.01 and ***p < 0.001. G04: 4 days, control; G34: 4 days post exposure to 3.0 Gy gamma radiation; G74: 4 days post exposure to 7.8 Gy gamma radiation; G01: 11 days, control; G31: 11 days post exposure to 3.0 Gy gamma radiation; P31: 11 days post exposure to 3.0 Gy proton radiation |
In Fig. 2, the separation of the 11 days post-irradiation for both 3.0 Gy gamma- and proton-exposed groups is clear. A set of discriminatory metabolites for separating the treated and control groups are identified with the corresponding correlation coefficients obtained from S-plot analysis, and listed in Table 4. These findings indicate that at the same dose, proton radiation is more potent than gamma radiation. The same conclusion can be drawn from the fold changes plot (Fig. 3).
| Table 4 Radiation induced metabolic changes in kidney tissue extracts after 11 days exposure between 3.0 Gy gamma- or 3.0 Gy proton-exposed mice and control group |
The major results from the combined 1H NMR metabolic profiling and multivariate date analysis of kidney tissue extracts are summarized. The treatment groups are well separated from the control group based on metabolites obtained from the hydrophilic extracts. A suit of discriminatory metabolites that play a critical role in separating the exposed groups have been identified. We find that the concentrations of 2-aminobutyrate, 3-hydroxybutyrate, homoserine, ascorbate, 1, 3-diaminopropane and β-alanine statistically significantly changed according to all the OPLS models across all exposed groups compared to control group. All of these results suggest that irradiation induced oxidative stress and broken DNA double strand in the mice kidney after whole body exposed to ionizing radiation. NMR based metabolomics approaches are a clinically useful diagnostic tool understanding the biochemical alterations and mechanisms of ionizing radiation.
3 ConclusionIn this study, we performed a quantitative metabolomics study on kidneys from ionizing radiation exposed mice to identify biomarkers based on NMR analysis. Both PCA and OPLS show that the exposed group is well separated from the control group based on the 75 metabolites detected in the kidney tissue extracts. We found that the concentrations of 2-aminobutyrate, 3-hydroxybutyrate, homoserine, ascorbate, 1, 3-diaminopropane and β-alanine were statistically significantly changed according to the various OPLS models across all exposed groups. Identification of these altered metabolites provides important information for improved understanding of the molecular mechanisms associated with ionizing radiation in kidney. These findings indicate that the NMR based metabolomics analysis is useful for detecting the effects of radiation which might be useful for diagnosing ionizing radiation.
| [1] | HALLIWELL B, ARUOMA O I. DNA damaged by oxygen-derived species-Its mechanism and measurement in mammalian systems[J]. Febs Lett, 1991, 281(1/2): 9-19. |
| [2] | NICHOLSON J K, LINDON J C, HOLMES E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data[J]. Xenobiotica, 1999, 29(11): 1181-1189. DOI: 10.1080/004982599238047. |
| [3] | SMOLINSKA A, BLANCHET L, BUYDENS L M, et al. NMR and pattern recognition methods in metabolomics: from data acquisition to biomarker discovery: a review[J]. Anal Chim Acta, 2012, 750: 82-97. DOI: 10.1016/j.aca.2012.05.049. |
| [4] | LENZ E M, WILSON I D. Analytical strategies in metabonomics[J]. J Proteome Res, 2007, 6(2): 443-458. DOI: 10.1021/pr0605217. |
| [5] | SOLANKY K S, BAILEY N J C, HOLMES E, et al. NMR-based metabonomic studies on the biochemical effects of epicatechin in the rat[J]. J Agric Food Chem, 2003, 51(14): 4139-4145. DOI: 10.1021/jf025677f. |
| [6] | BOLLARD M E, STANLEY E G, LINDON J C, et al. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition[J]. NMR Biomed, 2005, 18(3): 143-162. DOI: 10.1002/(ISSN)1099-1492. |
| [7] | ZHU X Y, LEI H H, WU J F, et al. Systemic responses of BALB/c mice to Salmonella typhimurium infection[J]. J Proteome Res, 2014, 13(10): 4436-4445. DOI: 10.1021/pr500770x. |
| [8] | BUNDY J G, SPURGEON D J, SVENDSEN C, et al. Earthworm species of the genus Eisenia can be phenotypically differentiated by metabolic profiling[J]. Febs Lett, 2002, 521(1/2/3): 115-120. |
| [9] | GRATTON J, PHETCHARABURANIN J, MULLISH B H, et al. Optimized sample handling strategy for metabolic profiling of human feces[J]. Anal Chem, 2016, 88(9): 4661-4668. DOI: 10.1021/acs.analchem.5b04159. |
| [10] | TRYGG J, WOLD S. Orthogonal projections to latent structures (O-PLS)[J]. J Chemometr, 2002, 16(3): 119-128. DOI: 10.1002/(ISSN)1099-128X. |
| [11] | JONSSON P, JOHANSSON E S, WUOLIKAINEN A, et al. Predictive metabolite profiling applying hierarchical multivariate curve resolution to GC-MS datas - A potential tool for multi-parametric diagnosis[J]. J Proteome Res, 2006, 5(6): 1407-1414. DOI: 10.1021/pr0600071. |
| [12] | XIAO X J, HU M, ZHANG X, et al. NMR-based metabolomics analysis of liver from C57BL/6 mouse exposed to ionizing radiation[J]. Radiat Res, 2017, 188(1): 44-55. DOI: 10.1667/RR14602.1. |
| [13] | BECKONERT O, KEUN H C, EBBELS T M D, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts[J]. Nat Protoc, 2007, 2(11): 2692-2703. DOI: 10.1038/nprot.2007.376. |
| [14] | XIAO X J, HU M, LIU M L, et al. 1H NMR metabolomics study of spleen from C57BL/6 mice exposed to gamma radiation[J]. Metabolomics, 2016, 6(1): 1-11. |
| [15] | WORLEY B, POWERS R. Multivariate analysis in metabolomics[J]. Curr Metabolomics, 2013, 1(1): 92-107. |
| [16] | ERIKSSON L, TRYGG J, WOLD S. CV-ANOVA for significance testing of PLS and OPLS (R) models[J]. J Chemometr, 2008, 22(11/12): 594-600. |
| [17] | WIKLUND S, JOHANSSON E, SJOSTROM L, et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models[J]. Anal Chem, 2008, 80(1): 115-122. DOI: 10.1021/ac0713510. |
| [18] | FAN T W M, LANE A N. Structure-based profiling of metabolites and isotopomers by NMR[J]. Prog Nucl Mag Res Sp, 2008, 52(2/3): 69-117. |
| [19] | MARTIN F P J, SPRENGER N, YAP I K S, et al. Panorganismal gut microbiome-host metabolic crosstalk[J]. J Proteome Res, 2009, 8(4): 2090-2105. DOI: 10.1021/pr801068x. |
| [20] | MARTIN F P J, DUMAS M E, WANG Y L, et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model[J]. Mol Syst Biol, 2007, 3(1): 1-16. |
 2019, Vol. 36
2019, Vol. 36 
