2. Jiangsu Provincial Institute of Materia Medica, Nanjing 210009, China;
3. Center for Instrument Analysis, China Pharmaceutical University, Nanjing 210009, China
2. 江苏省药物研究所, 江苏 南京 210009;
3. 中国药科大学, 分析测试中心, 江苏 南京 210009
Selaginellins are a rare group of natural pigments with p-quinone methide (p-QM) and alkynylphenol functionalities, elaborated by plants of the genus Selaginella[1-6]. Both tautomerism and atropisomerism have been observed among the structures[7]. Recently, selaginellins have gained interest as lead molecules for the development of phosphodiesterase-4 inhibitor[8, 9], soluble epoxide hydrolase inhibitor[10] and protein tyrosine phosphatase 1B inhibitor[11, 12]. X-ray crystallography has confirmed that the reported selaginellins possessing p-QM exist as racemates[1-3, 7]. And our previous study[7] using chiral separation and electronic circular-dichroism (ECD) method has proved that C-10-ether-containing selaginellin derivatives undergo racemization process concurrently induced by axial chirality and molecular rotation[7]. On the other hand, due to rapid QM-phenol tautomerism at room temperature, C-10-OH-containing selaginellins exist as a pair of enantiomers which cannot be separated into enantiomerically pure compound. In essence, the QM-phenol tautomeric equilibrium is a complex phenomenon, which involves proton transfer and the extensive delocalization between QM and phenol rings. The effect is translated to the nuclear magnetic resonance (NMR) signals, namely signals of the atoms on the aforementioned two rings displayed severe line broadening and thus indistinguishable. The basic knowledge of tautomerism has already been known[13-15], however, controversial interpretations with respect to the stereochemical implications of tautomeric selaginellins still remain[11, 12], that seriously mislead the study of structure-activity relationship[16]. Therefore, we aim in this study to characterize the tautomeric process in selaginellins, to illustrate the effects of pH, solvents, and temperature on tautomeric equilibrium, and to give a better understanding of the stereochemistry of tautomeric selaginellin derivatives.
1 Materials and methodsSamples of selaginellin (1) and selaginisoquinoline A (2) (Fig. 1) were prepared as the previous report[7]. Compounds 1 and 2 possessed hydroxyl group at C-10, representing the tautomeric selaginellins; compound 2 contained isoquinoline ring in selaginellin skeleton.
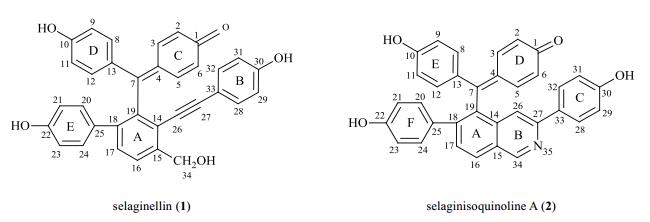
|
Fig. 1 Chemical structures of selaginellin (1) and selaginisoquinoline A (2) |
Ultraviolet-visible (UV-vis) spectra of compounds 1 and 2 were recorded using a Varian Cary 60 spectrophotometer (Agilent Technologies Inc., USA) equipped with a 1 cm path length cell at pH 3, 7 and 13, respectively.
For compound 1 and 2, 1H (500 MHz) and 13C NMR (125 MHz) spectra were acquired with a Bruker AV-500 spectrometer (Bruker, Switzerland) in DMSO-d6 at different pH environments, and in aprotic (CD3COCD3) and protic (CD3OD) solvents at pH of 7, respectively. 2D NMR spectra (HSQC and HMBC) were recorded at standard conditions. All the detections were carried out at 300 K.
The variable-temperature 1H NMR spectra of compound 2 were recorded on a Bruker AV-400 spectrometer (Bruker, Switzerland) in CD3COCD3-C5D5N (3:1) at 323 K, 300 K, 273 K, 253 K and 233 K, respectively.
2 Results and discussion 2.1 Effect of pH on tautomerismThe UV-vis spectrum of these selaginellins in methanol (Fig. 2) revealed intense absorption peaks at approximately 260~300 nm attributed to phenyl groups.
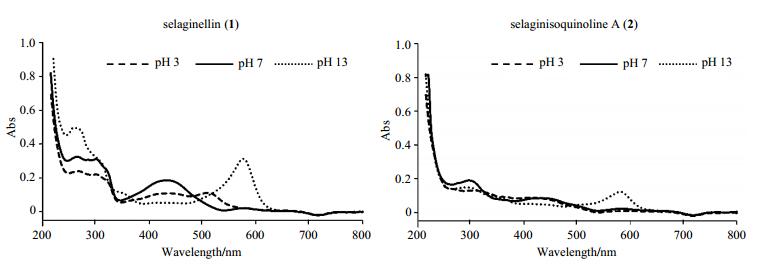
|
Fig. 2 UV-vis spectra of selaginellin (1) and selaginisoquinoline A (2) in methanol at different pH environments |
Fig. 2 showed that the spectral profile representing keto groups of compound 1 was significantly influenced by pH variation. At pH of 3, the maximum absorption peak appeared at about 510 nm. At pH of 7, the peak was observed at around 430 nm. However, with the increase of pH to 13, the maximum absorption peak in region of 400~600 nm moved to 575 nm. During the experimental process, it was observed that the pink acidic selaginellins methanol solution changed to red at neutral environment, and then to purple at basic condition. This bathochromic effect characteristic of selaginellins has been reported in previous reference[1].
In the case of compound 2, the change of the spectral profile and solution color were not obvious along with the pH decline, however, when pH increased to 13, the maximum absorption peak likewise appeared at about 580 nm, and solution color changed from yellow (pH 3~7) to purple. Maybe it is because polar solvent promotes intermolecular hydrogen bond formation between the nitrogen atom of the isoquinoline and protons in the solvents at acidic environment, whereas conjugated QM form can be stabilized at basic condition.
To further clarify the interesting acid-base properties of selaginellin, we recorded the NMR spectra of selaginellin (1) in neutral, acidic and basic solutions at 300 K, respectively (Fig. 3). The NMR parameters of selaginellin in DMSO-d6 at pH of 3, 7, and 13 were summarized in Table 1.
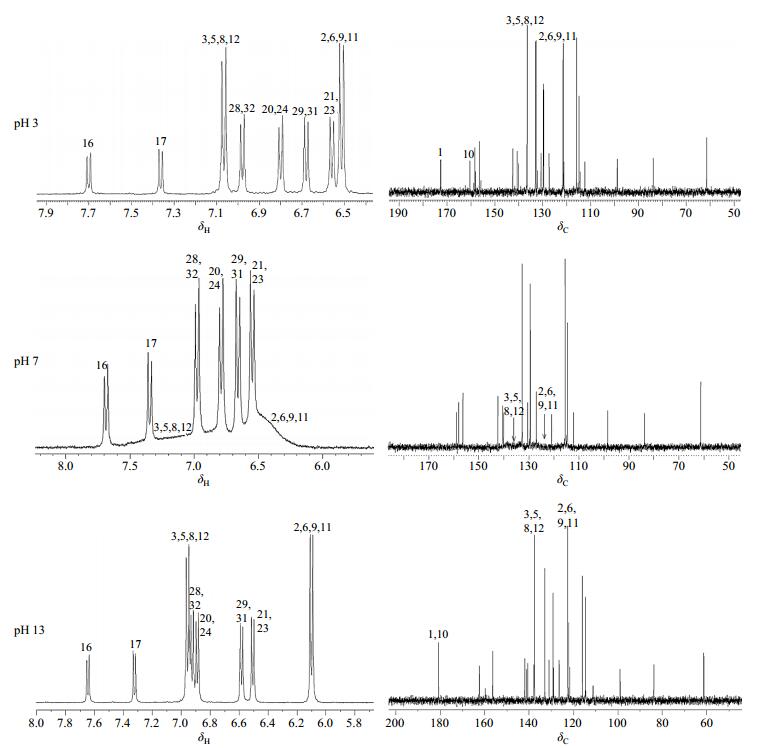
|
Fig. 3 Partial 1H and 13C NMR spectra of selaginellin (1) in DMSO-d6 at different pH environments |
| Table 1 1H and 13C NMR assignments of selaginellin (1) in DMSO-d6 at different pH environments |
In neutral solution, peaks of H-2/6/9/11 and H-3/5/8/12 collapsed into broad signals at about δH 6.48 and δH 7.04, respectively; and the 13C NMR spectra displayed severe line broadening of either QM or phenol rings, some of them almost obscured by the baseline. It could be rationalized that interconversion of the two enantiomers is a relatively fast process on the NMR time scale at room temperature, and causing no splitting of the NMR signals.
However, interconversion was accelerated in acidic or alkaline solutions, subsequently eliminated the NMR anomalies and led to the signal average. Zhang et al.[1] has reported that the pH range of compound 1 with the color change from red to pink was 1.5~1. But the presence of impurity signals reminded us the potential occurrence of chemical decomposition when solution pH decreased to such low values. Therefore, we set the pH of acidic solution to 3. The chemical shifts of H-2/6/9/11 and H-3/5/8/12 moved to δH 6.51 and δH 7.07, respectively. And narrower singlets of C-2/6/9/11 and C-3/5/8/12 appeared at δC 121.5 and δC 136.5 individually. The signals of C-1 and C-10 were observed separately at δC 172.7 and δC 160.5. In such acidic solution, keto moiety does not get pronated as reported[1], while forming the intermolecular hydrogen bond between the oxygen at keto group and proton in solvents (Fig. 4).
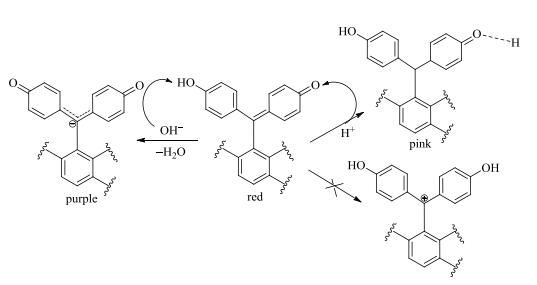
|
Fig. 4 Tautomerism mechanism of selaginellins introduced by pH variation |
In alkaline solution, the protons on the rings of C and D and the carbon atoms of C-4 and C-13 shifted to the high field region in the NMR spectra. The signals of C-1/10 appearing at δC 180.7 were assigned as carbonyl carbons. Alkaline environment changed the electron distribution. Apparently, the deprotonation nature of hydroxyl stabilized the keto form exclusively, producing a large conjugate system with carbon anion (Fig. 4). The donor conjugation effect of the carbon anion enhanced the electron density of rings C and D, therefore resulted in upfield shift of H2/6/9/11, H-3/5/8/12, C4 and C-13. In acidic and alkaline solutions, occurrence of extensive delocalization resulted in the averaged aromatic signals between rings C and D without notable influence on the residual atoms.
2.2 Effect of solvent on tautomerismThe polarity and hydrogen-bonding abilities of solvents affect tautomeric process significantly. At room temperature, tautomerism resulted in the averaged aromatic signals between quinone and phenyl units, thus the ketone-form was not visible in NMR spectra in either aprotic or protic solvents. In CD3COCD3, protons on rings D and E of compound 2 coalesce into two broad signals at δH 6.54 (H-2/6/9/11) and 7.17 (H-3/5/8/12), respectively. However, in protic solvent CD3OD, signals for H-2/6/9/11 and H-3/5/8/12 got sharpened and well-resolved (Fig. 5). This is because CD3OD functions as weak acid to provide proton, thus accelerates tautomerism. It was also observed that chemical shifts of H-26 and H-28/32 moved to high field region in CD3OD due to the effect of the intermolecular hydrogen bond between the nitrogen atom of the isoquinoline and proton of solvents. The NMR parameters of compound 2 in CD3COCD3 and CD3OD were summarized in Table 2.
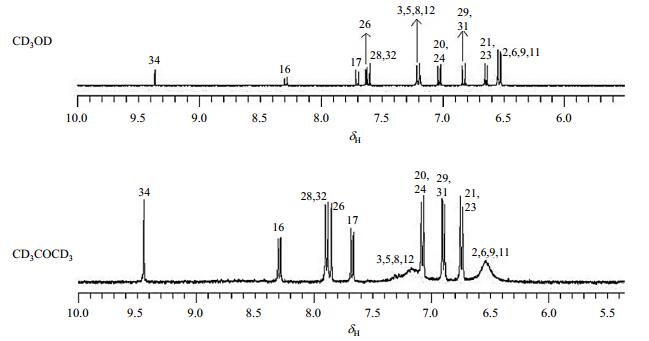
|
Fig. 5 Partial 1H NMR spectra of selaginisoquinoline A (2) at 300 K in CD3OD and CD3COCD3 |
| Table 2 1H NMR assignment of selaginisoquinoline A (2) in CD3OD and CD3COCD3 |
Tautomerism is known to be temperature-dependent. We examined the temperature influence on tautomerism process for compound 2 using variable-temperature NMR method (Fig. 6). The aprotic solvent CD3COCD3 was used to eliminate the participation of active hydrogen in media, with addition of C5D5N for better solubility of sample. The 1H NMR spectrum of compound 2 at 323 K displayed one broad peak at δH 6.61 assigned as H-2/6/9/11, corresponding to a time-averaged contribution of the tautomers with fast chemical exchange compared with those at lower temperature. However, the signals of H-3/5/8/12 was almost obscured by the baseline. As the temperature decreased to 300 K, the signals of H-2/6/9/11 and H-3/5/8/12 disappeared. These peaks emerged when the temperature reached 273 K. In this case, the tautomeric rate was slow relative to the NMR time scale at any temperature below that of coalescence (about 300 K). Our previous study[3, 7] revealed a series of non-tautomeric selaginellin derivatives with ether linkage at C-10. In particularly, protons of their C-rings resonated as a characteristic ABXY system. Finally, tautomerism was almost blocked at 233 K, when proton resonance suggested an ABXX' system (δH 6.50, brs, H-2; δH 6.31, brs, H-6; δH 7.10, d, J=7.7 Hz, H-3/5) indicative of a hexa-dienone ring (D), and an AA'XX' system (δH 6.87, d, J=8.4 Hz, H-9/11; δH 7.10, d, J=8.4 Hz, H-8/12) were readily assigned to ring E. However, that is far from the well-dispersed spectral characteristic of non-tautomeric selaginellin patterns (Fig. 7), probably because the temperature is not low enough to block the tautomerism.
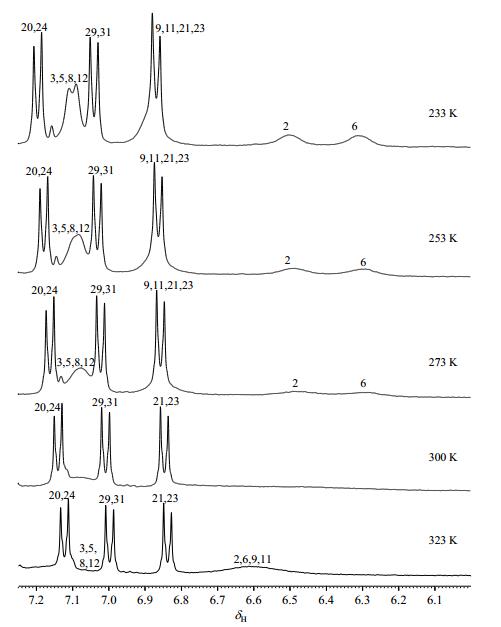
|
Fig. 6 Partial variable-temperature 1H NMR spectra of selaginisoquinoline A (2) in CD3COCD3-C5D5N (3:1) |
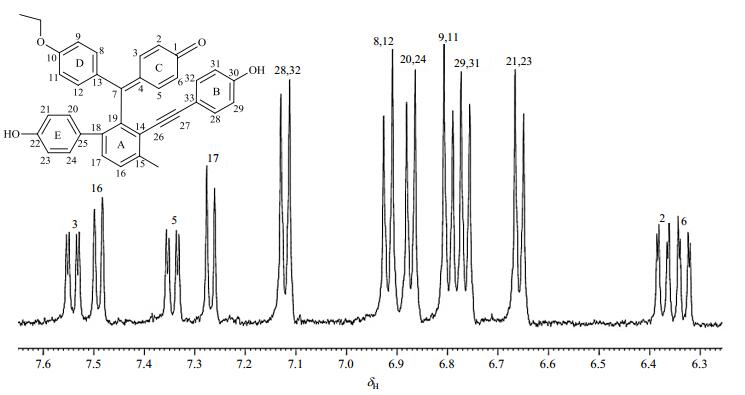
|
Fig. 7 Partial 1H NMR spectrum of selaginellin R (a non-tautomeric selaginellin derivative) at 300 K in CD3COCD3 |
This study addressed the main issues of tautomeric process, and gave valuable information on structure elucidation and stereochemical assignment of selaginellins. UV-vis and NMR experiments showed that pH values had profound influence on the tautomeric equlibrium due to the catalyzation by both acid and base. In neutral solution, tautomer exchange was at intermediate rate, while in acidic or basic media, in faster equilibria. Bathochromism of selaginellins with the increment of pH values rationalized the formation of intermolecular hydrogen-bonding in acidic condition, and conjugated QM in basic condition. Furthermore, 1H NMR data suggested that protic solvent could accelerate the tautomeric process compared to aprotic solvent. Finally, variable-temperature 1H NMR experiment provided full evidence that the tautomerism can be blocked below a certain temperature threshold. Given that tautomerism is an intrinsic property of selaginellins, it imparts each of the tautomers their respective physiochemical properties and plays a significant role in their biochemistry. Our investigation on this equilibrium would give a better understanding of their absorption and metabolism in vivo.
| [1] | ZHANG L P, LIANG Y M, WEI X C, et al. A new unusual natural pigment from Selaginella sinensis and its noticeable physicochemical properties[J]. J Org Chem, 2007, 72(10): 3921-3924. DOI: 10.1021/jo0701177. |
| [2] | CHENG X L, MA S C, YU J D, et al. Selaginellin A and B, two novel natural pigments isolated from Selaginella tamariscina[J]. Chem Pharm Bull (Tokyo), 2008, 56(7): 982-984. DOI: 10.1248/cpb.56.982. |
| [3] | CAO Y, CHEN J J, TAN N H, et al. Antimicrobial selaginellin derivatives from Selaginella pulvinata[J]. Bioorg Med Chem Lett, 2010, 20(8): 2456-2460. DOI: 10.1016/j.bmcl.2010.03.016. |
| [4] | XU K P, ZOU H, LI F S, et al. Two new selaginellin derivatives from Selaginella tamariscina (Beauv.) Spring[J]. J Asian Nat Prod Res, 2011, 13(2): 93-96. DOI: 10.1080/10286020.2010.536535. |
| [5] | YANG C, SHAO Y T, LI K, et al. Bioactive selaginellins from Selaginella tamariscina (Beauv.) Spring[J]. Beilstein J Org Chem, 2012, 8(1): 1884-1889. |
| [6] | ZHANG G G, JING Y, ZHANG H M, et al. Isolation and cytotoxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina[J]. Planta Med, 2012, 78(4): 390-392. DOI: 10.1055/s-0031-1298175. |
| [7] | CAO Y, YAO Y, HUANG X J, et al. Four new selaginellin derivatives from Selaginella pulvinata: mechanism of racemization process in selaginellins with quinone methide[J]. Tetrahedron, 2015, 71(10): 1581-1587. DOI: 10.1016/j.tet.2015.01.017. |
| [8] | LIU X, LUO H B, HUANG Y Y, et al. Selaginpulvilins A-D, new phosphodiesterase‑4 inhibitors with an unprecedented skeleton from Selaginella pulvinata[J]. Org Lett, 2014, 16(1): 282-285. DOI: 10.1021/ol403282f. |
| [9] | HUANG Y Y, LIU X, WU D Y, et al. The discovery, complex crystal structure, and recognition mechanism of a novel natural PDE4 inhibitor from Selaginella pulvinata[J]. Biochem Pharmacol, 2017, 130: 51-59. DOI: 10.1016/j.bcp.2017.01.016. |
| [10] | NGUYEN P H, ZHAO B T, ALI M Y, et al. Insulin-mimetic selaginellins from Selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity[J]. J Nat Prod, 2015, 78: 34-42. DOI: 10.1021/np5005856. |
| [11] | KIM J H, CHO C W, TAI B H, et al. Soluble epoxide hydrolase inhibitory activity of selaginellin derivatives from Selaginella tamariscina[J]. Molecules, 2015, 20: 21405. DOI: 10.3390/molecules201219774. |
| [12] | LE D D, NGUYEN D H, ZHAO B T, et al. PTP1B inhibitors from Selaginella tamariscina (Beauv.) Spring and their kinetic properties and molecular docking simulation[J]. Bioorg Chem, 2017, 72: 273-281. DOI: 10.1016/j.bioorg.2017.05.001. |
| [13] | RAUF M A, HISAINDEE S, SALEH N. Spectroscopic studies of keto-enol tautomeric equilibrium of azo dyes[J]. RSC Adv, 2015, 5: 18097-18110. DOI: 10.1039/C4RA16184J. |
| [14] |
ZHANG G, WANG M C, LIU H N, et al. NMR studies of lansoprazole and tautomerism in solution[J].
Chinese J Magn Reson, 2008, 25(2): 217-227.
张皋, 王民昌, 刘红妮, 等. 兰索拉唑的NMR分析及其在溶液中的互变异构现象[J]. 波谱学杂志, 2008, 25(2): 217-227. DOI: 10.3969/j.issn.1000-4556.2008.02.009. |
| [15] |
YANG X Y, WU X Y, AN Y P, et al. An NMR study on keto-enol tautomerism of indole-3-pyruvic acid[J].
Chinese J Magn Reson, 2014, 31(1): 81-91.
杨晓艳, 吴香玉, 安艳捧, 等. 吲哚丙酮酸的酮-烯醇互变异构化的NMR研究[J]. 波谱学杂志, 2014, 31(1): 81-91. DOI: 10.3969/j.issn.1000-4556.2014.01.009. |
| [16] | WANG L, WANG Y, MENG D, et al. Study on the interactions of two isomer selaginellins as novel small molecule inhibitors targeting PTP1B by docking and molecular dynamics simulations[J]. OALib J, 2018, 5(2): e4277. |
 2019, Vol. 36
2019, Vol. 36 
