2. 云南大学 化学科学与工程学院, 云南省高校功能分子分析与生物转化重点实验室, 云南 昆明 650091
2. Key Laboratory of Functional Molecules Analysis and Biotransformation of Universities in Yunnan Province, School of Chemical Science and Technology, Yunnan University, Kunming 650091, China
二萜生物碱(diterpenoid alkaloids)是四环或五环二萜的C-19或C-20与一分子β-氨基乙醇、甲胺或乙胺的氮原子联结而成的杂环化合物,可分为四种结构类型:C18-、C19-、C20-和双二萜生物碱[1].C19-二萜生物碱是其中数量最多的一类,主要分布在毛茛科(Ranunculaceae)的乌头属(Aconitum)、翠雀属(Delphinium)及飞燕草属(Consolida)植物中,具有显著的抗炎、镇痛和抗心律失常作用,目前有3-乙酰乌头碱和草乌甲素临床用作无成瘾性镇痛药[2, 3].C19-二萜生物碱结构复杂而生理作用显著,一直吸引着药学工作者的广泛关注.近年随着色谱学和波谱学等分离分析技术的发展,C19-二萜生物碱研究进展迅速,积累了丰富的基础数据.本文对2010 ~2018年国内外报道的天然C19-二萜生物碱的结构特点、核磁共振(nuclear magnetic resonance,NMR)波谱特征及解析方式进行总结,为C19-二萜生物碱的深入研究及合理开发利用提供参考依据.
1 化学成分研究进展C19-二萜生物碱可分为六种子类型:乌头碱型(Aconitine type,Ⅰ)、牛扁碱型(Lycaconitine type,Ⅱ)、热解型(Pyro type,Ⅲ)、内酯型(Lactone type,Ⅳ)、7, 17-次裂型(7, 17-Seco type,Ⅴ)和重排型(Rearranged type,Ⅵ).表 1列出了2010~2018年报道的167个天然来源的C19-二萜生物碱.
| 表 1 2010~2018年报道的天然来源C19-二萜生物碱 Table 1 Natural C19-diterpenoid alkaloids reported from 2010 to 2018 |
乌头碱型和牛扁碱型涵盖了绝大多数的C19-二萜生物碱,二者区别在于有无7-含氧取代:前者不含有7-含氧基;而后者含有.2010~2018年报道乌头碱型有105个(图 1),牛扁碱型有41个(图 2),它们大多数的区别在于常见取代基种类、数量和位置的不同.
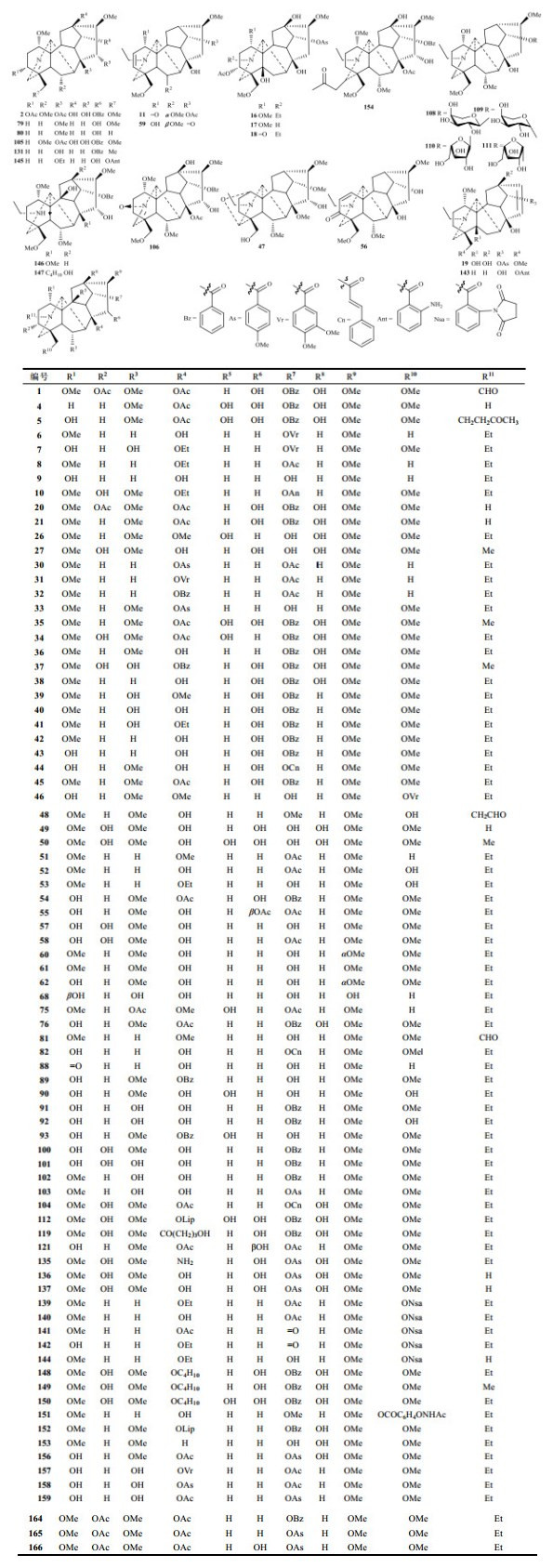
|
图 1 2010~2018年报道的天然来源的C19-乌头碱型二萜生物碱 Fig. 1 Natural aconitine type C19-diterpenoid alkaloids reported from 2010 to 2018 |
|
图 2 2010~2018年报道的天然来源的C19-牛扁碱型二萜生物碱 Fig. 2 Natural lycaconitine type C19-diterpenoid alkaloids reported from 2010 to 2018 |
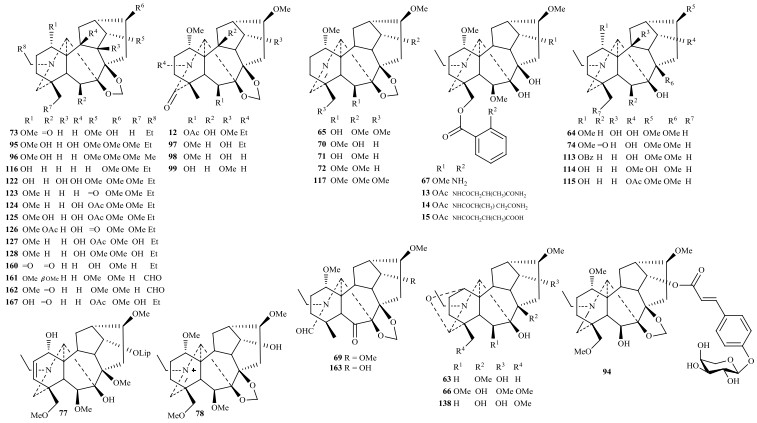
乌头碱型C19-二萜生物碱多见羟基、甲氧基、乙酰基(Ac)、苯甲酰基(Bz)、大茴香酰基(As)、藜芦酰基(Vr)取代[32, 35],也可见双键、酮羰基、亚胺(N=C(21))、氮杂缩醛、肉桂酸酯基(Cn)等基团取代;牛扁碱型C19-二萜生物碱多见邻氨基苯甲酸酯衍生物(Ant、Nsa)、7, 8-次甲二氧基、OMe-14等取代,而几乎不见Ac、Bz和As取代.乌头碱型和牛扁碱型C19-二萜生物碱几乎都含甲氧基取代,乌头碱型化合物16β-hydroxycardiopetaline是首个不含甲氧基的C19-二萜生物碱,其差向异构体1-epi-16β-hydroxycardiopetaline (68)近年也从黄草乌中分离得到,该化合物还具罕见的1β-含氧基[34].近年来,C19-二萜生物碱中也发现有新颖取代基.Meng等[51]从附子首次发现的乌头碱型C19-二萜生物碱苷aconicarmichosides A~D (108~111)分别取代有α-、β-的L -阿拉伯吡喃糖和L-阿拉伯呋喃糖;Zhang等[45]从伊犁翠雀花(D. iliense)发现的牛扁碱型iliensine A (94)取代有肉桂酸葡萄糖基;D. elatum中的牛扁碱型C19-二萜生物碱N-formyl-4, 19-secopacinine (69)首次发现具有CHO-19基团(甲酰胺)[35],而之前报道多为CHO-20基团,如乌头碱型化合物brachyaconitine (1)、alexhumboldtine (48)等.此外还有N-丁酮[5]、4-羟基丁酰基[55]、丁氧基[63]等取代基的报道.
乌头碱型C19-二萜生物碱常见取代基位置为C-1、C-3、C-6、C-8、C-14、C-16和C-18.牛扁碱型取代位置与乌头碱型类似,但其6-含氧基为β构型,而乌头碱型的多为α,仅极少数为β,本课题组从宾川乌头(A. duclouxii)分离的乌头碱型C19-二萜生物碱ducludine F (59)具有OCH3-6β取代[30].
C19-二萜生物碱酯化程度常见醇胺、单酯或双酯,具有三酯取代的C19-二萜生物碱如乌头碱型brachyaconitine B (2)、N-deethyl-3-O-acetylchasmaconitine (164)、N-deethyl-3-O-acetylyunaconitine (165)、N-deethyl-3-O-acetyljesaconitine (166)较为罕见[5, 68].
1.2 7, 17-次裂型7, 17-次裂型C19-二萜生物碱是乌头碱型的C(7)-C(17)键经Grob裂解形成双键△7, 8,然后OH-6α进攻N+=C(17)亚胺盐形成C(17)-O-C(6)键而得,典型化合物为大渡乌碱类,目前报道的大渡乌碱型约有10个,近年发现有guiwuline (28)(图 3)[17].部分化合物Grob裂解后保留N=C(17)亚胺结构,如brachyacintine C (3) [4];或形成C(3)-O-C(17)-N氮杂缩醛结构如secoaconitine (22)[12].本课题组从黄草乌和展毛黄草乌发现的vilmorine A (83)、vilmotenitines A和B (86, 87)具有△7, 8,但没有C(17)-O-C(6)键,且C(8)-C(9)键重排为C(8)-C(10)形成六元B环,是一类新颖的7, 17-次裂型骨架[42].
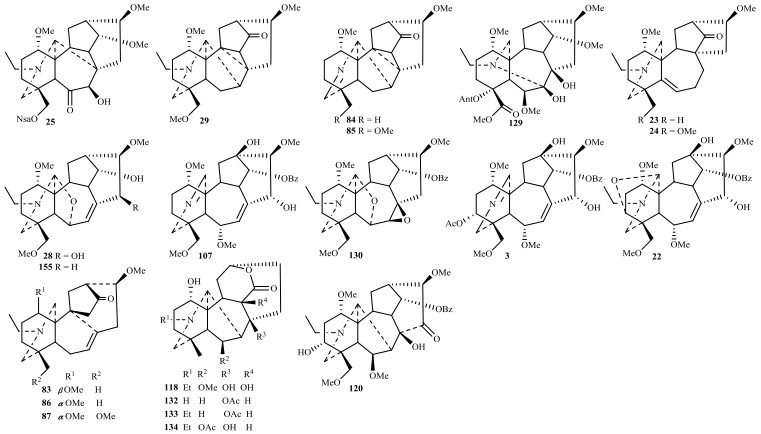
|
图 3 2010~2018年报道的天然来源的7, 17-次裂型、重排型、内酯型和热解型C19-二萜生物碱 Fig. 3 Natural 7, 17-seco type, rearranged type, lactone type and pyro type C19-diterpenoid alkaloids reported from 2010 to 2018 |
典型重排型C19-二萜生物碱acoseptine类由具有7, 8-邻二羟基牛扁碱型C19-二萜生物碱经纳咵醇重排成C(17)-C(8)键而得,目前约5个,近年发现的有D. yunnanense中的yunnanenseine A (25)[14].具B环C(8)-C(9)-C(10)三元环的vilmoraconitine类重排C19-二萜生物碱由谭宁华等[70]从黄草乌首次发现,同类化合物有aconitramine A (29)及本课题组从黄草乌分离的vilmorines B和C (84, 85) [18, 42].此外D. grandiflorum中的grandiflodine B (129)的N-C(19)和C(7)-C(17)键均断裂重排为N-C(7)键,是首次发现的重排型C19-二萜生物碱[58].
1.4 热解型和内酯型热解型C19-二萜生物碱指具有△8, 15或15-酮结构的C19-二萜生物碱,源于乌头碱型消除8-OAc或15-含氧基而得.近年来发现的有保山乌头(A. nagarum)中的nagaconitine B (120)[55][49].
内酯型C19-二萜生物碱指具有六元内酯C环的C19-二萜生物碱,由乌头碱型14-酮经Bayer-Viliger氧化而得.天然来源的内酯型C19-二萜生物碱数量极少,目前报道的约为11个,近年仅有从A. heterophyllum分离的9β-dihydroxylheteratisine (118) [54].
2 C19-二萜生物碱的NMR波谱特征和结构解析 2.1 C18-、C19-、C20-和双二萜生物碱的区分二萜生物碱结构复杂、解析困难,其1H NMR谱图高场区域重叠严重,而13C NMR谱图分辨率更高,而且结合DEPT技术可区分伯碳、仲碳、叔碳和季碳,对二萜生物碱的骨架解析极有帮助.C19-二萜生物碱的结构解析可首先根据其来源、母核碳原子数目、特征性季碳信号和取代基信号区分C18-、C19-、C20-和双二萜生物碱.
C19-二萜生物碱是含19个碳原子母核的四环二萜与β-氨基乙醇联结成的杂环衍生物,其不含氧取代的季碳C-4、C-11和含氧取代的季碳C-8在骨架无变化情况下相对恒定(表 2),是C19-二萜生物碱结构类型判断的重要依据.C19-二萜生物碱氧化程度较高,通常有多个含氧取代(δH 3.0~5.0;δC 70~90);醇羟基易与醋酸、苯甲酸衍生物成酯,几乎都有甲氧基取代(δH 3.2~3.6,s;δC 55~59,q),可作为判断依据.
| 表 2 C19-二萜生物碱常见季碳化学位移范围(δC) Table 2 Chemical shift (δC) ranges of common quaternary carbons in C19-diterpenoid alkaloids |
C18-二萜生物碱为C19-二萜生物碱失去18-碳原子的降解产物,母核碳数为18,数目较少,多存在于牛扁亚属.C18-二萜生物碱根据C-7含氧取代的有无分为高乌碱型和冉乌碱型,分别对应C19-乌头碱型和牛扁碱型.C18-二萜生物碱取代方式和C19-相似,区别在于缺少C-18角甲基(δH 0.8~1.1,s;δC 21~28,q)或含氧亚甲基(δH 2.8~3.4,ABq;δC 72~80,t),C-4为含氧季碳或不含氧的次甲基.C18-二萜生物碱通常包含不含氧取代季碳C-11和含氧取代季碳C-8、C-4.此外3, 4-环氧取代仅存在于C18-二萜生物碱,而C19-和C20-二萜生物碱均不存在.
C20-二萜生物碱结构类型较多,主要可分为阿替生型、海替生型、海替定型、光翠雀碱型、维特钦型、纳哌啉型六类.母核碳数为20,其不含氧取代季碳C-4(δC 30~40,s)、C-8(δC 30~50,s)和C-10(δC 35~50,s)相对恒定,可能存在C-15含氧取代季碳,这是判断该类型结构的主要依据.与C19-二萜生物碱相比,C20-二萜生物碱的含氧取代较少,而且不含甲氧基取代,且大多数C20-二萜生物碱具有环外双键△16, 17(δH ~5,brs;δC ~110,t;δC ~143,s).
双二萜生物碱数量极少,通常由1分子的C19-二萜生物碱与1分子或2分子的C20-二萜生物碱缩合而成,母核碳数为二者之和,解析可依据其组成单体的NMR特征进行.
2.2 C19-二萜生物碱子类型的确定C19-二萜生物碱是乌头属、翠雀属和飞燕草属植物的特征成分,其种类与植物进化程度有密切关系.C19-二萜生物碱的结构解析,尤其是骨架类型的推测,首先应考虑植物来源:如牛扁碱型几乎全分布于翠雀属;内酯型几乎全分布于乌头亚属比较原始的类群,如甘青乌头系.C19-二萜生物碱子类型的区分主要根据季碳和特征性取代基NMR特征信号,其中季碳信号在C19-二萜生物碱的骨架推测中起关键作用(表 2).
乌头碱型C19-二萜生物碱常具有不含氧取代季碳C-4、C-11和含氧取代季碳C-8;而且因含氧取代的引入,可能存在季碳C-9、C-10和C-13(δC > 70).牛扁碱型C19-二萜生物除含有季碳C-4、C-11、C-8外,NMR谱图低场区域还可见C-7β取代季碳(δC 86~90,s)信号,若存在7, 8-次甲二氧基取代,则见于更低场区域(δC 91~93,s).受7β-含氧取代影响,与乌头碱型C19-二萜生物碱相比,牛扁碱型季碳C-8的NMR信号向低场位移,据此可区分二者.另外,也可利用取代基种类、位置和构型区分乌头碱型和牛扁碱型C19-二萜生物碱:乌头碱型C19-二萜生物碱常见的Ac、As和Bz取代几乎不出现在牛扁碱型中;而邻氨基苯甲酸酯衍生物和次甲二氧基(δH 3.2~3.6, s;δC 55~59, t)取代几乎都存在牛扁碱型C19-二萜生物碱中;乌头碱型C-14多为羟基或成酯,而牛扁碱型多为甲氧基;乌头碱型6-含氧基多为α构型,极少数为β构型,而牛扁碱型6-含氧基多为β构型;乌头碱型C-13、C-15常有羟基或醋酸酯取代,而牛扁碱型C-13、C-15则不含取代基.
与乌头碱型相比较,虽然大渡乌碱类7, 17-次裂型C19-二萜生物碱也具有较为恒定的季碳C-4(δC 37~38,s)和C-11(δC 50~51,s),但△7, 8(C-8,δC 137~139)替代了C-8含氧取代季碳.此外,7, 17-次裂型常具有N=C(17)亚胺、C(17)-O-C(6)等基团.与大渡乌碱型7, 17-次裂型C19-二萜生物碱相比较,Vilmorine A类7, 17-次裂型则多出不含氧取代季碳C-10(δC 44~45,s),此外,C-14酮羰基取代(δC 216~218)也是该类化合物的特征信号.
Acoseptine类重排型C19-二萜生物碱除含有季碳C-4、C-11外,C-8由于重排变为不含氧取代季碳(δC 54~55,s).具有C(8)-C(9)-C(10)三元环结构的重排型C19-二萜生物碱与乌头碱型相比,除C-4、C-11外,多出不含氧季碳C-8和C-10,而且与vilmorine A类7, 17-次裂型C19-二萜生物碱类似,具有C-14酮羰基.内酯型C19-二萜生物碱的季碳和乌头碱型一致,区别在于结构中多出内酯键(δC 175,s).热解型C19-二萜生物碱中△8, 15替代了C-8含氧取代季碳.
2.3 C19-二萜生物碱结构解析通过季碳及取代基确定C19-二萜生物碱子类型后,需综合利用1H、13C和2D NMR进行结构解析,确定取代基种类、数目、位置及构型.取代基种类主要通过取代基的特征1H和13C NMR信号确定(表 3).取代基数目可通过13C NMR谱中δC 70~90含氧取代碳数、1H NMR谱中δH 3.0~5.0含氧取代质子数,结合高分辨质谱(high revolution mass spectrum,HR-MS)所得分子式来确定[71].
| 表 3 C19-二萜生物碱常见取代基特征核磁共振波谱信号 Table 3 Chemical shifts of common substituent groups in C19-diterpenoid alkaloids |
取代位置主要根据含氧取代碳的级数和化学位移、取代质子化学位移和耦合常数等,参照文献类似化合物的NMR数据判断.应充分利用不同取代基产生的化学位移效应进行取代位置的判断,如C-8取代羟基或乙酰基化学位移相差6~8.首先考虑常见取代位置,如乌头碱型常见取代顺序为C-8/C-16/C-14→C-18→C-1→C-3/C-13→C-15.2D HMBC谱在取代位置判断中作用极大,如甲氧基位置可根据取代位置甲基质子与13C核相关峰确定,酯基取代可根据取代位置质子与羰基的相关峰确定.
取代构型可根据取代位置13C核化学位移判断,如乌头碱型C19-二萜生物碱含1β-含氧基时,C-1向高场位移4~5,C-6含氧基取代构型不同亦有明显化学位移差异.值得注意的是,利用化学位移判断取代构型时,需排除溶剂效应[72].NOESY谱在取代基构型判断中作用较大,如C-1取代构型可通过NOESY中H-1/H-5、H-1/H-11相关确定,C-6取代构型可通过NOESY中H-6/H-9相关确定.而化合物的绝对构型则一般通过X-单晶衍射实验确定.
3 总结近年来,二萜生物碱的研究进展迅速,许多结构新颖的化合物被报道,反映了C19-二萜生物碱丰富的结构多样性,具有巨大的研究潜力与价值.C19-二萜生物碱结构复杂、鉴定困难,本文对其化学结构和NMR波谱特征进行了总结,为该类化合物的快速鉴定提供了参考依据.随着高分辨NMR波谱仪及多维NMR技术的推广与应用[73],C19-二萜生物碱的鉴定将更加快速而准确,且微量二萜生物碱的鉴定也将更加方便.因此可预见将有愈来愈多的C19-二萜生物碱新结构被发现,为药物研发提供更多的活性先导化合物.
| [1] | WANG F P, CHEN Q H, LIU X Y. Diterpenoid alkaloids[J]. Nat Prod Rep, 2009, 27(4): 529-570. |
| [2] | REINA M, GONZÁLEZ-COLOMA A. Structural diversity and defensive properties of diterpenoid alkaloids[J]. Phytochem Rev, 2007, 6(1): 81-95. DOI: 10.1007/s11101-006-9013-5. |
| [3] | HAO D C, GU X J, XIAO P G, et al. Recent advances in the chemical and biological studies of Aconitum pharmaceutical resources[J]. J Chin Pharm Sci, 2013, 22(3): 209-221. |
| [4] | SHEN Y, ZUO A X, JIANG Z Y, et al. Four new nor-diterpenoid alkaloids from Aconitum brachypodum[J]. Helv Chim Acta, 2010, 93(5): 863-869. DOI: 10.1002/(ISSN)1522-2675. |
| [5] | SHEN Y, ZUO A X, JIANG Z Y, et al. Two new diterpenoid alkaloids from Aconitum brachypodum[J]. B Kor Chem Soc, 2010, 31(11): 3301-3303. DOI: 10.5012/bkcs.2010.31.11.3301. |
| [6] | SHEN Y, ZUO A X, JIANG Z Y, et al. Five new C19-diterpenoid alkaloids from Aconitum hemsleyanum[J]. Helv Chim Acta, 2010, 93(3): 482-489. DOI: 10.1002/hlca.v93:3. |
| [7] | ZHANG Z T, LIU X Y, CHEN D L, et al. New diterpenoid alkaloids from Aconitum liangshanicum[J]. Helv Chim Acta, 2010, 93(4): 811-817. DOI: 10.1002/hlca.v93:4. |
| [8] | GABBASOV T M, TSYRLINA E M, SPIRIKHIN L V, et al. 19-Oxodeltaline, a norditerpene alkaloid from the aerial part of Delphinium uralense[J]. Chem Nat Comp, 2010, 46(1): 158-159. |
| [9] | CHEN F Z, CHEN Q H, WANG F P. C19-diterpenoid alkaloids from Delphinium umbrosum[J]. J Asian Nat Prod Res, 2010, 12(6): 498-504. DOI: 10.1080/10286020.2010.489827. |
| [10] | GAO F, ZHU S A, WU W, et al. C19-diterpenoid alkaloids from the roots of Aconitum hemsleyanum var.hanyuanum and their chemotaxonomic significance[J]. Biochem Syst Ecol, 2010, 38(5): 1052-1055. DOI: 10.1016/j.bse.2010.09.003. |
| [11] | GAO F, LIU X Y, WANG F P. Three new C19-diterpenoid alkaloids from Aconitum hemsleyanum var.circinatum[J]. Helv Chim Acta, 2010, 93: 785-790. DOI: 10.1002/hlca.v93:4. |
| [12] | WANG Y J, ZHANG J, ZENG C J, et al. Three new C19-diterpenoid alkaloids from Aconitum pendulum[J]. Phytochem Lett, 2011, 4(2): 166-169. |
| [13] | SHEN Y, ZUO A X, JIANG Z Y, et al. Hemsleyaconitines F and G, two novel C19-diterpenoid alkaloids possessing a unique skeleton from Aconitum hemsleyanum[J]. Helv Chim Acta, 2011, 94(2): 268-272. DOI: 10.1002/hlca.v94.2. |
| [14] | CHEN F Z, CHEN Q H, WANG F P. Diterpenoid alkaloids from Delphinium yunnanense[J]. Helv Chim Acta, 2011, 94(2): 254-260. DOI: 10.1002/hlca.v94.2. |
| [15] | FORGO P, BORCSA B, CSUPOR D, et al. Diterpene alkaloids from Aconitum anthora and assessment of the hERG-inhibiting ability of Aconitum alkaloids[J]. Planta Med, 2011, 77(4): 368-373. DOI: 10.1055/s-0030-1250362. |
| [16] | USMANOVA S K, AISA H A. Karaconitine, a new C19-norditerpenoid alkaloid from Aconitum karakolicum roots[J]. Chem Nat Comp, 2011, 47(2): 265-267. |
| [17] | WANG D P, LOU H Y, HUANG L, et al. A novel franchetine type norditerpenoid isolated from the roots of Aconitum carmichaeli Debx.with potential analgesic activity and less toxicity[J]. Bioorg Med Chem Lett, 2012, 22(13): 4444-4446. DOI: 10.1016/j.bmcl.2012.04.132. |
| [18] | YONG S, AI H L, CAO T W, et al. Three new C19-diterpenoid alkaloids from Aconitum transsectum[J]. Helv Chim Acta, 2012, 95(3): 509-513. DOI: 10.1002/hlca.v95.3. |
| [19] | SHEN Y, AI H L, ZI S H, et al. Two new C19-diterpenoid alkaloids from Aconitum transsectum[J]. J Asian Nat Prod Res, 2012, 14(3): 244-248. DOI: 10.1080/10286020.2011.648623. |
| [20] | JIANG B, LIN S, ZHU C, et al. Diterpenoid alkaloids from the lateral root of Aconitum carmichaelii[J]. J Nat Prod, 2012, 75(6): 1145-1159. DOI: 10.1021/np300225t. |
| [21] | ZHANG Z T, WANG L, CHEN Q F, et al. Revisions of the diterpenoid alkaloids reported in a JNP paper (2012, 75, 1145-1159)[J]. Tetrahedron, 2013, 69(29): 5859-5866. DOI: 10.1016/j.tet.2013.05.029. |
| [22] | WANG F P, CHEN D L, DENG H Y, et al. Further revisions on the diterpenoid alkaloids reported in a JNP paper (2012, 75, 1145-1159)[J]. Tetrahedron, 2014, 70(15): 2582-2590. DOI: 10.1016/j.tet.2014.01.066. |
| [23] | PIRILDAR S, UNSAL-GURER C, KOCYIGIT M, et al. Norditerpenoid alkaloids from Delphinium flexuosum Bieb[J]. Z Naturforsch C, 2012, 67(11/12): 541-544. |
| [24] | MERICLI A H, CAGAL-YURDUSEVER N, OZCELIK H, et al. A new diterpenoid alkaloid from the roots of a white-flowering Aconitum orientale sample[J]. Helv Chim Acta, 2012, 95: 314-319. DOI: 10.1002/hlca.v95.2. |
| [25] | KURTOĞLU S, SEN B, MELIKOĞLU G, et al. Diterpenoid alkaloids of Aconitum vulparia rchb[J]. Z Naturforsch C, 2012, 67(3-4): 103-106. DOI: 10.1515/znc-2012-3-401. |
| [26] | LIU X X, JIAN X X, CAI X F, et al. Cardioactive C19-diterpenoid alkaloids from the lateral roots of Aconitum carmichaeli "Fu Zi"[J]. Chem Pharm Bull, 2012, 60(1): 144-149. DOI: 10.1248/cpb.60.144. |
| [27] | XU J J, ZHAO D K, AI H L, et al. Three new C19-diterpenoid alkaloids from Aconitum forrestii[J]. Helv Chim Acta, 2013, 96(11): 2155-2159. DOI: 10.1002/hlca.v96.11. |
| [28] | YIN T P, CAI L, LEI G, et al. Two new diterpenoid alkaloids from the roots of Aconitum duclouxii Levl[J]. Chin J Org Chem, 2013, 33(12): 2528. DOI: 10.6023/cjoc201307025. |
| [29] | YIN T P, CAI L, HE J M, et al. Three new diterpenoid alkaloids from the roots of Aconitum duclouxii[J]. J Asian Nat Prod Res, 2014, 16(4): 345-350. DOI: 10.1080/10286020.2014.881802. |
| [30] | YIN T P, CAI L, ZHOU H, et al. A new C19-diterpenoid alkaloid from the roots of Aconitum duclouxii[J]. Nat Prod Res, 2014, 28(19): 1649-1654. DOI: 10.1080/14786419.2014.927463. |
| [31] | GUO Z J, XU Y, ZHANG H, et al. New alkaloids from Aconitum taipaicum and their cytotoxic activities[J]. Nat Prod Res, 2014, 28(3): 164-168. DOI: 10.1080/14786419.2013.861832. |
| [32] | ZHAO B, USMANOVE S, AISA H A. Three new C19-diterpenoid alkaloids from Delphinium tianshanicum W.T.Wang[J].Phytochem Lett[J]. Phytochem Lett, 2014, 10: 189-192. DOI: 10.1016/j.phytol.2014.09.010. |
| [33] | BEGUM S, ALI M, LATIF A, et al. Pharmacologically active C19-diterpenoid alkaloids from the aerial parts of Aconitum laeve Royle[J]. Rec Nat Prod, 2014, 8(2): 83-92. |
| [34] | TANG T X, CHEN D L, WANG F P. A new C19-diterpenoid alkaloid from Aconitum vilmorinianum[J]. Chin J Org Chem, 2014, 34(5): 909. DOI: 10.6023/cjoc201401013. |
| [35] | WADA K, CHIBA R, KANAZAWA R, et al. Six new norditerpenoid alkaloids from Delphinium elatum[J]. Phytochem Lett, 2015, 12: 79-83. DOI: 10.1016/j.phytol.2015.02.010. |
| [36] | SHAHEEN F, AHMAD M, RIZVI T S, et al. Norditerpenoid alkaloids from Delphinium kohatense Munz[J]. Rec Nat Prod, 2015, 51(2): 337-340. |
| [37] | CHEN L, SHAN L H, ZHANG J F, et al. Diterpenoid alkaloids from Aconitum soongaricum var.pubescens[J]. Nat Prod Commun, 2015, 10(12): 2063-2065. |
| [38] | ZHAO Q, GOU X J, LIU W, et al. Majusine D:a new C19-diterpenoid alkaloid from Delphinium majus[J]. Nat Prod Commun, 2015, 10(12): 2069-2070. |
| [39] | ZHAO B, USMANOVA S K, YILI A, et al. New C19-norditerpenoid alkaloid from Delphinium shawurense[J]. Chem Nat Comp, 2015, 51(3): 519-522. DOI: 10.1007/s10600-015-1328-2. |
| [40] | CHEN C L, TAN W H, WANG Y, et al. New norditerpenoid alkaloids from Aconitum vilmorinianum Komarov[J]. J Nat Med, 2015, 69(4): 601-607. DOI: 10.1007/s11418-015-0926-4. |
| [41] | YIN T P, CAI L, LI Y, et al. New alkaloids from Aconitum stapfianum[J]. Nat Prod Bioprosp, 2015, 5(6): 271-275. DOI: 10.1007/s13659-015-0075-1. |
| [42] | YIN T P, CAI L, FANG H X, et al. Diterpenoid alkaloids from Aconitum vilmorinianum[J]. Phytochem, 2015, 116: 314-319. DOI: 10.1016/j.phytochem.2015.05.002. |
| [43] | CAI L, FANG H X, YIN T P, et al. Unusual C19-diterpenoid alkaloids from Aconitum vilmorinianum var.patentipilum[J]. Phytochem Lett, 2015, 14: 106-110. DOI: 10.1016/j.phytol.2015.09.010. |
| [44] | QIN X D, YANG S, ZHAO Y, et al. Five new C19-diterpenoid alkaloids from Aconitum carmichaeli[J]. Phytochem Lett, 2015, 13: 390-393. DOI: 10.1016/j.phytol.2015.07.017. |
| [45] | ZHANG J F, DAI R Y, SHAN L H, et al. Iliensines A and B:two new C19-diterpenoid alkaloids from Delphinium iliense[J]. Phytochem Lett, 2016, 17: 299-303. DOI: 10.1016/j.phytol.2016.08.014. |
| [46] | WADA K, ASAKAWA E, TOSHO Y, et al. Four new diterpenoid alkaloids from Delphinium elatum[J]. Phytochem Lett, 2016, 17: 190-193. DOI: 10.1016/j.phytol.2016.06.009. |
| [47] | QI Y, ZHAO D K, ZI S H, et al. Two new C19-diterpenoid alkaloids from Aconitum straminiflorum[J]. J Asian Nat Prod Res, 2016, 18(4): 366-370. DOI: 10.1080/10286020.2015.1107545. |
| [48] | LI G Q, ZHANG L M, ZHAO D K, et al. Two new C19-diterpenoid alkaloids from Aconitum tsaii[J]. J Asian Nat Prod Res, 2016, 19(5): 457-461. |
| [49] | XU W L, SHAN L H, HUANG S, et al. New ditetpenoid alkaloid from the whole plant of Aconitum franchetii var.villosulum[J]. Chin J Org Chem, 2016, 36(11): 2739. DOI: 10.6023/cjoc201604055. |
| [50] | WANG F, YUE Z G, XIE P, et al. C19-norditerpenoid alkaloids from Aconitum szechenyianum and their effects on LPS-activated NO production[J]. Molecules, 2016, 21(9): 1175. DOI: 10.3390/molecules21091175. |
| [51] | MENG X H, GUO Q L, ZHU C G, et al. Unprecedented C19-diterpenoid alkaloid glycosides from an aqueous extract of "fu zi" :neoline 14-O-L-arabinosides with four isomeric L-anabinosyls[J]. Chin Chem Lett, 2017, 28(8): 1705-1710. DOI: 10.1016/j.cclet.2017.04.026. |
| [52] | LIANG X X, CHEN L, SONG L, et al. Diterpenoid alkaloids from the root of Aconitum sinchiangense W.T.Wang with their antitumor and antibacterial activities[J]. Nat Prod Res, 2017, 31(17): 2016-2023. DOI: 10.1080/14786419.2016.1272113. |
| [53] | ZHANG J F, SHAN L H, GAO F, et al. Five new C19-diterpenoid alkaloids from Delphinium tianshanicum W.T.Wang[J]. Chem Biodivers, 2017, 14(4). DOI: 10.1002/cbdv.201600297. |
| [54] | AHMAD H, AHMAD S, SHAH S A A, et al. Antioxidant and anticholinesterase potential of diterpenoid alkaloids from Aconitum heterophyllum[J]. Bioorg Med Chem, 2017, 25(13): 3368-3376. DOI: 10.1016/j.bmc.2017.04.022. |
| [55] | ZHAO D K, SHI X Q, ZHANG L M, et al. Four new diterpenoid alkaloids with antitumor effect from Aconitum nagarum var.heterotrichum[J]. Chin Chem Lett, 2017, 28(2): 358-361. DOI: 10.1016/j.cclet.2016.09.012. |
| [56] | LIN C Z, LIU Z J, BAIRI Z D, et al. A new diterpenoid alkaloid isolated from Delphinium caeruleum[J]. Chin J Nat Med, 2017, 15(1): 45-48. |
| [57] | SHAN L H, ZHANG J F, GAO F, et al. Diterpenoid alkaloids from Delphinium anthriscifolium var.majus[J]. Sci Rep, 2017, 7(1): 60-63. |
| [58] | CHEN N H, ZHANG Y B, LI W, et al. Grandiflodines A and B, two novel diterpenoid alkaloids from Delphinium grandiflorum[J]. RSC Adv, 2017, 7(39): 24129-24132. DOI: 10.1039/C7RA02869E. |
| [59] | GUO R H, GUO C X, HE D, et al. Two new C19-diterpenoid alkaloids with anti-inflammatory activity from Aconitum iochanicum[J]. Chin J Chem, 2017, 35(10): 1644-1647. DOI: 10.1002/cjoc.v35.10. |
| [60] | ZHOU X L, XU W L, FREJAT F O, et al. Three new lactone-type diterpenoid alkaloids from Aconitum rotundifolium Kar. & Kir[J]. Heterocycles, 2017, 94(10): 1903-1908. DOI: 10.3987/COM-17-13768. |
| [61] | YAMASHITA H, TAKEDA K, HARAGUCHI M, et al. Four new diterpenoid alkaloids from Aconitum japonicum subsp.subcuneatum[J]. J Nat Med, 2018, 72(1): 230-237. DOI: 10.1007/s11418-017-1139-9. |
| [62] | ZHANG J F, CHEN L, HUANG S, et al. Diterpenoid alkaloids from two Aconitum species with antifeedant activity against Spodoptera exigua[J]. J Nat Prod, 2017, 80(12): 3136-3142. DOI: 10.1021/acs.jnatprod.7b00380. |
| [63] | ZONG X X, YAN G Y, WU J L, et al. New C19-diterpenoid alkaloids from the parent roots of Aconitum carmichaelii[J]. Tetrahedron Lett, 2017, 58(16): 1622-1626. DOI: 10.1016/j.tetlet.2017.03.033. |
| [64] | JIANG H Y, HUANG S, GAO F, et al.Diterpenoid alkaloids from Aconitum brevicalcaratum as autophagy inducers[J].Nat Prod Res, 2018, https://doi.org/10.1080/14786419.2018.1437435. |
| [65] | LIU W Y, HE D, ZHAO D K, et al. Four new C19-diterpenoid alkaloids from the roots of Aconitum ouvrardianum[J]. J Asian Nat Prod Res, 2018. DOI: 10.1080/10286020.2018.1451520. |
| [66] | YIN T P, HU X F, MEI R F, et al. Four new diterpenoid alkaloids with anti-inflammatory activities from Aconitum taronense Fletcher et Lauener[J]. Phytochem Lett, 2018, 25: 152-155. DOI: 10.1016/j.phytol.2018.04.001. |
| [67] | YAMASHITA H, KATOH M, KOKUBUN A, et al. Four new C19-diterpenoid alkaloids from Delphinium elatum[J]. Phytochem Lett, 2018, 24: 6-9. DOI: 10.1016/j.phytol.2017.12.013. |
| [68] | WANG X Y, WANG D X, LAI G F, et al. Diterpenoid alkaloids from Aconitum brachypodum[J]. Chem Nat Comp, 2018, 54(1): 137-141. |
| [69] | AHMAD H, AHMAD S, SHAH S A A, et al. Selective dual cholinesterase inhibitors from Aconitum laeve[J]. J Asian Nat Prod Res, 2018, 20(2): 172-181. DOI: 10.1080/10286020.2017.1319820. |
| [70] | XIONG J, TAN N H, JI C J, et al. Vilmoraconitine, a novel skeleton C19-diterpenoid alkaloid from Aconitum vilmorinianum[J]. Tetrahedron Lett, 2008, 49(33): 4851-4853. DOI: 10.1016/j.tetlet.2008.06.008. |
| [71] |
WEI W, LI X W, YANG R J, et al. Structural elucidation of three monoester-diterpenoid aconines by NMR spectroscopy[J].
Chinese J Magn Reson, 2010, 27(2): 238-248.
魏巍, 李绪文, 杨瑞杰, 等. 3种单酯型乌头碱的NMR研究[J]. 波谱学杂志, 2010, 27(2): 238-248. DOI: 10.3969/j.issn.1000-4556.2010.02.012. |
| [72] |
YIN T P, CHEN Y, LUO P. Structural elucidation and NMR spectral assignments of two C19-diterpenoid alkaloids[J].
Chinese J Magn Reson, 2018, 35(1): 90-97.
尹田鹏, 陈阳, 罗萍, 等. 两个C19-二萜生物碱的结构鉴定和nmr信号归属[J]. 波谱学杂志, 2018, 35(1): 90-97. |
| [73] |
SUN L J, HU X F, CHENG X, et al. NMR characterization of flavanone naringenin 7-O-glycoside diastereomer[J].
Chinese J Magn Reson, 2017, 34(4): 465-473.
孙丽娟, 胡小芳, 程寻, 等. 柚皮素7-O-葡萄糖苷非对映异构体的NMR波谱分析[J]. 波谱学杂志, 2017, 34(4): 465-473. |
 2019, Vol. 36
2019, Vol. 36 
