2. 大连理工大学, 精细化工国家重点实验室, 辽宁 大连 116012;
3. 大连理工大学 化学分析测试中心, 辽宁 大连 116023
2. State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116012, China;
3. Chemistry Analysis and Inspection Center, Dalian University of Technology, Dalian 116023, China
硫代碳酰腙(TCHs)是一类具有广谱生物活性的化合物,其衍生物具有抗癌[1-3]、抗糖尿病[4]、抗病毒[5]、抗菌[6-8]和抗氧化[9]等活性.此外,硫代碳酰腙也是构建各种生物活性杂环化合物的结构单元[10].靛红含有不同的功能化基团,具有独特的电子特性,因而靛红衍生物具有多种有益的生物特性,如抗癌[11, 12]、抗糖尿病[13]、抗菌[14]和抗氧化[15]等活性.咔唑及其衍生物是非常重要的含氮芳香族杂环化合物,此类化合物显示出抗癌[16, 17]、抗糖尿病[18, 19]、抗菌[20]、抗氧化[21-23]、抗结核[24]等特性.
鉴于上述3类化合物的重要生物活性,本课题组在前期工作的基础上[25, 26],设计将硫代碳酰腙、靛红和咔唑3个药效团进行杂交,合成出了一个新型的基于咔唑-靛红双-硫代碳酰腙衍生物,即1-[(3Z)-2-氧代吲哚-3-亚基]-5-[(9-己基-3-咔唑基)亚基]硫代碳酰腙(化合物2,化学结构见图 1).本文利用元素分析、红外吸收光谱(IR)、多种核磁共振(NMR)技术(包括1H NMR、13C NMR、1H-1H COSY、1H-13C HSQC和1H-13C HMBC)确定了新化合物2的结构,并对其1H和13C NMR信号进行了全归属.本研究对此类化合物的1H和13C NMR信号归属,以及其结构与活性关系的揭示具有一定的指导意义.
|
图 1 1-[(3Z)-2-氧代吲哚-3-亚基]-5-[(9-己基-3-咔唑基)亚基]硫代碳酰腙(新化合物2)的合成路线 Fig. 1 Synthetic route of 1-[(3Z)-2-oxoindoline-3-ylidene]-5-[(9-hexyl-3-carbazolyl)ylidene]thiocarbohydrazone (new compound 2) |
NMR实验均在Bruker 500型超导NMR谱仪上完成;IR光谱利用Bruker TENSOR27型红外分光光度计获得,KBr压片;元素分析在Elementar Vario EL III元素分析仪上完成.含0.03%四甲基硅烷(TMS)的DMSO-d6(氘代率为99.9%,CIL)购自北京恒思锐科贸有限公司.
1.2 NMR实验样品溶于DMSO-d6,以TMS为内标.实验温度为297.5 K,1H和13C NMR的工作频率分别为500.03 MHz和125.73 MHz,谱宽分别为10 330.6 Hz和29 761.9 Hz.2D NMR(1H-1H COSY、1H-13C HSQC和1H-13C HMBC)实验均采用标准脉冲序列.1H-1H COSY的F2(1H)维和F1(1H)维谱宽均为8 474.6 Hz,采样数据点阵为t2 × t1 = 2 048 × 256;1H-13C HSQC的F2(1H)维和F1(13C)维谱宽分别为8 474.6 Hz和20 827.8 Hz,采样数据点阵为t2 × t1 = 2 048 × 256;1H-13C HMBC的F2(1H)维和F1(13C)维谱宽分别为8 474.6 Hz和27 927.4 Hz,采样数据点阵为t2 × t1 = 2 048 × 256.
2 结果与讨论 2.1 新化合物2的合成向50 mL干燥的三口瓶中,加入0.30 mmol靛红和10 mL EtOH,室温搅拌至固体全部溶解,再加入2滴冰醋酸和0.30 mmol化合物1[27],加热回流5 h.反应结束后,将混合物倒入冰水中,用饱和Na2CO3溶液调节溶液pH至7~8,抽滤,用冰水洗涤滤饼,抽干后得到橙红色固体粗产物,空气中晾干后用DMF-EtOH-H2O重结晶提纯,得到0.09 g橙红色粒状晶体目标化合物2,产率为60.4%,熔点为262.4~263.1 ℃.合成路线见图 1.
2.2 元素分析和IR光谱分析元素分析C28H28N6OS实测值(%):C 67.47,H 5.86,N 16.71,新化合物2理论值(%):C 67.72,H 5.68,N 16.92.
IR光谱:3 137(vN-H),3 054(v=CH),2 928和2 856(vC-H),1 708(vC=O),1 618(vC=N),1 597、1 495和1 467(vC=C),1 517(δN-H),1 340(vC=S).1 708 cm-1处的峰归属为酰胺羰基的伸缩振动吸收峰,由此证明目标化合物2已被合成.酰胺羰基吸收峰通常在1 640~1 680 cm-1处,但在此化合物中由于五元环环张力的影响使其吸收峰向高波数移动.
元素分析和IR光谱数据初步描绘了此化合物的结构.
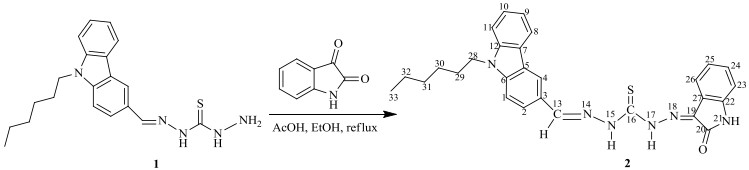
2.3 NMR波谱分析观察新化合物2的DMSO-d6溶液测得的1H NMR谱(图 2)和13C NMR谱(图 3)发现,咔唑和靛红环的1H或13C NMR信号因化学位移比较接近难于归属.

|
图 2 新化合物2的1H NMR谱. (a) 全谱;(b)和(c)分别为(a)的中低场(δH 6.9~8.5)和高场区(δH 0.4~4.5)的放大谱 Fig. 2 1H NMR spectra of new compound 2. (a) Full spectrum; (b) Partial enlarged spectrum (δH 6.9~8.5); (c) Partial enlarged spectrum (δH 0.4~4.5) |

|
图 3 新化合物2的13C NMR谱. (a) 全谱;(b) 局部放大谱(δC 109~123) Fig. 3 13C NMR spectra of new compound 2. (a) Full spectrum; (b) Partial enlarged spectrum (δC109~123) |
2D NMR谱对于确定化合物的结构及1H和13C NMR信号的准确归属非常有帮助[28-31].因此,本文利用1D和2D NMR波谱(包括1H NMR、13C NMR、1H-1H COSY、1H-13C HMBC和1H-13C HSQC)对新化合物2进行了结构确定,并对其1H和13C NMR数据进行了全归属.
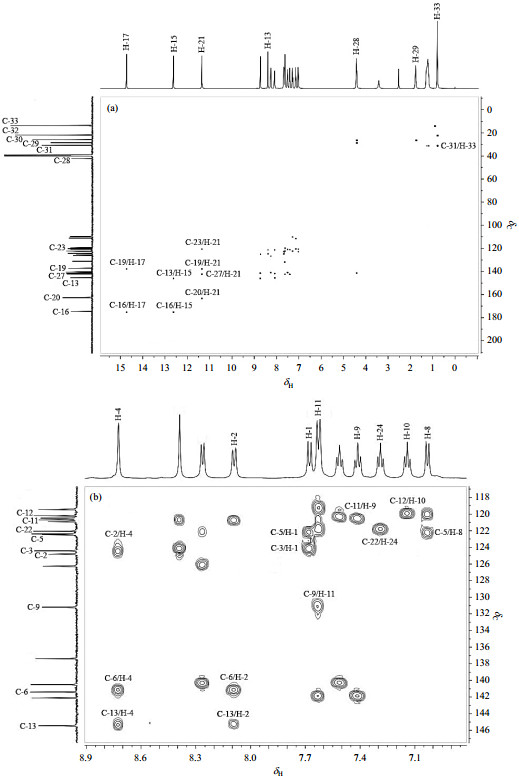
根据化学位移规律可知,13C NMR谱中最低场的δC 174.7可归属为C-16,次低场δC 162.7可归属为C-20.根据HMBC谱(图 4)可知,C-16(δC 174.7)与δH 12.62(1H, s)和δH 14.72(1H, s),δH 12.62与δC 145.4,δH 14.72与δC 137.4存在着远程相关,而在HSQC谱(图 5)中δC 137.4无相关峰存在,δC 145.4与δH 8.39(1H, s)存在着相关峰,由此即可确定δC 137.4为C-19,δC 145.4为C-13,δH 8.39为H-13,进一步确定出δH 12.62为H-15,δH 14.72为H-17.1H NMR谱中低场δH 11.00~δH 15.00之间共有三个吸收峰,其中H-17(δH 14.72)和H-15(δH 12.62)已归属,信号峰δH 11.35(1H, s)在HMBC谱中与C-19(δC 137.4)和C-20(δC 162.7)存在着远程相关,所以δH 11.35归属为H-21.在HMBC谱中这种诸如C-20与H-21的邻位相关是常见的[29].
|
图 4 新化合物2的1H-13C HMBC谱. (a) 全图;(b) 部分放大谱 Fig. 4 1H-13C HMBC spectra of new compound 2. (a) Full spectrum; (b) Partial enlarged spectrum |

|
图 5 新化合物2的1H-13C HSQC谱.(a) 全谱;(b) 部分放大谱 Fig. 5 1H-13C HSQC spectra of new compound 2. (a) Full spectrum; (b) Partial enlarged spectrum |
靛红环的归属:由HMBC谱可知,H-21还与δC 120.5和δC 142.1分别存在着远程耦合,而δC 120.5与HSQC谱中δH 8.26(1H, d)存在相关峰,所以δC 120.5和δH 8.26可分别归属为C-23和H-23.由1H-1H COSY谱(图 6)可知,H-23与δH 7.29(1H, t)、δH 7.29与δH 7.51(1H, t)、δH 7.51与δH 7.63(1H, d)相关,因此δH 7.29、δH 7.51和δH 7.63可分别归属为H-24、H-25、H-26.再根据HSQC谱,即可归属C-24(δC 119.4)、C-25(δC 126.3)、C-26(δC 109.7).由HMBC谱可知,H-24与δC 122.1,H-26与δC 142.1存在远程相关,则δC 122.1和δC 142.1可分别归属为C-22和C-27.

|
图 6 新化合物2的1H-1H COSY谱.(a) 全谱;(b) 部分放大谱 Fig. 6 1H-1H COSY spectra of new compound 2. (a) Full spectrum; (b) Partial enlarged spectrum |
咔唑环的归属:在HMBC谱中,C-13与δH 8.09(1H, d)和δH 8.72(1H, s),δH 8.09和δC 141.4存在着远程耦合,所以δH 8.09、δH 8.72和δC 141.4可分别归属为H-2、H-4和C-6.在1H-1H COSY谱中,H-2与δH 7.68(1H, d)存在交叉峰,则δH 7.68归属为H-1.根据HSQC谱,可归属C-1(δC 109.8)、C-2(δC 124.8)、C-4(δC 120.9).在HMBC谱中,H-1还与δC 124.4和δC 122.5,δC 122.5与δH 7.03(1H, d)远程相关,因此δC 124.4、δC 122.5和δH 7.03可分别归属为C-3、C-5和H-8.在1H-1H COSY谱中,H-8与δH 7.41(1H, t)、δH 7.41与δH 7.14(1H, t)、δH 7.14与δH 7.63(1H, d)存在着交叉峰,则δH 7.41、δH 7.14、和δH 7.63可分别归属为H-9、H-10和H-11.根据HSQC谱,可归属C-8(δC 111.1)、C-9(δC 131.2)、C-10(δC 122.4)和C-11(δC 120.7).在HMBC谱中,H-10与δC 120.2远程相关,则δC 120.2可归属为C-12.低场区剩余的δC 140.5即为C-7.
烷基链的归属:根据化学位移规律,1H NMR谱中的δH 0.79(3H, t)和δH 4.42(2H, t)可归属为H-33和H-28.在1H-1H COSY谱中,H-28与δH 1.77(2H, m)、δH 1.77与δH 1.29(2H, m)、δH 1.29与δH 1.22(2H, m)、δH 0.79与δH 1.19(2H, m)存在着交叉峰,因此δH 1.77、δH 1.29、δH 1.22和δH 1.19可分别归属为H-29、H-30、H-31和H-32.根据HSQC谱,可归属C-28(δC 42.4)、C-29(δC 28.5)、C-30(δC 26.1)、C-31(δC 30.9)、C-32(δC 22.0)和C-33(δC 13.8).
至此,新化合物2的1H和13C NMR信号归属完毕(表 1).
| 表 1 新化合物2的1H和13C NMR归属(DMSO-d6, 500 MHz) Table 1 1H and 13C NMR data of new compound 2 (DMSO-d6, 500 MHz) |
本文综合利用元素分析、IR光谱、NMR波谱技术确定了新化合物2的结构,并对其1H和13C NMR信号进行了全归属.本文的研究结果对此类化合物1H和13C NMR信号的归属以及结构与活性关系的研究具有指导意义.
| [1] | BROCKMAN R W, THOMSON J R, BELL M J, et al. Observations on the antileukemic activity of pyridine-2-carboxaldehyde thiosemicarbazone and thiocarbohydrazone[J]. Cancer Res, 1956, 16(2): 167-170. |
| [2] | BOŽIĆ A, MARINKOVIĆ A, BJELOGRLIĆ S, et al. Quinoline based mono-and bis-(thio) carbohydrazones:synthesis, anticancer activity in 2D and 3D cancer and cancer stem cell models[J]. RSC Adv, 2016, 6(106): 104763-104781. DOI: 10.1039/C6RA23940D. |
| [3] | GABR M T, EL-GOHARY N S, EL-BENDARY E R, et al. Isatin-β-thiocarbohydrazones:Microwave-assisted synthesis, antitumor activity and structure-activity relationship[J]. Eur J Med Chem, 2017, 128: 36-44. DOI: 10.1016/j.ejmech.2017.01.030. |
| [4] | TEJASREE C, KIRAN G, RAJYALAKSHMI G, et al. Antidiabetic activity of 1-(4-(dimethylamino) benzylidene)-5-(2-oxo indolin-3-ylidene) thiocarbohydrazone in rats[J]. Int J Pharm Sci Res, 2014, 5(7): 2738-2743. |
| [5] | GANGARAPU K, MANDA S, JALLAPALLY A. Synthesis of thiocarbohydrazide and carbohydrazide derivatives as possible biologically active agents[J]. Med Chem Res, 2014, 23(2): 1046-1056. DOI: 10.1007/s00044-013-0684-3. |
| [6] | ESSA H H, KANDIL F, FALAH A. Synyhesis and identification of schiff bases and bilogical activity new study[J]. Iraqi J Sci, 2012, 53(2): 230-240. |
| [7] | SHI Z C, ZHAO Z G, LIU M, et al. Solvent-free synthesis of novel unsymmetric chenodeoxycholic acid bis thiocarbazone derivatives promoted by microwave irradiation and evaluation of their antibacterial activity[J]. CR Chimie, 2013, 16(11): 977-984. DOI: 10.1016/j.crci.2013.05.009. |
| [8] | LI L, JIANG Y J, Liu X L, et al. Microwave-assisted synthesis and antibacterial activity of unsymmetrical indolyl/aryl bis-thiosemicarbazones[J]. J Chem Res, 2013, 37(6): 372-374. DOI: 10.3184/174751913X13688155249983. |
| [9] | KIRAN G, MANESHWAR T, RAJESHWAR Y, et al. Microwave-assisted synthesis, characterization, antimicrobial and antioxidant activity of some new isatin derivatives[J]. J Chem, 2013. DOI: 10.1155/2013/192039. |
| [10] | RIYADH S M, GOMHA S M, MAHMMOUD E A. Recent trends in chemistry of ethylidene thiocarbonohydrazides[J]. World J Pharm Pharm Sci, 2015, 4(6): 192-201. |
| [11] | WANG J B, YUN D, YAO J L, et al. Design, synthesis and QSAR study of novel isatin analogues inspired Michael acceptor as potential anticancer compounds[J]. Eur J Med Chem, 2018, 144: 493-503. DOI: 10.1016/j.ejmech.2017.12.043. |
| [12] | SOLOMON V R, HU C, LEE H. Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity[J]. Bioorg Med Chem, 2009, 17(21): 7585-7592. DOI: 10.1016/j.bmc.2009.08.068. |
| [13] | WANG G C, CHEN M, QIU J, et al. Synthesis, in vitro α-glucosidase inhibitory activity and docking studies of novel chromone-isatin derivatives[J]. Bioorg Med Chem Lett, 2018, 28(2): 113-116. DOI: 10.1016/j.bmcl.2017.11.047. |
| [14] | TEHRANI K H M E, HASHEMI M, Hassan M, et al. Synthesis and antibacterial activity of schiff bases of 5-substituted isatins[J]. Chin Chem Lett, 2016, 27(2): 221-225. DOI: 10.1016/j.cclet.2015.10.027. |
| [15] | ANDREANI A, BURNELLI S, GRANAIOLA M, et al. New isatin derivatives with antioxidant activity[J]. Eur J Med Chem, 2010, 45: 1374-1378. DOI: 10.1016/j.ejmech.2009.12.035. |
| [16] | MURALI K, SPARKES H A, PRASAD K J R. Synthesis of hetero annulated isoxazolo-, pyrido-and pyrimido carbazoles:Screened for in vitro antitumor activity and structure activity relationships, a novel 2-amino-4-(3'-bromo-4'-methoxyphenyl-8-chloro-11H-pyrimido[4, 5-a]carbazole as an antitumor agent[J]. Eur J Med Chem, 2017, 128: 319-331. DOI: 10.1016/j.ejmech.2017.02.009. |
| [17] | SUN L Q, WU Y B, LIU Y H, et al. Novel carbazole sulfonamide derivatives of antitumor agent:Synthesis, antiproliferative activity and aqueous solubility[J]. Bioorg Med Chem Lett, 2017, 27(2): 261-265. DOI: 10.1016/j.bmcl.2016.11.068. |
| [18] | DINESHKUMAR B, MITRA A, MAHADEVAPPA M. Antidiabetic and hypolipidemic effects of mahanimbine (carbazole alkaloid) from murraya koenigii (rutaceae) leaves[J]. Int J Phytomed, 2010, 2(1): 22-30. |
| [19] | WANG G C, WANG J, HE D X, et al. Synthesis and biological evaluation of novel 1, 2, 4-triazine derivatives bearing carbazole moiety as potent a-glucosidase inhibitors[J]. Bioorg Med Chem Lett, 2016, 26(12): 2806-2809. DOI: 10.1016/j.bmcl.2016.04.071. |
| [20] | KONG X Q, ZHANG H Z, CAO C S, et al. Synthesis of fluorinated carbazoles via C-H arylation catalyzed by Pd/Cu bimetal system and their antibacterial activities[J]. Bioorg Med Chem, 2016, 24(6): 1376-1383. DOI: 10.1016/j.bmc.2016.02.013. |
| [21] | BANDGAR B P, ADSUL L K, CHAVAN H V, et al. Synthesis, biological evaluation, and docking studies of 3-(substituted)-aryl-5- (9-methyl-3-carbazole)-1H-2-pyrazolines as potent anti-inflammatory and antioxidant agents[J]. Bioorg Med Chem Lett, 2012, 22(18): 5839-5844. DOI: 10.1016/j.bmcl.2012.07.080. |
| [22] | ZHU D Q, CHEN M H, LI M, et al. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity[J]. Eur J Med Chem, 2013, 68: 81-88. DOI: 10.1016/j.ejmech.2013.07.029. |
| [23] | HIEDA Y, ANRAKU M, CHOSHI T, et al. Antioxidant effects of the highly-substituted carbazole alkaloids and their related carbazoles[J]. Bioorg Med Chem Lett, 2014, 24(15): 3530-3533. DOI: 10.1016/j.bmcl.2014.05.050. |
| [24] | BÖRGER C, BRÜTTING C, JULICH-GRUNER K K, et al. Anti-tuberculosis activity and structure-activity relationships of oxygenated tricyclic carbazole alkaloids and synthetic derivatives[J]. Bioorg Med Chem, 2017, 25(22): 6167-6174. DOI: 10.1016/j.bmc.2016.12.038. |
| [25] |
LI Y J, WANG S Y, JIN K, et al. Synthesis and cell division cycle 25B phosphatase/protein tyrosine phosphatase 1B inhibitory activity evaluation of novel acylthiourea derivatives[J].
Chin J Org Chem, 2018, 38(5): 1242-1250.
李英俊, 王思远, 靳焜, 等. 新型酰基硫脲衍生物的合成及细胞分裂周期25B磷酸酶和蛋白酪氨酸磷酸酶1B抑制活性研究[J]. 有机化学, 2018, 38(5): 1242-1250. |
| [26] | 李继阳.新型基于咔唑-希夫碱/酰腙衍生物的合成、表征及性能[D].大连: 辽宁师范大学, 2017. |
| [27] | LI Y Q, SHI W, MA J C, et al. A novel optical probe for Hg2+ in aqueous media based on mono-thiosemicarbazone Schiff base[J]. J Photochem Photobioi A, 2017, 338: 1-7. DOI: 10.1016/j.jphotochem.2017.01.026. |
| [28] |
LI Y J, LUO T C, XU Y T, et al. NMR spectroscopic characterization of 2-aryloxymethyl-1H-benzimidazole acetic acid hydrazides[J].
Chinese J Magn Reson, 2013, 30(2): 233-246.
李英俊, 罗潼川, 许永廷, 等. 2-芳氧甲基苯并咪唑-1-乙酰肼的NMR谱的表征[J]. 波谱学杂志, 2013, 30(2): 233-246. DOI: 10.3969/j.issn.1000-4556.2013.02.008. |
| [29] |
LI Y J, LI J Y, JIN K, et al. An NMR study of a novel N-acylhydrazone derivative containing benzimidazole moiety[J].
Chinese J Magn Reson, 2017, 34(1): 25-34.
李英俊, 李继阳, 靳焜, 等. 含苯并咪唑环N-酰腙衍生物的NMR研究[J]. 波谱学杂志, 2017, 34(1): 25-34. |
| [30] |
ZHOU Z G, YUAN Y Y, LIU H B, et al. An NMR study on prucalopride[J].
Chinese J Magn Reson, 2018, 35(1): 119-127.
周中高, 元洋洋, 刘红波, 等. 普卡必利的NMR研究[J]. 波谱学杂志, 2018, 35(1): 119-127. |
| [31] |
FAN H Y. Spectral analyses of a novel Ibuprofen-phillygenin ester[J].
Chinese J Magn Reson., 2018, 35(1): 98-108.
樊宏宇. 新型连翘脂素-布洛芬酯合物的波谱学数据解析[J]. 波谱学杂志, 2018, 35(1): 98-108. |
 2019, Vol. 36
2019, Vol. 36 
