With the development of portable devices and electric vehicles, lithium-ion batteries have been widely used. Conventionally, LiCoO2 is used as a cathode material in commercial cells. However, the low capacity and high cost of LiCoO2 limit its wider application. Cathode materials with better performance/properties need to meet the increasing demand of batteries. Therefore, developing new cathode materials and establishing basic structure-function relationships of the electrode materials have become the key issues.
The title material, monoclinic Li3V2(PO4)3 (LVP), is a promising cathode material with high theoretical capacity (197 mAh/g, 3~4.8 V), relatively high operating voltage, good ionic mobility and excellent low temperature performance. The electrochemical charge (delithiation) curve of LVP is multi-staged involving several two-phase reactions, while the initial stage of the discharge (relithiation) curve presents a typical "S" shape, suggesting a solid solution pathway for the first step of lithium reinsertion. The structure changes during the phase transition processes need to be analyzed to obtain understanding of the charge-discharge mechanism. Meanwhile, Li+ dynamics, e.g. the mobility of lithium ion is important to excellent rate performance, which is highly related with the framework structure of the material. Revealing the hopping rates and preferred paths of lithium ions is important to explain the electrochemical behavior as well as improve the performance of LVP. Due to the framework formed by VO6 octahedra corner-sharing with PO4 tetrahedra, the electronic conductivity of LVP is very poor. In order to improve the electronic conductivity, carbon coating and ion doping modifications of LVP are widely adopted, and the structure of interface and modified framework need to be carefully analyzed.
The monoclinic LVP framework formed by VO6 octahedra corner-sharing with PO4 tetrahedra and its complicate phase transitions upon cycling have greatly challenged the traditional diffraction methods. Solid-state nuclear magnetic resonance (SSNMR) has been demonstrated a powerful tool for investigating the local structure changes and Li+ dynamics of electrode materials, including LVP[1, 2]. Moreover, the density functional theory (DFT) calculation of 7Li chemical shifts in complicate phosphate like LVP is also a challenging topic. The present review will be organized as three sessions to cover most of the progresses achieved by SSNMR in LVP system. The first session is about mechanism investigation, including charge-discharge mechanisms and Li+ dynamics. Then we will move on to understand the effect of routine material modification methods, for instance carbon coating and cation doping. In the final session, a brief introduction will be given on the history and progress of DFT calculation of 7Li NMR shifts in LVP.
1 Mechanism investigation 1.1 Charge-discharge mechanismsWith the help of X-ray/neutron diffraction and 7Li SSNMR, Nazar et al.[3] have studied the charge mechanisms of LVP. As shown in Fig. 1(a), three lithium signals of δLi 103, 52, and 17 are resolved by 7Li NMR spectrum of pristine LVP which arisen from the well-known Fermi contact shift[3]. In Fig. 1(b), for Li2V2(PO4)3, two isotropic signals are observed at δLi 77 and 143 in spite of the almost identical geometry of the two lithium sites. The 7Li NMR shift difference of Li2V2(PO4)3 is due to the magnitude of the hyperfine interaction between the two sites [one electron difference in orbital transfer between Li1-O-V(1) d1 and Li2-O-V(2) d2], which demonstrates the charge ordering of the Vn+ clearly. On the basis of this observation, Nazar et al. proposed a vanadium charge ordering associated with lithium site ordering in partially delithiated Li2V2(PO4)3, which can possibly be the driving force the phase transition.

|
Fig. 1 Description of delithiated phases LixV2(PO4)3 where i)-structure, ii)- 7Li NMR spectra, and iii)- coordination environment of Li: (a) x=3.0; (b) x=2.0. Isotropic peaks are marked in the spectra; the remainders are spinning sidebands (Reproduced from Ref. [3]) |
Later, they described the subtle relation between structure and electrochemical properties in the full electrochemical curve on oxidation (Li extraction) and reduction (Li insertion) by 7Li SSNMR and X-ray/neutron diffraction of different samples[4]. A series of 7Li NMR spectra of deinserted ("D") LixV2(PO4)3 (x=3.0, 2.0, 1.0, and 0) single-phase compositions are shown in Fig. 2. The 7Li NMR of D-LVP and D-Li2V2(PO4)3 are shown in Fig. 2(a) and 2(b), respectively, which are nearly the same as that in Ref. [2]. The 7Li NMR result again proves the vanadium charge ordering in D-Li2V2(PO4)3. The 7Li NMR of D-LiV2(PO4)3 is shown in Fig. 2(c). There is one isotropic chemical shift at δLi 138. According to the previous results, Li-O-V d1 interaction can give rise to a 7Li NMR shift of about δLi 77, an additional positive contribution of 7Li NMR shift results from the additional oxygen interaction providing electron density transfer from V(1). Additionally, the line broadening of D-LiV2(PO4)3 spectrum may result from the local Li disorder[3]. The 7Li NMR of D-V2(PO4)3 is shown in Fig. 2(d). There is no obvious 7Li signal in the spectrum, suggesting the complete extraction of Li from the framework. According to the calculated result based on X-ray diffraction (XRD), the average bond distances of V(1)-O and V(2)-O are fairly close, and average vanadium valencies are +4.5, i.e., this phase does not show the V4+/V5+ charge ordering.

|
Fig. 2 7Li MAS NMR spectra of D-LixV2(PO4)3. (a) x=3.0; (b) x=2.0; (c) x=1.0; (d) x=0. The isotropic shift values are labeled; all other peaks are spinning sidebands (Reproduced from Ref. [4], the chemical shifts were referenced to LiC3H5O3) |
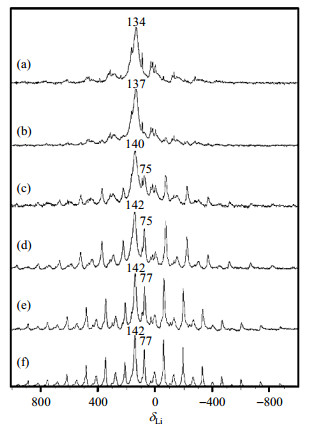
A series of compositions (x=0.5, 0.75, 1.0, 1.25, 1.5, 2.0) in LixV2(PO4)3 were prepared via chemical route, i.e., reinserted samples ("R"), in order to investigate the Li insertion process in the solid solution region by 7Li NMR. In Fig. 3, there are two dominant isotropic chemical shifts at δLi 134 and 75 that resemble the 7Li shift in D-LiV2(PO4)3 and in D-Li2V2(PO4)3, respectively. During the discharge process, the continuous change in the NMR shift from δLi 134 to δLi 142 (referenced to LiC3H5O3) and narrowing in line width is due to the subtle framework transformations in the solid solution regime. Because the vanadium charge ordering is absent, reinsertion of Li leads to disorder over two sites, which resulting to a solid solution regime. The low population of Li+ and variable vanadium valence states cannot drive the charge ordering. Beyond x=1.25, due to the additional contributions of the incorporation of both Li and reduced vanadium to the lattice potential, vanadium charge ordering is reestablished thus two-phase behavior is reappeared.
|
Fig. 3 7Li MAS NMR spectra of R-LixV2(PO4)3. (a) x=0.5; (b) x=0.75; (c) x=1.0; (d) x=1.25; (e) x=1.5; (f) x=2.0 (Reproduced from Ref. [4], the chemical shifts were referenced to LiC3H5O3) |
Li+ mobility plays an important role in controlling the rate performance of LVP, which has been monitored by many different methods. For examples, electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) can be used to measure ionic diffusion coefficient, and theoretical calculation can be used to predict the preferred pathways of lithium ions[5, 6]. Goward et al.[7] have investigated the lithium dynamics of LVP including the mechanism of lithium hopping and the time scale of the processes through variable-temperature 7Li magic-angle spinning (MAS) NMR spectra and 7Li 2D exchange spectroscopy.
The variable-temperature 7Li NMR spectra from 276 K to 364 K are shown in Fig. 4. The linear temperature dependence of the 7Li chemical shift indicates Curie-Weiss behavior[7]. Three lithium peaks gradually coalesce with increasing temperature, indicates three lithium sites are exchanging at a rate of πν/21/2 comparable to the separation, between three resonances. Additionally, a small peak at δLi 0, which can be seen at 313 K, is assigned to Li2CO3.

|
Fig. 4 Variable-temperature 7Li NMR spectra acquired at 25 kHz for Li3V2(PO4)3 (Reproduced from Ref. [7]) |
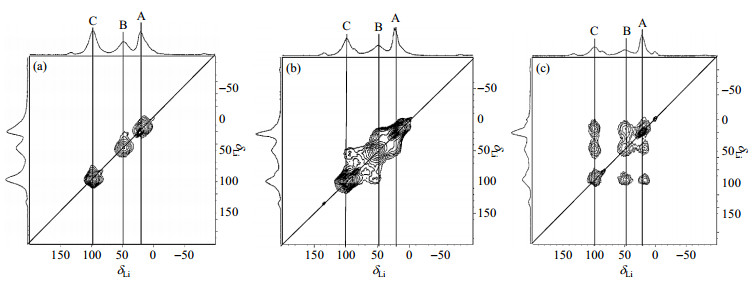
In the 7Li 2D exchange spectra, the direct exchange of different lithium sites can be observed as cross peaks. 7Li 2D spectrum for LVP was acquired at room temperature with a mixing time (τmix) of 1 μs, as shown in Fig. 5(a). No exchange is taking place at τmix of 1 μs because there are only individual signals appear along the diagonal. When τmix achieving 50 μs [Fig. 5(b)], cross peaks are shown between the B and C sites as well as A and B sites (weakly). A, B and C sites are lithium sites which corresponding to Li1, Li2 and Li3 in Nazar's literature[3], respectively. It indicates a microsecond time scale for Li hopping, which is consistent with the conclusion from variable-temperature experiment by Goward et al.[7] In Fig. 5(c), the cross peaks between all three sites are shown at τmix of 500 μs.
|
Fig. 5 7Li 2D exchange spectra of LVP acquired under ambient bearing gas temperature at 25 kHz with τmix of (a) 1 μs, (b) 50 μs, and (c) 500 μs (Reproduced from Ref. [7]) |
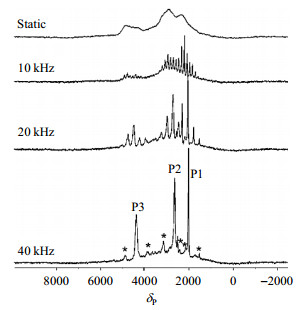
Later, Goward et al.[8] adopted low-temperature 6Li{31P} rotational-echo, double-resonance (REDOR) to identify the spatial adjacent 7Li-31P environments and further investigate lithium mobility of the three Li sites in monoclinic LVP. In Fig. 6, the 31P NMR spectra of LVP with different spinning speeds are shown. The three crystallographic sites (site P1, P2, and P3 with increasing frequency) can be distinguished well with the help of high spinning speed (40 kHz). Due to the Fermi-contact shift from the hyperfine transfer of electron density, which derives from the paramagnetic nuclei V3+, the 31P chemical shifts range at δP 2 000 ~ 4 500[9, 10]. The 31P resonances highly depend on temperature[11].
|
Fig. 6 31P NMR spectra for LVP acquired at spinning speeds of 0 kHz (static), 10 kHz, 20 kHz and 40 kHz, respectively. The spinning sidebands are marked with asterisks. The three resonances correspond to P1, P2, and P3 from the lowest to the highest frequency (Reproduced from Ref. [8]) |
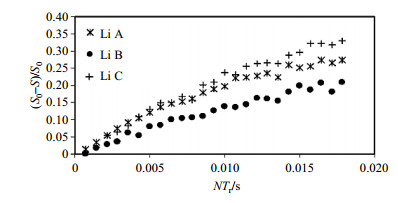
The REDOR curves for the three Li sites at 185 K are shown in Fig. 7. All three Li sites experience different attenuation with recoupling time, indicating they each have a different dipolar coupling with the nearest 31P nucleus. Based on the known Li-P internuclear distances, the 31P resonances can be assigned by setting each isotropic 31P shift on-resonance and observing the degree of signal attenuation. The strongest REDOR transfer for all three Li sites is observed when pulsing on the 31P resonance at δP 2 650, suggesting all three Li sites are closest to P2. The REDOR transfer for Li-C was investigated, which was selected because it is the easiest to distinguish due to the difference in distance between Li C-P1 and Li C-P3 is more than 0.05 nm, in order to assign the remaining two 31P resonances. Site C experienced a larger transfer from the 31P resonance at the lowest frequency which was the crystallographic site P1. The three sites are therefore labeled P1, P2, and P3 with increasing frequency.
|
Fig. 7 6Li{31P} REDOR curves for LVP acquired at 14 kHz MAS (temperature=185 K) Plot of signal attenuation, (S0-S)/S0, for sites Li A (asterisk), Li B (dots), and Li C (plus signs) as a function of dipolar evolution time (NTr) (Reproduced from Ref. [8]) |
The simulated curves, the fits, and the experimental data for Li A, Li B, and Li C are shown in Fig. 8. The simulated curves indicate Li ions are static within the cage. Meanwhile, the fits show the correlation between reduced dipolar coupling and mobility at each Li site. The ratio of the reduced dipolar coupling to the static coupling indicates the attenuation degree of the dipolar coupling. The relative mobility at each Li site in LVP can be quantified by the degree of attenuation. As the increase of the ratio of the reduced dipolar coupling to the static coupling, the dipolar coupling decreases, i.e., the greater the ratio, the smaller the change in the dipolar coupling, which reveals a slower mobility. The mobility at each Li site increases from A < B < C which corresponding to the increase of the shortest Li-O contact within each void. The related data are summarized in Table 1.
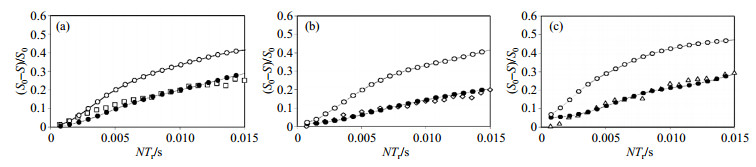
|
Fig. 8 6Li{31P} REDOR curves acquired at 14 kHz MAS (temperature=185 K) for (a) Li A (squares), (b) Li B (diamonds), and (c) Li C (triangles). The static dipolar coupling curve (open circles) and reduced dipolar coupling fit (closed circles) were simulated using SIMPSON (Reproduced from Ref. [8]) |
| Table 1 Summary of the ratio values of reduced 6Li-31P dipolar couplings to the static dipolar couplings (Reproduced from Ref. [8]) |
LVP is a complex system involving Li dynamic processes at different timescales, such as chemical exchange, spin diffusion and long range Li migration. These dynamic processes can be investigated by combining multiple NMR techniques including variable-temperature NMR, 2D EXSY and REDOR.
2 Understanding the effect of material modification 2.1 Carbon coatingDue to the poor electronic conductivity of pristine LVP, the materials in practical applications of lithium ion batteries are carbon coated LVP. Carbon coating can effectively enhance the electronic conductivity as well as alleviate the aggregation of LVP particles. Therefore, it is essential to understand the interface structure between carbon coating and LVP. SSNMR can help characterize the coating interface structure between carbon and LVP. Especially, 13C NMR can help analyze the composition of carbon coating, i.e., degree of graphitization (sp2/sp3 ratio). Lin et al.[12] conducted 7Li, 31P and 13C SSNMR to study the structure of carbon-LVP interface.
In Fig. 9(a), the 7Li NMR signals at δLi 0 is probably Li3PO4 because the relative intensity of the signal differs upon changing carbon sources, which indicates some Li-containing species are related to the carbon sources. A special broad signal centered around δLi 70 appears in the 7Li NMR spectrum of LVP/CA (citric acid) sample. Furthermore, in the 7Li 2D exchange spectrum [Fig. 9(c)], the signal at δLi 70 cannot exchange with other signals, suggesting the peak at δLi 70 is the LixC intermetallic compound formed at the LVP-carbon interface rather than in the LVP phase. When 7Li NMR spectra were acquired at higher spinning speed of 60 kHz [Fig. 9(b)], the broadening and coalescence appeared in the spectra of all samples, which can be related to multiple factors including the heat conductivity of carbon coating, Li+ pathway at the LVP-carbon interface and the size of the primary particles. The 31P NMR spectra are shown in Fig. 9(d). A 31P signal at δP 10 presenting in all spectra with the same trend of intensity varies with the carbon source, which is similar to the 7Li NMR spectra. According to the reference and the NMR integration results, the signal at δLi 0 of 7Li NMR spectra and δP 10 of 31P NMR spectra can be attributed to Li3PO4[13]. In Fig. 9(e), the 13C NMR spectra of LVP/CA, LVP/Glu (glucose), and LVP/GO (graphene oxide) at the spinning rate of 20 kHz is shown. In all 13C NMR spectra, the regions of δC 8~80 and 90~180 can be assigned to sp3 carbon and sp2 carbon, respectively. LVP/GO, LVP/Glu and LVP/CA composites contain significant amounts of sp2 and sp3 carbon. The sp3 carbon signal of LVP/Glu is broad, which relies on the chemical shift dispersion of amorphous/disordered carbon. On the contrary, the sp3 carbon signal of LVP/CA is sharp at δC 59 (C-C, C-O), δC 30 (C-C), and δC 16 (C-C, C-H), suggesting the ordered sp3 carbon in LVP/CA sample[14, 15].

|
Fig. 9 SSNMR spectra of different LVP/carbon samples. (a) 7Li NMR at the spinning rate of 30 kHz; (b) 7Li NMR at the spinning rate of 60 kHz; (c) 7Li 2D exchange spectrum of the LVP/CA composite with τmix of 1 ms; (d) 31P NMR at the spinning rate of 60 kHz, the spinning sidebands are labeled with "*"; (e) 13C NMR at the spinning rate of 20 kHz (Reproduced from Ref. [12]) |
By combining 7Li, 31P and 13C multi-nuclei NMR, the interfacial structure between carbon coating and LVP can be characterized. Additionally, the composition of carbon coating (sp2/sp3 carbon ratio) can be investigated. This method can be further extended into the study of other composite materials with carbon coating.
2.2 Cation dopingIt is a widely adopted method to improve the electrochemical performance of LVP by cation doping. Unfortunately, XRD usually cannot characterize the local structure changes resulting from doping cations, due to the low doping level. As a useful tool, NMR can help analyze the subtle structure changes of cation doped LVP.
Dai et al.[16] characterized the local structure changes of Mg doped LVP and Mg-Cl co-doped LVP samples by 31P NMR. In Fig. 10, the 31P NMR spectra of LVP/C are similar to the results of the LVP/carbon samples discussed above. However, the spectra of Mg doped LVP (LVMP/C) and Mg-Cl co-doped LVP (LVMPCl/C) are obviously broad because of the doped effect of Mg or Cl, which greatly changed the electronic environment of P. Meanwhile, the effect of Cl doping on the local environment of P is relatively small because the 31P spectra of LVMP/C and LVMPCl/C are almost the same.
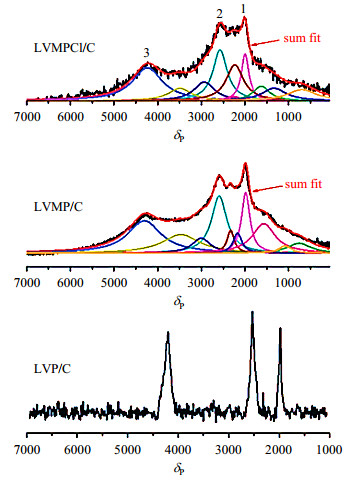
|
Fig. 10 31P NMR spectra acquired on as-synthesized LVP/C, Mg doped LVP (LVMP/C) and Mg-Cl co-doped LVP (LVMPCl/C) (Reproduced from Ref. [16]) |
31P NMR has been demonstrated a sensitive indicator of the structure change caused by cation doping in LVP. In future, NMR can effectively characterize structure of the ion doped LVP, and other ion doping cathode materials.
3 DFT calculation of NMR shiftsThe localization of the single electron center on the V atom/ion in the LVP system and the edge-sharing/plane-sharing special structure between the VO polyhedron and the LiO polyhedron result in a difficult correlation between the 7Li NMR shifts and the individual Li+ environments. In 2010, Carlier et al.[17] have analyzed the 7Li NMR signals by the electron spin density transfer mechanisms. Based on DFT calculations, the local geometrical analysis results in the δ (Li1) > δ (Li3)≈δ (Li2) assignment of three Li site NMR shifts in LVP. However, DFT calculations of the spin density on Li by Vienna Ab-initio Simulation Package (VASP) cannot conclude the assignment, because this depends on the integration radius and the method.
In 2018, computations of NMR shifts for paramagnetic materials were reported using the Gaussian-augmented-plane-wave (GAPW) approach available within the CP2K code. Kaupp et al.[18] computed 7Li NMR shifts for LVP and Li3Fe2(PO4)3 using CP2K, for which detailed experimental data were available. The total 7Li isotropic shifts for the three lithium sites due to different contributions have been summarized in Fig. 11. Assignment of the shifts to their corresponding sites gives the order of δ (Lia) > δ (Lic) > δ (Lib). It would appear difficult to make assignment just by visual inspection of the structure. This work provides a deeper study of different approaches and a thorough analysis of different contributions to the NMR shifts.
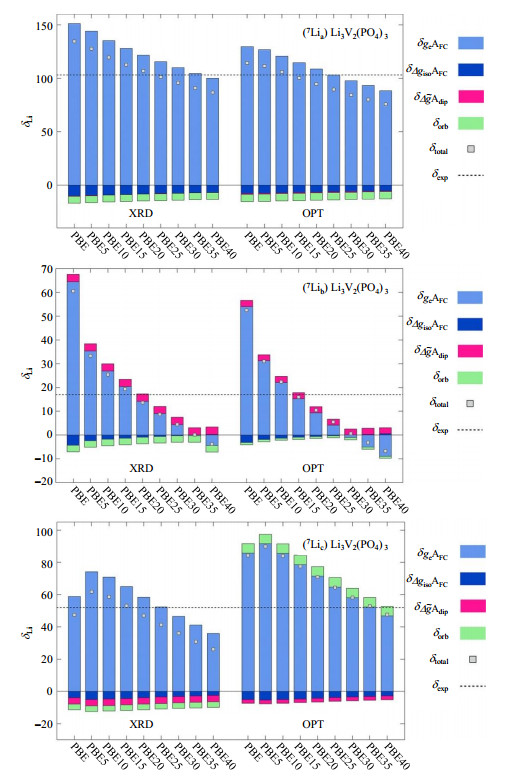
|
Fig. 11 Comparison of 7Li chemical shifts of three distinct lithium sites, Lia (top), Lib (middle), and Lic (bottom) for LVP computed for both XRD and optimized (OPT) structures. Variations with EXX admixture to PBE-based functions for the HFC tensors are shown. g-tensor and orbital shielding obtained at PBE level. Shields converted to shifts relative to solid lithium lactate (LiC3H5O3) (Reproduced from Ref. [18]) |
DFT calculation enables the possible correlation between 7Li NMR shifts and different Li+ environments in LVP. DFT modeling based on VASP cannot conclude the assignment of lithium sites in LVP. Furthermore, GAPW with the CP2K code can compute the 7Li NMR shifts and give the unambiguous assignments.
4 ConclusionIn summary, by combining multiple NMR techniques, including MAS NMR, variable temperature test and 2D spectrum, with other structure characterization methods, vanadium charge ordering and lithium site ordering of LVP during charge and discharge, dynamic of Li hopping processes, structure of LVP-carbon interface, structural changes in doped LVP, and 7Li NMR calculation have been systematically investigated. SSNMR is becoming an important tool investigating the reaction process of lithium ion batteries due to its precise and non-destructive properties. NMR plays a vital role in the modification and development of lithium vanadium phosphate cathode material. In the future, in-situ NMR can be used to analyze LVP materials to further investigate the real-time changes during charge-discharge process rather than ex-situ method or merely use chemical oxidation-reduction sample. NMR methods of variable temperature test and 2D spectrum can also study the dynamics of Li+/Na+/K+ in other materials. NMR can study the coating/interface structure of different materials and the effect of ion doping on different materials. Additionally, DFT calculation can help to establish correlation between NMR shifts and crystallographic sites. Using the CP2K code, relatively precise computation of NMR shifts for paramagnetic materials can be achieved. After a complete NMR study of lithium vanadium phosphate, many problems, such as mechanisms of LVP during charge and discharge, Li+ dynamics, carbon coating, and cation doping can be solved, which is beneficial to the materials and industry development of lithium ion battery.
| [1] |
LEI Z Y, LIANG X M, LEI Y Y, et al. Process in solid-state NMR studies on carbon anode materials for lithium/sodium-ion batteries[J].
Chinese J Magn Reson, 2020, 37(1): 28-39.
雷振宇, 梁欣苗, 雷友义, 等. 固体核磁共振技术在锂/钠离子电池碳负极中的应用及研究进展[J]. 波谱学杂志, 2020, 37(1): 28-39. |
| [2] |
JIANG T T, FU X B, WU J Z, et al. Structure and dynamics of polymer-ceramic interface in Li1.5Al0.5Ge1.5P3O12/polyether solid electrolyte:a solid-state NMR study[J].
Chinese J Magn Reson, 2017, 34(4): 429-438.
姜婷婷, 付晓彬, 吴金泽, 等. Li1.5Al0.5Ge1.5P3O12/高分子固体电解质表界面结构与分子运动的固体NMR研究[J]. 波谱学杂志, 2017, 34(4): 429-438. |
| [3] | YIN S C, GRONDEY H, STROBEL P, et al. Charge ordering in lithium vanadium phosphates:electrode materials for lithium-ion batteries[J]. J Am Chem Soc, 2003, 125(2): 326-327. DOI: 10.1021/ja028973h. |
| [4] | YIN S C, GRONDEY H, STROBEL P, et al. Electrochemical property:structure relationships in monoclinic Li3-YV2(PO4)3[J]. J Am Chem Soc, 2003, 125(34): 10402-10411. DOI: 10.1021/ja034565h. |
| [5] | RUI X H, DING N, LIU J, et al. Analysis of the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 cathode material[J]. Electrochim Acta, 2010, 55(7): 2384-2390. DOI: 10.1016/j.electacta.2009.11.096. |
| [6] | LEE S, PARK S S. Atomistic simulation study of monoclinic Li3V2(PO4)3 as a cathode material for lithium ion battery:structure, defect chemistry, lithium ion transport pathway, and dynamics[J]. J Phys Chem C, 2012, 116(48): 25190-25197. DOI: 10.1021/jp306105g. |
| [7] | CAHILL L S, CHAPMAN R P, BRITTEN J F, et al. 7Li NMR and two-dimensional exchange study of lithium dynamics in monoclinic Li3V2(PO4)3[J]. J Phys Chem B, 2006, 110(14): 7171-7177. DOI: 10.1021/jp057015+. |
| [8] | CAHILL L S, KIRBY C W, GOWARD G R, et al. 6Li{31P} rotational-echo, double-resonance studies of lithium ion site dynamics in Li3V2(PO4)3[J]. J Phys Chem C, 2008, 112(6): 2215-2221. DOI: 10.1021/jp077254s. |
| [9] | ROCA M, AMORÓS P, CANO J, et al. Prediction of magnetic properties in oxovanadium(IV) phosphates:the role of the bridging PO4 anions[J]. Inorg Chem, 1998, 37(13): 3167-3174. DOI: 10.1021/ic971210o. |
| [10] | SANANES M T, TUEL A. Study by 31P NMR spin echo mapping of vanadium phosphorus oxide catalysts[J]. Solid State Nucl Magn Reson, 1996, 6(2): 157-166. DOI: 10.1016/0926-2040(95)01215-X. |
| [11] | GEE B, HORNE C R, CAIRNS E J, et al. Supertransferred hyperfine fields at 7Li:variable temperature 7Li NMR studies of LiMn2O4-based spinels[J]. J Phys Chem B, 1998, 102(50): 10142-10149. DOI: 10.1021/jp982301p. |
| [12] | HUO H, LIN Z Y, WU D, et al. Investigating the structure of an active material-carbon interface in the monoclinic Li3V2(PO4)3/C composite cathode[J]. ACS Appl Energy Mater, 2019, 2(5): 3692-3702. DOI: 10.1021/acsaem.9b00410. |
| [13] | CHEETHAM A K, CLAYDEN N J, DOBSON C M, et al. Correlations between 31P N.M.R. chemical shifts and structural parameters in crystalline inorganic phosphates[J]. J Chem Soc Chem Commun, 1986: 195-197. |
| [14] | ISHII Y, YANG D, VELAMAKANNI A, et al. Structural characterization of C-labeled graphite oxide[J]. Science, 2008, 321: 1815-1818. DOI: 10.1126/science.1162369. |
| [15] | JAKUPOVIC J, SCHUSTER A, BOHLMANN F, et al. Lumiyomogin, ferreyrantholide, fruticolide and other sesquiterpene lactones from ferreyranthus fruticosus[J]. Phytochemistry, 1988, 27(4): 1113-1120. DOI: 10.1016/0031-9422(88)80285-1. |
| [16] | SUN S T, LI R H, MU D Y, et al. Magnesium/chloride co-doping of lithium vanadium phosphate cathodes for enhanced stable lifetime in lithium-ion batteries[J]. New J Chem, 2018, 42(16): 13667-13673. DOI: 10.1039/C8NJ02165A. |
| [17] | CASTETS A, CARLIER D, TRAD K, et al. Analysis of the 7Li NMR signals in the monoclinic Li3Fe2(PO4)3 and Li3V2(PO4)3 phases[J]. J Phys Chem C, 2010, 114(44): 19141-19150. DOI: 10.1021/jp106871z. |
| [18] | MONDAL A, GAULTOIS M W, PELL A J, et al. Large-scale computation of nuclear magnetic resonance shifts for paramagnetic solids using CP2K[J]. J Chem Theory Comput, 2018, 14(1): 377-394. |
 2020, Vol. 37
2020, Vol. 37 
