2. Laboratory of Magnetic Resonance Spectroscopy and Imaging, Suzhou Institute of Nano-tech and Nano-bionics, Chinese Academy of Sciences, Suzhou 215123, China
2. 磁共振波谱与影像实验室, 中国科学院 苏州纳米技术与纳米仿生研究所, 江苏 苏州 215123
Tautomers are constitutional isomers of organic compounds that readily interconvert. In solution or the melting state, different tautomers of one compound can exist in a dynamic equilibrium and react with each other, which is a commonly observed phenomenon, i.e., tautomerism[1, 2].The most observed form of tautomerism is prototropy, such as keto-enol, amine-imine, lactam-lactim, amino acid-ammonium carboxylate tautomerism, and so on[3]. Molecules in different tautomeric state exhibit not only unique spectral fingerprints, but also different physicochemical properties[4-6]. Though tautomerism is widely detected and discussed in solution, solid state tautomerism is less reported. A recent survey shows that only 0.05% of the compounds in the Cambridge Structural Database (CSD) are found in different tautomeric forms, which is much lower than the theoretical ratio, ~10%[7].
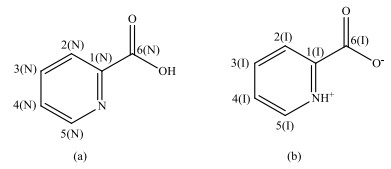
Investigation on solid state tautomerism is an extremely important topic for chemists and material scientists for the curiosity towards structure-property relationship. It's well known that one compound may exist in different crystal forms. For tautomeric compound, the limited reports reveal that each tautomer is often contained in a distinct crystal phase, such as irbesartan and clonixin[8, 9]. Only a handful of crystal structures contain different isomers in one crystal lattice[10-12]. 2-picolinic acid (PCA, Fig. 1) is such a rare case. The co-existence of neutral PCA molecules and zwitterions in the same crystal structure has been clearly revealed by 15N solid state nuclear magnetic resonance (NMR)[13], Fourier transform infrared (FTIR) spectroscopy[14], and partly by the crystal structure solved from the single crystal X-ray diffraction (XRD) data[13].
|
Fig. 1 Molecular structures of neutral 2-picolinic acid (PCA) (a) and PCA in zwitterion form (b) |
PCA is an endogenous bidentate chelating agent in the human body[15]. It's currently used as a popular cocrystal former for pharmaceuticals due to the safety and its various functional groups (Fig. 1). The determination of tautomeric/ionization state is one of the essential tasks for cocrystal analysis. Even a single crystal structure is solved, supplementary information derived from other spectroscopy methods is also indispensable. For example, two reported structures of the same form of albendazole (CSD refcodes: SUTWIO and BOGFUZ) show different proton location. In addition, single crystal samples are often difficult to be obtained. The analysis needs on powder samples further highlight the importance of spectroscopy methods.
Obviously, solid state NMR deserves the central position for the analysis of molecular states in the solid state if a single crystal is not available. 15N solid state NMR spectra are often, but not always, very sensitive for the change of chemical environments, and can provide important clues for the judgment of molecular states. While, the collection of 15N NMR spectrum is often extremely time-consuming because the low natural abundance (0.36%) of this isotope. Also, crystal samples of small molecules often hold long 1H spin-lattice relaxation time (T1)[16], which further blocks its application as a routine method. Fortunately, tautomeric/ionization state changes can also give some featured resonances on the 13C NMR spectra. For example, the keto tautomer, enol tautomer, and anion of acesulfame in the solid state can be clearly distinguished via 13C NMR fingerprints[17-19]. For PCA, such 13C NMR features are also desired. Anhydrous PCA sample gives the opportunity to learn the 13C chemical shift information of the neutral and zwitterion molecular states. Though PCA is a very simple molecule, the accurate 13C chemical shift assignments have not yet been achieved straightforward by either experimental or theoretical methods.
1 Materials and methods 1.1 MaterialsPCA (≥99%) was purchased from Maya Reagent Co., Ltd. Analytical grade CH3Cl and ethyl acetate were obtained from Sinopharm Chemical Reagent Co., Ltd. PCA was recrystallized in CH3Cl/ethyl acetate (1:9, v/v) solution via slow solvent evaporation to obtain the single crystal and powder samples. The crystal form of PCA was confirmed by powder XRD using a Bruker D8 Advance diffractometer.
1.2 Single crystal XRD and virtual structure fileThe diffraction data for PCA was obtained on a Saturn724+ diffractometer with Mo-Kα (λ = 0.071 073 nm) radiation at 123 K. The crystal structure was solved by direct methods and refined on F2 by full-matrix least squares using SHELXL-2017[20]. All non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms associated with carbon atoms were placed in geometrically calculated positions and position of O and N bonded hydrogens were located from the difference electron density maps. The active hydrogen atoms on O and N display positional disorder with a refined relative ratio of 0.50/0.50. This structural model was named PCA-R (R: Real). Additionally, another model, PCA-V (V: Virtual) was constructed via removing the positional disorder and reducing the structural symmetry. Crystallographic data for PCA-R and PCA-V are summarized in Table 1 in the Results and Discussion section. The fractional atomic coordinates, occupancies, and atomic displacement parameters of these two models are provided in Table S1 and S2 (available in the online version).
| Table 1 Crystallographic data and structure refinement parameters for PCA |
13C solid-state NMR spectra of PCA were collected on a Bruker AvanceIII-500 spectrometer using a 4 mm double-resonance magic angle spinning (MAS) probe. The cross-polarization (CP) pulse sequence was selected and a total sideband suppression (TOSS) frame[21] was added in the CP program to remove the spinning sidebands. The Hartmann-Hahn conditions were optimized by using adamantine. The MAS rate, contact time, and recycle delay were set to 8 kHz, 2.0 ms and 300 s, respectively. This 13C CP/MAS TOSS NMR experiment for PCA took about 15 h. 13C non-quaternary suppression (NQS)[22] spectrum was also collected via adding a π pulse and a 60 μs of dephasing delay in the CP/MAS TOSS program before the signal acquisition. The 13C chemical shift was externally referenced to tetramethylsilane (TMS).
1.4 Chemical shift calculationThe virtual structure of PCA was used for the NMR shielding parameter calculation using CASTEP program[23]. Geometry optimization was firstly performed using GGA/PBE functional with Grimme dispersion correction. The energy cutoff was set to 300 eV, and ultrasoft pseudopotential was employed. For shielding calculation, a fine K-point, energy cutoff of 550 eV, and on-the-fly pseudopotential were selected. Nicotinamide is a structural analog of PCA, which was used for the 15N chemical shift calibration in this study.
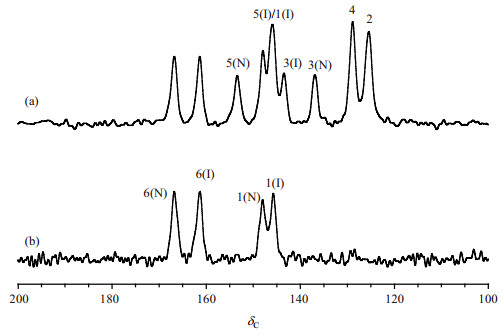
2 Results and discussionFig. 2(a) shows the 13C CP/MAS TOSS NMR spectrum of PCA. For this sample, nine resonance peaks can be clearly observed. For a crystalline sample, if the number of molecules per asymmetric unit (Z') is equal to 1, i.e., only one symmetry-independent molecule containing in the crystal structure, each chemically distinct carbon should be represented by a single resonance signal. Z' > 1 samples often show splitting in the 13C solid state NMR spectra[24]. Since PCA contains six carbon atoms in the molecule, the 13C NMR spectrum indicates PCA molecules should be in a Z' > 1 structure.
|
Fig. 2 13C CP/MAS TOSS (a) and CP/MAS NQS TOSS (b) NMR spectra of PCA |
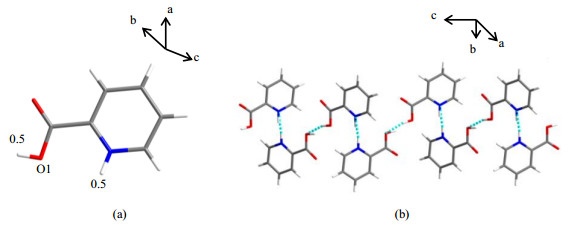
The crystal form of commercially available PCA (CSD refcode: PICOLA02) was reported, and was used also in this study. This sample crystallizes in a monoclinic crystal system, C2/c space group, with eight asymmetric units in a unit cell (Z=8, Z is the number of formula units per cell). Each asymmetric unit contains one PCA molecule [Z' = 1, Fig. 3(a)] with unusual proton positional disorder (0.50:0.50) at both N1 and O1 sites. The result indicates that PCA molecules exist in both the neutral and zwitterion forms, but the single crystal measurement can only give such an averaged result. Fortunately, the co-existence of neutral molecules and zwitterions in the same crystal structure has been firmly verified by 15N solid state NMR and FTIR spectroscopy[13, 14]. The PCA molecules are linked by N–H⋯N and O–H⋯O hydrogen bonds, forming a zig-zag chain along the c axis [Fig. 3(b)]. The π-π stacking of PCA in adjacent chains (not shown here) can also be observed with a centroid-centroid distance of 0.376 1 nm, which is one of the indispensable interactions for the stabilization of the current crystal structure. The previously reported PCA structure was solved at room temperature. Then, our first thought is whether structural disorders can be eliminated by a low temperature test. Diffraction data were recollected at about 120 K (Table 1), and similar result (PCA-R) was obtained. Perhaps, test at lower temperature (e.g., 10 K)[25] may output desired structure model, which needs special XRD apparatus. The other approach is using advanced electron diffraction method at liquid N2 temperature, which is currently under way.
|
Fig. 3 The asymmetric unit (a) and the 1D zig-zag chain structure (b) of PCA-R |
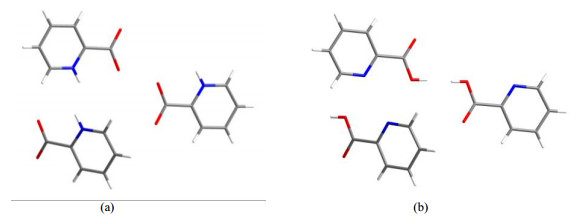
As mentioned above, the 13C chemical shift assignment is an urgent task due to the actual application requirements. Such issue can be fixed by using DFT calculation on a periodic model, i.e., crystallographic information file (CIF), very accurately and quickly. For example, using this protocol, Zheng et al.[26] demonstrated the excellent agreements between the calculated and experimental 13C NMR parameters for various amino acids and peptides. Though PCA is a very simple molecule and PCA-R is a very simple structural model, this CIF cannot be submitted directly for the calculation task due to the proton positional disorder. Deleting each disordered proton on this Z'=1 structure will lead to unreasonable structural models (Fig. 4). Making P1 symmetry on PCA-R before the proton deletion can generate reasonable models, while these ones have a higher Z' value, 8. Calculation on such high Z' models often need more powerful computing capability, and the accuracy is yet to be verified.
|
Fig. 4 Fragments of two unreasonable structural models for PCA: (a) Removing disordered protons on carboxyl groups; (b) Removing disordered protons on pyridine N |
Here, a virtual structure, PCA-V (Fig. 5), was constructed during the refinement. Proton positional disorder was removed and neutral molecules and zwitterions exist independently. Though PCA-V (Table 1) holds different space group and cell parameters, the packing arrangements of PCA-R and PCA-V are consistent. Also this model has low residual values (R1/wR2 = 0.060 6 / 0.157 5), which may be regarded as an alternative representation for the PCA structure. Then, PCA-V was subjected to DFT calculations.
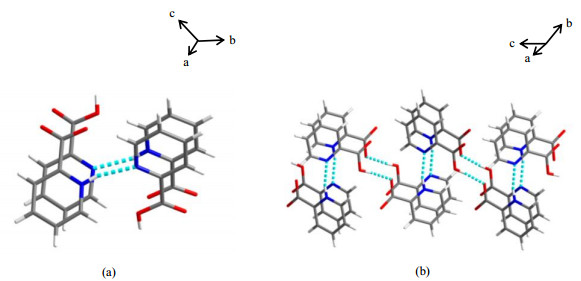
|
Fig. 5 The asymmetric unit (a) and 1D zig-zag chain structures (b) of PCA-V |
The experimental (δCexp) and calculated (δCcal) 13C isotropic chemical shifts of PCA are summarized in Table 2, and their correlations are displayed in Fig. 6. The calculated 13C chemical shifts δCcal = − (σCcal −σCref), where σCcal is the calculated shielding value, and σCref = 167.3. This value was calculated from addition of the mean average of δCexp values and the mean average of σCcal values. The theoretical 13C chemical shifts are all well consistent with the experimental values with a standard deviation of ΔδC 2.1. The maximal ΔδC value is less than 5, which is within the level of calculation error, indicating a reasonable computational accuracy. We should point out that the NQS experiment [Fig. 2(b)] is very necessary for the correct identifying C-1(I) and C-3(I) signals (Fig. 6) for these sites have very similar theoretical δCcal values (Table 2).
| Table 2 The experimental and calculated 13C isotropic chemical shifts of PCA |
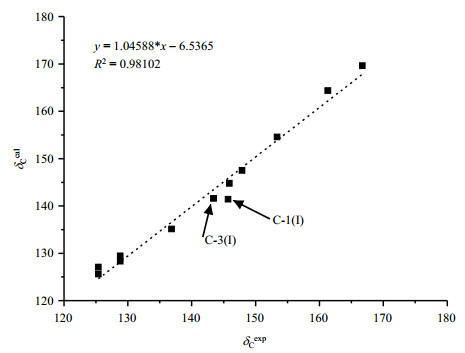
|
Fig. 6 Correlations of experimental and calculated 13C isotropic chemical shifts of PCA |
The 15N chemical shifts of PCA were not reported previously though Hamazaki et al.[13] claimed there are "two separate signals of protonated and nonprotonated N atoms" on the 15N CP/MAS NMR spectrum. Here, the theoretical 15N chemical shifts of PCA were obtained using the similar calculation approach for 13C. The σNref value, −160.3, was derived from δNexp+σNcal of nicotinamide (CSD refcode: NICOAM01), a structural analog of PCA. This σNref value is consistent with the reported one (−160.4) by other research group[27]. For the protonated and nonprotonated 15N, the predicted chemical shifts are δN −179.6 and δN −90.0, respectively.
According to the theoretical assignment (Fig. 2), neutral PCA isomers display its featured resonances at C-3, C-5, and C-6 sites, and zwitterions show a featured C-6 signal. Such information can be used to analyze the molecular states of PCA in its crystalline complexes, especially when a single crystal is not available. For example[14], though C-6 signal of PCA in naringenin-PCA cocrystal cannot be clearly assigned, the absence of featured C-3 and C-5 peaks of neutral PCA solidly confirms the existence of PCA molecules in the zwitterionic form in this sample. Additionally, since π-π stacking is one of the indispensable interactions for the stabilization of the current crystal form, the use of aromatic solvents during the crystal growth procedure may produce different polymorphs or solvates (perhaps contain different tautomeric forms)[28], or change the crystallization kinetics, which deserves further investigation.
3 ConclusionPCA is an important chemical and also a very rare case which contains both the neutral molecules and zwitterions in the same crystal structure. In this contribution, the 13C chemical shift assignment of this sample was performed by periodic DFT calculation using a crystallography virtual structure. Theoretical 13C chemical shifts are well consistent with the experimental values. Neutral molecules and zwitterions rise their fingerprint resonances at several carbon sites. The chemical shift information obtained here will provide important spectral evidence for the investigation of tautomerism of PCA in its crystalline complexes.
Acknowledgement The authors acknowledge the National Natural Science Foundation of China (No. 21673279) and the Youth Innovation Promotion Association of CAS (No. 2012242) for financial support for this work. The authors also thank Prof. ZHENG An-min, Wuhan Institute of Physics and Mathematics, CAS, for the calculation supporting.| [1] | ANTONOV L. Tautomerism: methods and theories[M]. Weinheim: Wiley-VCH, 2003. |
| [2] | ELGUERO J, KATRITZKY A R, DENISKO O V. Tautomerism of heterocycles[M]. New York: Academic Press, 1976. |
| [3] | SMITH M B, MARCH J. March's advanced organic chemistry:reactions, mechanisms, and structure[M]. 6th ed. New York: Wiley-Interscience, 2007. |
| [4] |
WU Z W, ZHANG R, RONG Z M. Azo-hydrazone tautomerism of azo dyes[J].
J Chem Ind Eng, 2015, 66(1): 52-59.
吴祖望, 张蓉, 荣泽明. 论偶氮染料的偶氮-腙互变异构[J]. 应用化学, 2015, 66(1): 52-59. |
| [5] | MASAND V H, MAHAJAN D T, HADDA T B, et al. Does tautomerism influence the outcome of QSAR modeling?[J]. Med Chem Res, 2014, 23: 1742-1757. DOI: 10.1007/s00044-013-0776-0. |
| [6] | IVANOVA D, DENEVA V, NEDELTCHEVA D, et al. Tautomeric transformations of piroxicam in solution:a combined experimental and theoretical study[J]. RSC Adv, 2015, 5(40): 31852-31860. DOI: 10.1039/C5RA03653D. |
| [7] | CRUZ-CABEZA A J, GROOM C R. Identification, classification and relative stability of tautomers in the Cambridge structural database[J]. CrystEngComm, 2011, 13(1): 93-98. DOI: 10.1039/C0CE00123F. |
| [8] | BAUER M, HARRIS R K, RAO R C, et al. NMR study of desmotropy in Irbesartan, a tetrazole-containing pharmaceutical compound[J]. J Chem Soc, Perkin Trans, 1998, 2(3): 475-482. |
| [9] | KUMAR S S, NANGIA A. A solubility comparison of neutral and zwitterionic polymorphs[J]. Cryst Growth Des, 2014, 14(4): 1865-1881. DOI: 10.1021/cg5000205. |
| [10] | TOTHADI S, BHOGALA B R, GORANTLA A R, et al. Triclabendazole:an intriguing case of co-existence of conformational and tautomeric polymorphism[J]. Chem Asian J, 2012, 7(2): 330-342. DOI: 10.1002/asia.201100638. |
| [11] | MIRMEHRABI M, ROHANI S, MURTHY K S K, et al. Characterization of tautomeric forms of ranitidine hydrochloride:thermal analysis, solid-state NMR, X-ray[J]. J Cryst Growth, 2004, 260(3-4): 517-526. DOI: 10.1016/j.jcrysgro.2003.08.061. |
| [12] | WANG X J, KONG M M, LI D X, et al. Stanozolol-aromatic carboxylic acid crystalline complexes:flexible tautomeric/ionization states and supramolecular synthons[J]. Cryst Eng Comm, 2019. DOI: 10.1039/C8CE01439F. |
| [13] | HAMAZAKI H, HOSOMI H, TAKEDA S, et al. 2-Pyridinecarboxylic acid[J]. Acta Cryst Sect C:Cryst Struct Commun, 1998, 54(10): IUC9800049. |
| [14] | LUO C, LIANG W D, CHEN X, et al. Pharmaceutical cocrystals of naringenin with improved dissolution performance[J]. CrystEngComm, 2018, 20(22): 3025-3033. DOI: 10.1039/C8CE00341F. |
| [15] | GRANT R S, COGGAN S E, SMYTHE G A. The physiological action of picolinic acid in the human brain[J]. Int J Tryptophan Res, 2009, 2(2): 71-79. |
| [16] | LOU X B, SHEN M, LI C, et al. Reduction of the 13C cross-polarization experimental time for pharmaceutical samples with long T1 by ball milling in solid-state NMR[J]. Solid State Nucl Magn Reson, 2018, 94(16): 20-25. |
| [17] | WANG L, LUO M, LI J H, et al. Sweet theophylline cocrystal with two tautomers of acesulfame[J]. Cryst Growth Des, 2015, 15(6): 2574-2578. DOI: 10.1021/acs.cgd.5b00207. |
| [18] | LI J H, FU X, LI J Y, et al. Quinine acesulfamates[J]. Cryst Growth Des, 2016, 17(1): 58-66. |
| [19] | YUAN Y, WANG L, LI D X, et al. How many parameters can affect the solid form of cocrystallization products in mechanochemical reactions? a case study[J]. Cryst Growth Des, 2018, 18(12): 7244-7247. DOI: 10.1021/acs.cgd.8b01320. |
| [20] | SHELDRICK G M. Crystal structure refinement with SHELXL[J]. Acta Cryst Sect C:Struct Chem, 2015, 71(1): 3-8. DOI: 10.1107/S2053229614024218. |
| [21] | SCHAEFER J, SEFCIK M D, STEJSKAL E O, et al. Total suppression of sidebands in CPMAS C-13 NMR[J]. J Magn Reson, 1982, 49(2): 341-345. |
| [22] | BALIMANN G E, GROOMBRIDGE C J, HARRIS R K, et al. Chemical applications of high-resolution 13C NMR spectra for solids[J]. Philos Trans R Soc Lond A, 1981, 299: 643-663. DOI: 10.1098/rsta.1981.0040. |
| [23] | CLARK S J, SEGALL M D, PICKARD C J, et al. First principles methods using CASTEP[J]. Z Kristallogr, 2005, 220(5-6): 567-570. |
| [24] | STEED K M, STEED J W. Packing problems:high Z' crystal structures and their relationship to cocrystals, inclusion compounds, and polymorphism[J]. Chem Rev, 2015, 115(8): 2895-2933. DOI: 10.1021/cr500564z. |
| [25] | HOSER A A, SOVAGO I, LANZA A, et al. A crystal structure prediction enigma solved:the gallic acid monohydrate system-surprises at 10 K[J]. Chem Commun, 2017, 53(5): 925-928. DOI: 10.1039/C6CC06799A. |
| [26] | ZHENG A M, LIU S B, DENG F. 13C shielding tensors of crystalline amino acids and peptides:theoretical predictions based on periodic structure models[J]. J Comput Chem, 2009, 30(2): 222-235. |
| [27] | TATTON A S, PHARM T N, VOGT F G, et al. Probing hydrogen bonding in cocrystals and amorphous dispersions using 14N-1H HMQC solid-state NMR[J]. Mol Pharmaceutics, 2013, 10(3): 999-1007. DOI: 10.1021/mp300423r. |
| [28] | JOSEPH A, ALVES J S R, BERNARDES C E S, et al. Tautomer selection through solvate formation:the case of 5-hydroxynicotinic acid[J]. CrystEngComm, 2019, 21(13): 2220-2233. DOI: 10.1039/C8CE02108B. |
 2020, Vol. 37
2020, Vol. 37 
