2. CAS Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan(Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences), Wuhan 430071, China
2. 中国科学院生物磁共振分析重点实验室, 波谱与原子分子物理国家重点实验室, 武汉磁共振中心(中国科学院 武汉物理与数学研究所), 湖北 武汉 430071
Polyhydroxyalkanoates (PHAs) represent some unique biopolymeric metabolites of microorganism in terms of their biosynthesis, biological functions and functionality in their applications. They are naturally synthesized by bacteria serving as energy storage and are often semi-crystalline. These biopolymers are increasingly becoming a focus of research due to their biodegradability and biocompatibility[1]. As renewable materials, they have shown some potential to replace, at least in partial, the non-degradable petroleum-based ones[2]. Moreover, these biopolymers are the environmentally friendly materials, even when degraded; the products are hydroxycaboxylic acids, some of which are normal biological metabolite such as 3-hydroxybutyric acid also known as “ketone body”. Ultimately, the degradation products are carbon dioxide and water which were from environment in the first place during biosynthesis, thus leading to no new or extra CO2 load to the environment[3].
Amongst them, poly(3-hydroxybutyrate) (PHB) may also have some potential as biomedical materials since its biodegraded product, 3-hydroxybutyrate, is the ketone body present in human blood plasma. However, its industrial application remains limited, owing to several inherent deficiencies[3] including poor mechanical properties such as brittleness due to its high crystallinity and narrow thermal processing window[4, 5]. To overcome these drawbacks, a number of strategies have been developed including preparing miscible blends with other flexible biodegradable polymers or plasticizer of low molecular weight [5-8], and using chemical and biological routes to synthesize block copolymers[9-11]. A number of copolymers with other similar hydroxyalkanoate monomer units have been developed, such as poly(3-hydroxybutyrate-co-3- hydroxyvalerate) ([P(HB-HV)])[12-14], poly(3-hydroxybutyrate-co-4-hydroxybutyrate) ([P(3-HB-4-HB)])[15-17], poly(3-hy-droxybutyrate-co-3-hydroxypropionate) ([P(HB-HP)])[18, 19] and poly(3-hydrorate-co-3- hydroxyhexanoate) ([P(HB-HHX)])[20, 21], via biosynthetic routes of a variety of bacteria. Compared with PHB, these copolymers exhibit decreased segmental rotation temperature (Ts), melting point (Tm) and crystallinity, leading to reduced brittleness and enhanced flexibility. These studies have shown that the above limitations can be overcome by the preparation of multi-block copolymers from PHB with segments of other biodegradable macromolecules.
It is conceivable that the structural, dynamic and interactional properties of PHB and its copolymers are key factors governing their properties in biological function and functionality in applications. Therefore, the elucidation of such properties is fundamentally important to understand the mode of actions of these polyesters in the living organisms and the functionality in their applications. Extensive researches have been carried out on structure and molecular dynamics of PHB and some of its copolymers using a variety of techniques including dielectric relaxation[22], dynamic mechanical analysis (DMA)[23] and nuclear magnetic resonance (NMR) spectroscopy[24-26].
For PHB, of the various cold-crystallized samples, dielectric relaxation results have shown that the presence of semi-crystalline morphology such as crystallinity had a significant effect on the glass-rubber relaxation characteristics as compared to the wholly amorphous material[27]. DMA results showed that Tm, Ts, temperature to flow (Tflow), and moduli decrease as the content of 3-hydroxyvalerate (HV) increases in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) random copolymers. These techniques, however, can provide limited information about the structural features related to dynamics. In addition, Klinowski et al.[28] had reported another two crystalline phases, α-orthorhombic phase and β-orthorhombic phase, existed apart from the amorphous phase by 13C NMR lineshape analysis and the normalized infrared spectroscopy. Using spectral deconvolution, the results indicated large differences in molecular mobility between the crystalline and amorphous regions of PHB. Our previous work[29] about the structural characteristics of the semi-crystalline biopolymer PHB and its copolymers PHBV showed that the domain sizes of the crystalline regions decreased whilst the mobile domain sizes increased with the HV co-monomer contents increasing. In addition, it had been observed that introduction of HV enhanced molecular mobility of the biopolymers and raised the ratio of the amorphous to the crystalline region[29].
However, less work has been conducted on the molecular dynamics of these materials. Little information is available on the relationship between the macroscopic properties and the molecular structure and dynamics at the molecular level, which are undoubtedly useful for understanding the materials and indeed important for the future design of new materials. Consequently, a number of questions remain unanswered, such as ‘what is the nature of the molecular dynamics for the different domains of these biopolymers’, ‘how does the content of 3-hydroxyvalerate affect the molecular dynamics’, and ‘is there any relationship between the molecular dynamics and their macroscopic properties’. To answer some of the questions, the molecular dynamics need to be studied, and a promising way for the study is NMR relaxation time measurement since it provides not only dynamic information but also related structural information. Normally such studies are carried out by measuring 1H spin-lattice relaxation times in the laboratory frame (T1) and rotating frame (T1ρ), and spin-spin relaxation time (T2) as a function of either magnetic field strength or temperature. However, the former requires a broad range of magnets, which is often unrealistically expensive though field cycling techniques are now available.
In the present study, T1, T1ρ and T2 measurements as a function of temperature combined with differential scanning calorimetry (DSC) analysis are employed to investigate the molecular dynamics of semi-crystalline PHB and copolymers PHBV containing 5 wt. % (PHBV5) and 12 wt. % (PHBV12) 3-hydroxyvalerate monomer in different regions and dynamic states.
2 Experimental section 2.1 MaterialsPHB (MW = 470 000 g/mol), PHBV5 and PHBV12 were purchased from Aldrich Chemical Co., Inc. (Milwaukee, WI.USA No. 80181-31-3) and used with further processing. Fig. 1 shows the schematic form of their chemical structures. All samples were dried for at least 24 h under vacuum at the temperature of 353 K and sealed into 10 mm NMR tubes for NMR measurements.
|
Fig. 1 Schematic chemical structures for PHB and PHBV biopolymers |
DSC analyses were conducted on a Perkin-Elmer DSC-4 with a heating rate of 10 K/min under liquid nitrogen. 5~10 mg of powdered sample was sealed in an aluminium pan and placed in the DSC instrument. Sample temperature was scanned between 223 K and 473 K as follows: samples were first heated from223 K to 473 K and maintained at 473 K for 1 min; then cooled to 223 K rapidly, and reheated to 473 K, after that cooled to 223 K again at a cooling rate of 100 K/min.
2.3 1H relaxation time measurementsT1, T1ρ, and proton second moment (M2r), were measured on a Bruker AC 80 NMR spectrometer operating at proton frequency of 80.13 MHz with 10 mm probe head over the temperature range of 150~370 K. The proton 90° pulse length was 10.8 μs. The sample temperature was controlled with a Bruker variable temperature unit, VT-2000, and stable within ± 1 K. An increment of 10 K was used and experiments were always started from the lowest temperature. A total of 15 min waiting time for each temperature was allowed to ensure temperature stabilization and equilibration. The actual sample temperatures on Bruker AC 80 spectrometer were calibrated by directly inserting a pre-calibrated thermal couple into a sample-filled tube in the probe head. The errors in the measurements of T1 and T1ρ were estimated to be about 5%. A total of 1 024 data points were collected with 32 scans and 100 kHz sweep width in these measurements.
T1 was measured using the standard inversion recovery method [recycle delay-180°x-τ-90°x-acquisition]. The recycle delay was at least 5 times of proton T1. Relaxation delay time, τ, was chosen from 0.1 ms to 10 s depending on the length of T1. The relaxation processes for all the polymers were found to be monoexponential over the whole temperature range studied.
1H T1ρ was measured using a standard spin-lock pulse sequence [recycle delay-90°x-τSL-acquisition] where spin lock time, τSL, was chosen from 0.1 ms to 15 ms depending on the length of T1ρ. The decays of nuclear magnetization for all these polymers were found to be bi-exponential over the whole temperature range studied.
M2r was measured using solid echo pulse sequence [recycle delay-90°x-τ1-90°y-τ-acquisition]. The recycle delay was 6 s. τ was chosen to be 5 μs, just slightly longer than the dead time (about 4.3 μs) of the spectrometer, and τ1 was used from 1 μs to 18 μs depending on the length of T2. The solid echoes were fitted to a modified Gaussian function[30] to obtain the values of M2r.
All relaxation time values were extracted by curve fitting using a Ladenburg-Marquardt nonlinear curve-fitting routine installed in a personal computer.
3 Results and discussion 3.1 DSCFig. 2 shows the DSC thermograms of PHB and its copolymers (PHBV5, PHBV12) in the temperature range of 223~473 K. There is no significant difference among the glass transition temperatures (Tg, about 270 K) of the three samples. The endothermic peaks at 439.7, 429.4 and 426.3 K indicate Tm values of the lamellar (α-orthorhombic) crystal of PHB, PHBV5 and PHBV12, respectively. The peaks at 417.8 K and 414.8 K in PHBV5 and PHBV12, respectively, are attributed to a transition of α-orthorhombic to β-orthorhombic form (from the amorphous domain between the orthorhombic α-form lamellar)[31]. Due to the coexistence of crystalline and amorphous phases in the PHB matrix and its relative higher degree of crystallinity, the melting peak of PHB was so broad that the β-orthorhombic form was not observed. Furthermore, with the increased content of HV, the β-orthorhombic form increases, this indicates that the conformational change of PHBV12 is more prominent than that of PHBV5 and PHB. Crystallization of the α-form of PHBV12 took place at 340.2 K on cooling, whereas those of PHB and PHBV5 were not observed. Taking 146 J/g for 100% crystalline of PHB[32], the DSC analysis indicated that the PHB sample had a degree of crystallinity α of 68.4%, which was calculated based on the formula α (%) = 100 *∆Hm/∆H [33, 34], where ∆Hm is the enthalpy of the peak and ∆H denotes the enthalpy of melting process of a pure crystalline sample[32]. According to the DSC experimental results, the crystallinity was estimated as 60.3% and 56.7% for PHBV5 and PHBV12, respectively. These DSC results were well consistent with our previous NMR measurements[29] further confirming that Tg, Tm and crystallinity of PHB decreased with the increased content of HV co-monomers.

|
Fig. 2 DSC curves for (a) PHB, (b) PHBV5 and (c) PHBV12 |
1H spin-lattice relaxation rate in the laboratory frame, R1, as a function of temperature is shown in Fig. 3. We can find that in all three biopolymers studied, a R1 maximum was observable at about 184 K, indicating the presence of a motion as an efficient proton relaxation process. This is probably associated with the three-fold reorientation of methyl groups in PHB or rotational motions of either methyl or ethyl moieties in PHBV5 and PHBV12. Although the R1 maxima were at about the same temperature (182~185 K), their maximum R1 values were obviously different (tabulated in Table 1). Another maximum appeared at about 323 K for PHBV12, which probably corresponded to the segmental backbone rotation process. While in PHB and PHBV5, the maxima moved to higher temperatures (> 350 K). This difference is probably attributable to the differences in their molecular packing and will be further discussed in later section. To evaluate the data quantitatively, the experimental data were fitted to the well-known Kubo-Tomita expression[35] [Eq. (1)]. We assumed that two relaxation processes corresponding to the side chain motion and segmental rotation respectively dominated the spin-lattice relaxation time. The observed spin-lattice relaxation rate
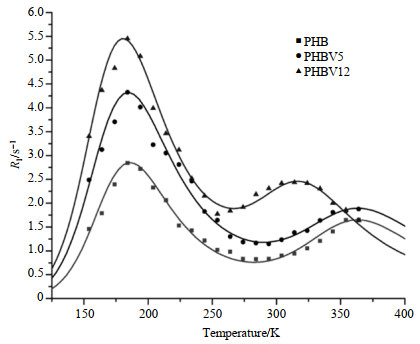
|
Fig. 3 Temperature dependence of the proton spin-lattice relaxation rates in the laboratory frame, R1, for PHB, PHBV5 and PHBV12. The solid lines are fitted data using two components form of relaxation theory |
| Table 1 1H relaxation parameters for PHB, PHBV5 and PHBV12 from spin-lattice relaxation in the laboratory frames |
| $ {R_{lobs}} = \frac{1}{{{T_1}}} = \sum\limits_{i \ge 1} {{C_i}} \left( {\frac{{{\tau _{ci}}}}{{1 + \omega _0^2\tau _{ci}^2}} + \frac{{4{\tau _{ci}}}}{{1 + 4\omega _0^2\tau _{ci}^2}}} \right) $ | (1) |
Where
| $ {\tau _{ci}} = {\tau _{0i}}\exp \left( {\frac{{{E_{{\rm{a}}i}}}}{{RT}}} \right) $ | (2) |
Where
| $ {C_i} = \frac{2}{3}\Delta {M_2}{\gamma ^2} = \frac{1}{2}\frac{{{n_i}}}{N}\frac{{{\gamma ^4}{\hbar ^2}}}{{^{{r^6}}}} $ | (3) |
Where N is the total number of protons in the molecule,
Fig. 3 shows the theoretical fitting of the experimental data for the three biopolymers by the two components form of the relaxation theory described in Eq. (1) and (2). For PHB, the 3-fold reorientation of methyl group has a τ0 value of (1.22±0.31)×10﹣12 s and Ea value of (10.74±0.40) kJ/mol, these values broadly agree with those of the polycrystalline methyl groups[36], and similar results also have been found for other methyl and ethyl moieties in the solid state[37]. At higher temperature (above 350 K), the process attributed to the segmental rotation has a τ0 value of (2.61±0.55)×10﹣12 s and Ea value of (21.08±4.61) kJ/mol. For PHBV5 and PHBV12, the τ0 values of side chain motion are (2.70±0.60)×10﹣12 and (1.55±0.36)×10﹣12 s, and Ea values are (9.40±0.36) and (10.03±0.36) kJ/mol, respectively; segmental rotation processes at higher temperature (359 K and 323 K for PHBV5 and PHBV12, respectively) have τ0 values of (1.71±4.68)×10﹣14 and (1.05±0.38)×10﹣13 s, and Ea values of (33.44±7.40) and (25.03±2.46) kJ/mol, respectively. The relaxation constant (C) and the maximum relaxation rate (R1max) of different motional processes are also obtained and tabulated in Table 1. Using the obtained values of Ci through the fitting procedure, the calculated average inter-proton distance of methyl group in PHB is 0.182 nm according to the Eq. (2), which agrees satisfactorily with that of other methyl group. These results showed that the weighted average relaxation rate R1 of two processes speeded up with the increasing of HV co-monomer content.
3.3 1H spin-lattice relaxation in the rotating frameFor all the three biopolymers, bi-exponential T1ρrelaxation times were observed over the temperature range of 150~370 K. These fast and slow components of T1ρ may correspond to amorphous and crystalline regions, respectively, since our previous study showed that the long 1H T1ρ components are associated with the crystalline structure while the short ones are associated with the amorphous regions. For both the crystalline (Fig. 4) and amorphous (Fig. 5) regions, the temperature dependence of R1ρ shows two maxima, implying the presence of two main relaxation processes: one occurs at the low temperature range of 150~250 K and the other one at the high temperature region of 250~370 K. The experimental data associated with the two regions were fitted to the two thermally-activated motions according to the relaxation theory described in Eq. (4) which is similar to that in R1 analysis:
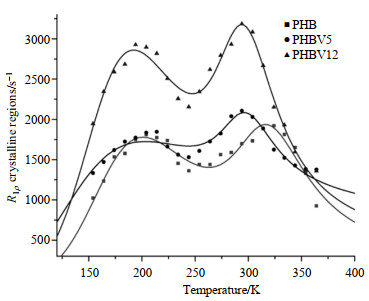
|
Fig. 4 Temperature dependence of the proton spin-lattice relaxation rates in the rotating frame, R1ρ, for the crystalline regions of PHB, PHBV5 and PHBV12. The solid lines are fitted data using two components form of relaxation theory |
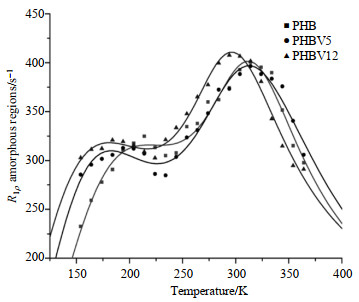
|
Fig. 5 Temperature dependence of the proton spin-lattice relaxation rates in the rotating frame, R1ρ, for the amorphous regions of PHB, PHBV5 and PHBV12. The solid lines are fitted data using two components form of relaxation theory |
| $ {R_{{\rm{1}}\rho }} = \frac{1}{{{T_{1\rho }}}} = \frac{3}{2}\sum\limits_{i \ge 1} {{C_i}\left( {\frac{{{\tau _{ci}}}}{{1 + 4\omega _{ei}^2\tau _{ci}^2}} + \frac{5}{3} \times \frac{{{\tau _{ci}}}}{{1 + \omega _0^2\tau _{ci}^2}} + \frac{2}{3} \times \frac{{{\tau _{ci}}}}{{1 + 4\omega _0^2\tau _{ci}^2}}} \right)} $ | (4) |
Where
In the present situation, the applied
| $ {\omega _{\rm{e}}} = \sqrt {\omega _{{\rm{SL}}}^2 + \omega _{\rm{L}}^2} $ | (5) |
In practice,
For PHB, PHBV5 and PHBV12, the experimental data agreed well with the theoretical ones for crystalline regions (shown in Fig. 4) and amorphous regions (shown in Fig. 5). Motional parameters obtained are tabulated in Table 2 and 3. The peak in the low temperature range should be attributed to side chain motion process owing to its activation energy (Ea≈4.17, 3.96, 3.14 kJ/mol in the crystalline regions; Ea≈6.62, 3.39, 6.23 kJ/mol in the amorphous regions) and correlation times of molecular motions (τ0≈9.70×10﹣8, 7.73×10﹣8, 1.31×10﹣7 s in the crystalline regions; τ0≈2.08×10﹣8, 1.53×10﹣7, 2.21×10﹣8 s in the amorphous regions). The high temperature processes (304 K) may be associated with segmental motions of backbone of the polymer chain. Their motional parameters (Ea≈22.24, 16.80, 19.46 kJ/mol; τ0≈2.40×10﹣10, 2.01×10﹣9, 4.70×10﹣10 s corresponding to the crystalline regions and Ea≈29.86, 43.63, 31.00 kJ/mol; τ0≈1.47×10﹣11, 2.38×10﹣14, 3.93×10﹣12 s corresponding to the amorphous regions of PHB, PHBV5 and PHBV12 respectively) were also obtained by curve fitting. For amorphous regions, the relaxation rates, R1ρ, of PHB, PHBV5 and PHBV12 increased with the increasing of the HV co-monomers contents (Fig. 4). For crystalline regions, R1ρ of the three materials present the similar tendency as amorphous regions (Fig. 5). However, over the whole temperature range, the relaxation rate of molecular motions in amorphous region is much faster than that in crystalline region (shown in Fig. 4 and 5). These results obviously indicate that the addition of HV co-monomers exerts more influences on the amorphous regions than crystalline regions. The molecular dynamic parameters obtained by R1ρ analysis can also be explained on the molecular level that the molecular structures are modified and molecular motions are enhanced by adding HV co-monomers in both the amorphous and crystalline regions.
| Table 2 1H relaxation parameters for crystalline regions of PHB, PHBV5 and PHBV12 from spin-lattice relaxation in the rotating frames |
| Table 3 1H relaxation parameters for amorphous regions of PHB, PHBV5 and PHBV12 from spin-lattice relaxation in the rotating frames |
The free induction decays of PHB, PHBV5 and PHBV12 cannot be described simply by either a Gaussian or an exponential decay function. Nevertheless, a much better approximation can be made by a modified Gaussian function[30], shown in Eq. (6):
| $ I(t) = {I_0}\exp (\frac{{ - {a^2}{t^2}}}{2})\frac{{\sin bt}}{{bt}} $ | (6) |
The rationale of using Eq. (6) is that the wide-line 1H NMR spectrum is assumed to be a rectangular line shape with a total width 2b, convoluted with a Gaussian line shape with a standard deviation a[40]. One of the most useful way to evaluate this kind of free induction decay (FID) is to use the residual second moment, M2r, which is a measurement of the strength of the dipolar interactions and can be calculated as M2r =
a2+b2/3. In general, M2r is expected to decrease when the motional correlation time
| $ {\tau _c} = {(\gamma \sqrt {{M_{2{\rm{r}}}}} )^{ - 1}} $ | (7) |
Temperature dependence of M2r for the PHB, PHBV5 and PHBV12 is shown in Fig. 6. For PHB, at low temperature (e.g. 150 K), the measured M2r value is about 0.132 mT2. It decreases to 0.101 mT2 as the temperature approaches 184 K and then remains almost constant (a first plateau) in the temperature range of 184~278 K. The reduction of M2r is due to the three-fold (C3) reorientation of methyl groups at about 180 K, which is consistent with the above R1 and R1ρanalyses. The M2r value further decreases gradually with increasing temperature and eventually reaches a second plateau of 0.071 mT2 at about 360 K. This reduction is attributed to the breakdown of the segments of the biopolymer chains above its segmental rotation temperature 278 K. M2r of PHBV5 and PHBV12 show similar tendency to that of PHB. But their values decrease from 0.11 mT2 and 0.092 mT2 at low temperature (about 150 K) to 0.083 mT2 and 0.067 mT2 at 184 K, respectively. Then they remain almost constant in the temperature range of 184~280 K. And M2r decrease gradually with a further increase of temperature and eventually reach the second plateaus of 0.05 mT2 and 0.044 mT2 at 350 K, respectively. From the experimental data, we can reasonably conclude that the three-fold reorientation of methyl groups plays a major role at temperatures below 280 K, while at temperatures above 350 K the segmental rotation process dominates the main relaxation pathway. Furthermore, as can be seen from the experimental results of these materials, the higher content of HV, the narrower of the 1H line-width of NMR spectrum and the faster molecular motions of them. This can assist the understanding of relationship between the macroscopic properties and molecular structures and dynamics on molecular level.
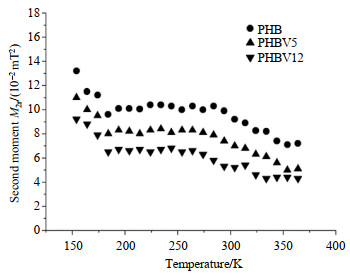
|
Fig. 6 Temperature dependence of proton second moments, M2r, of PHB, PHBV5 and PHBV12 |
The molecular dynamics of the semi-crystalline biopolymer PHB and its copolymers PHBV have been well characterized by means of 1H relaxation time measurements. Our results indicate that the major relaxation pathways in PHB and its copolymers PHBV are the side chain motion and segmental rotation of polymer backbone chain. The correlation times and activation energies of molecular motions for PHB and PHBV copolymers are determined by fitting the T1 and T1ρexperimental data with a theory formula. These results can be explained on molecular level that the structures of PHB are modified and molecular motions are enhanced by adding HV co-monomers, which is consistent with the DSC results.
Acknowledgement We acknowledge that DSC measurements were performed at the Analytical Centre, Wuhan University of Technology.| [1] | ANJUM A, ZUBER M, ZIA K M, et al. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers:A review of recent advancements[J]. Int J Biol Macromol, 2016(89): 161-174. |
| [2] | POLTRONIERI P, KUMAR P. Polyhydroxyalkanoates (PHAs) in industrial applications[M]//MARTÍNEZ L, KHARISSOVA O, KHARISOV B. Handbook of ecomaterials. Springer, Cham, 2018: 1-30. |
| [3] | CHANPRATEEP S. Current trends in biodegradable polyhydroxyalkanoates[J]. J Biosci Bioeng, 2010, 110(6): 621-632. DOI: 10.1016/j.jbiosc.2010.07.014. |
| [4] | NERKAR M, RAMSAY J A, RAMSAY B A, et al. Improvements in the melt and solid-state properties of poly(lactic acid), poly-3-hydroxyoctanoate and their blends through reactive modification[J]. Polymer, 2015(64): 51-61. |
| [5] | LI L, HUANG W, WANG B, et al. Properties and structure of polylactide/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PLA/PHBV) blend fibers[J]. Polymer, 2015(68): 183-194. |
| [6] | BASNETT P, CHING K Y, STOLZ M, et al. Novel poly(3-hydroxyoctanoate)/poly(3-hydroxybutyrate) blends for medical applications[J]. Reactive and Functional Polymers, 2013, 73(10): 1340-1348. DOI: 10.1016/j.reactfunctpolym.2013.03.019. |
| [7] | CORRE Y M, BRUZAUD S, GROHENS Y. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(propylene carbonate) blends:an efficient method to finely adjust properties of functional materials[J]. Macromol Mater Eng, 2013, 298(11): 1176-1183. DOI: 10.1002/mame.201200345. |
| [8] | ABDELWAHAB M A, FLYNN A, CHIOU B S, et al. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends[J]. Polym Degrad Stabil, 2012, 97(9): 1822-1828. DOI: 10.1016/j.polymdegradstab.2012.05.036. |
| [9] | WANG Q, YANG P, XIAN M, et al. Production of block copolymer poly(3-hydroxybutyrate)-block-poly(3-hydroxypropionate) with adjustable structure from an inexpensive carbon source[J]. ACS Macro Lett, 2013, 2(11): 996-1000. DOI: 10.1021/mz400446g. |
| [10] | TRIPATHI L, WU L P, MENG D, et al. Biosynthesis and characterization of diblock copolymer of p(3-hydroxypropionate)-block-p(4-hydroxybutyrate) from recombinant Escherichia coli[J]. Biomacromolecules, 2013, 14(3): 862-870. DOI: 10.1021/bm3019517. |
| [11] | LI S Y, DONG C L, WANG S Y, et al. Microbial production of polyhydroxyalkanoate block copolymer by recombinant Pseudomonas putida[J]. Appl Microbiol Biotechnol, 2011, 90(2): 659-669. DOI: 10.1007/s00253-010-3069-2. |
| [12] | LEE W H, LOO C Y, NOMURA C T, et al. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors[J]. Bioresour Technol, 2008, 99(15): 6844-6851. DOI: 10.1016/j.biortech.2008.01.051. |
| [13] | KOLLER M, BONA R, CHIELLINI E, et al. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora[J]. Bioresour Technol, 2008, 99(11): 4854-4863. DOI: 10.1016/j.biortech.2007.09.049. |
| [14] | PHUKON P, SAIKIA J P, KONWAR B K. Bio-plastic (P-3HB-co-3HV) from Bacillus circulans (MTCC 8167) and its biodegradation[J]. Colloids Surf B Biointerfaces, 2012(92): 30-34. |
| [15] | RAO U, SRIDHAR R, SEHGAL P K. Biosynthesis and biocompatibility of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil[J]. Biochem Eng J, 2010, 49(1): 13-20. |
| [16] | MENG D C, SHI Z Y, WU L P, et al. Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway[J]. Metab Eng, 2012, 14(4): 317-324. DOI: 10.1016/j.ymben.2012.04.003. |
| [17] | CHANPRATEEP S, BUASRI K, MUANGWONG A, et al. Biosynthesis and biocompatibility of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate)[J]. Polym Degrad Stabil, 2010, 95(10): 2003-2012. DOI: 10.1016/j.polymdegradstab.2010.07.014. |
| [18] | FUKUI T, SUZUKI M, TSUGE T, et al. Microbial synthesis of poly((r)-3-hydroxybutyrate-co-3-hydroxypropionate) from unrelated carbon sources by engineered cupriavidus necator[J]. Biomacromolecules, 2009, 10(4): 700-706. DOI: 10.1021/bm801391j. |
| [19] | WANG Q, YANG P, XIAN M, et al. Biosynthesis of poly(3-hydroxypropionate-co-3-hydroxybutyrate) with fully controllable structures from glycerol[J]. Bioresour Technol, 2013(142): 741-744. |
| [20] | OBRUCA S, BENESOVA P, PETRIK S, et al. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds[J]. Process Biochemistry, 2014, 49(9): 1409-1414. DOI: 10.1016/j.procbio.2014.05.013. |
| [21] | WONG Y M, BRIGHAM C J, RHA C, et al. Biosynthesis and characterization of polyhydroxyalkanoate containing high 3-hydroxyhexanoate monomer fraction from crude palm kernel oil by recombinant Cupriavidus necator[J]. Bioresour Technol, 2012(121): 320-327. |
| [22] | SIMON-COLIN C, GOUIN C, LEMECHKO P, et al. Biosynthesis and characterization of polyhydroxyalkanoates by Pseudomonas guezennei from alkanoates and glucose[J]. Int J Biol Macromol, 2012, 51(5): 1063-1069. DOI: 10.1016/j.ijbiomac.2012.08.018. |
| [23] | DE LIMA J A, FELISBERTI M I. Poly(hydroxybutyrate) and epichlorohydrin elastomers blends:Phase behavior and morphology[J]. Eur Polym J, 2006, 42(3): 602-614. DOI: 10.1016/j.eurpolymj.2005.09.004. |
| [24] | ALTHURI A, MATHEW J, SINDHU R, et al. Microbial synthesis of poly-3-hydroxybutyrate and its application as targeted drug delivery vehicle[J]. Bioresour Technol, 2013(145): 290-296. |
| [25] | MASOOD F, HASAN F, AHMED S, et al. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus cereus FA11 isolated from TNT-contaminated soil[J]. Ann Microbiol, 2011, 62(4): 1377-1384. |
| [26] | CERRONE F, S NCHEZ-PEINADO M D M, RODR GUEZ-D AZ M, et al. PHAs production by strains belonging to Massilia genus from starch[J]. Starch, 2011, 63(4): 236-240. DOI: 10.1002/star.201000132. |
| [27] | SHAFEE E E. The influence of semicrystalline morphology on the dielectric relaxation properties of poly(3-hydroxybutyrate)[J]. Eur Polym J, 2001, 37(8): 1677-1684. DOI: 10.1016/S0014-3057(01)00034-9. |
| [28] | NOZIROV F, SZCZESNIAK E, FOJUD Z, et al. H-1 and C-13 NMR studies of molecular dynamics in the biocopolymer of glycolide and epsilon-caprolactone[J]. Solid State Nucl Mag, 2002, 22(1): 19-28. DOI: 10.1006/snmr.2002.0056. |
| [29] | ZHANG L M, TANG H R, HOU G J, et al. The domain structure and mobility of semi-crystalline poly(3-hydroxybutyrate) and poly(3-hydroxybutyrateco-3-hydroxyvalerate):A solid-state NMR study[J]. Polymer, 2007, 48(10): 2928-2938. DOI: 10.1016/j.polymer.2007.03.026. |
| [30] | ABRAGAM A. The principles of nuclear magnetism[M]. Oxford University Press, 1961: 120. |
| [31] | NOZIROV F, FOJUD Z, KLINOWSKI J, et al. High-resolution solid-state C-13 NMR studies of poly (R)-3-hydroxybutyric acid[J]. Solid State Nucl Mag, 2002, 21(3/4): 197-203. |
| [32] | NOZIROV F, NAZIROV A, JURGA S, et al. Molecular dynamics of poly(L-lactide) biopolymer studied by wide-line solid-state H-1 and H-2 NMR spectroscopy[J]. Solid State Nucl Mag, 2006, 29(4): 258-266. DOI: 10.1016/j.ssnmr.2005.09.001. |
| [33] | ARRIETA M P, L PEZ J, HERN NDEZ A, et al. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications[J]. Eur Polym J, 2014(50): 255-270. |
| [34] | ZEMBOUAI I, KACI M, BRUZAUD S, et al. A study of morphological, thermal, rheological and barrier properties of Poly(3-hydroxybutyrate-Co-3-Hydroxyvalerate)/polylactide blends prepared by melt mixing[J]. Polym Test, 2013, 32(5): 842-851. DOI: 10.1016/j.polymertesting.2013.04.004. |
| [35] | TANG H R, BELTON P S. Molecular motions of D-alpha-galacturonic acid (GA) and methyl-D-alpha-galacturonic acid methyl ester (MGAM) in the solid state-A proton NMR study[J]. Solid State Nucl Mag, 1998, 12(1): 21-30. DOI: 10.1016/S0926-2040(98)00041-1. |
| [36] | WANG Y L, TANG H R, BELTON P S. Solid state NMR studies of the molecular motions in the polycrystalline alpha-L-fucopyranose and methyl alpha-L-fucopyranoside[J]. J Phys Chem B, 2002, 106(49): 12834-12840. DOI: 10.1021/jp0268617. |
| [37] | BECKMANN P A, BURBANK K S, LAU M M W, et al. Solid state proton spin-lattice relaxation in four structurally related organic molecules[J]. Chem Phys, 2003, 290(2/3): 241-250. |
| [38] | MCCALL D W, DOUGLASS D C. Molecular motion in polyethylene.V. (NMR-coparison with dielectric results-20 degrees-130 degreesc-e)[J]. Appl Phys Lett, 1965, 7(1): 12-14. DOI: 10.1063/1.1754231. |
| [39] | BECKMANN P A, BURBANK K S, CLEMO K M, et al. H-1 nuclear magnetic resonance spin-lattice relaxation, C-13 magic-angle-spinning nuclear magnetic resonance spectroscopy, differential scanning calorimetry, and x-ray diffraction of two polymorphs of 2, 6-di-tert-butylnaphthalene[J]. J Chem Phys, 2000, 113(5): 1958-1965. DOI: 10.1063/1.482000. |
| [40] | PACANSKY J, YOSHIMINE M. Theoretical-studies of the barriers for internal-rotation of methyl-groups in the tert-butyl radical[J]. J Phys Chem, 1986, 90(9): 1980-1983. DOI: 10.1021/j100400a050. |
 2019, Vol. 36
2019, Vol. 36 
