2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
仲氢诱导超极化(Parahydrogen-Induced Polarization,PHIP)[1-3]技术是核磁共振(Nuclear Magnetic Resonance, NMR)极化技术的一种,理论上可以使样品信号强度增强4~5个数量级,从而增强NMR检测灵敏度,提高谱图质量.PHIP以仲氢分子作为极化源,一般通过将仲氢分子与不饱和有机物分子发生加氢反应,使所得产物获得极化,从而实现信号增强.PHIP的产生需要满足以下三个基本条件:1)加氢反应过程中,成对的仲氢分子参与反应,即产物中新加成的氢原子来自于同一氢分子,相互之间保留自旋耦合相关性[1, 2];2)加成后的氢原子的自旋弛豫时间应该大于反应时间,保证极化不会丧失;3)在反应过程中[4]或反应后[2],新加成氢原子磁不等价.
多相催化-仲氢诱导超极化(HET-PHIP)实验技术是PHIP研究领域中的热点.它利用多相催化剂催化反应体系,是一种快速、可控且廉价地制取极化液体[5-7]和气体[8-11]的方式,广泛应用于催化反应机理研究和磁共振成像(Magnetic Resonance Imaging, MRI)领域.相对于均相PHIP技术,该技术还具有催化剂易与产物分离的优点.HET-PHIP技术常使用金属纳米颗粒或负载金属纳米颗粒作为催化剂[8, 11-17].鉴于金属钯(Pd)纳米颗粒在许多反应中都表现出较好的催化性能,因此许多HET-PHIP研究围绕Pd基催化剂进行.目前,在Pd纳米颗粒催化的不饱和烃加氢反应[18, 19]、α、β-不饱和羰基化合物加氢反应[20]、炔醇加氢反应[7]中都成功观测到了PHIP极化信号.金属纳米颗粒催化的多相加氢反应通常遵循Horiuti-Polanyi机理[21],即氢分子会首先吸附在金属表面并发生解离,生成的氢原子在金属表面自由移动,再与反应物进行反应,得到最终的加氢产物.然而,该过程中由于无法保证产物中新加成氢原子来源于同一仲氢分子,导致HET-PHIP技术存在极化效率不高、信号增强不明显的问题.
有研究发现通过改变催化剂负载金属的形貌,可有效提高催化剂的极化效率[7, 17, 22].而通过向单金属纳米颗粒中引入其它金属形成合金,可有效地改变纳米颗粒原本的组成和形貌. Kovtunov研究组[20]首先对Pd-Sn、Pd-Zn、Pd-Pb、Pd-Ag、Pd-Au、Pd-Mn3+,以及Pt-Y-Ce-Zr等多种含Pd的合金催化剂进行了PHIP极化性能的研究,发现金属纳米颗粒的金属组成对催化剂极化效率有较大的影响.2017年,Bowers等人[23]制备了Pt-Sn双金属催化剂,并观测到很大的极化增强.最近,Kovtunov等人[24]报道了Pd-In双金属催化剂,虽极化效率不及前面报道的Pt-Sn催化剂,但催化活性很好,因此观测到了更明显的极化增强信号.可见,在单金属催化剂中引入新的金属形成合金催化剂已成为提升催化剂极化效率的有效手段.我们在之前的工作中,系统性地研究了Pd-Au双金属催化剂极化效率的优化方法,发现催化剂颗粒形貌对极化效率有较大影响[17].
工业加氢反应中,Pd-Cu合金催化剂受到了广泛的关注与研究[25, 26].由于Pd与Cu之间存在的协同效应,使其在氧还原[27, 28]、一氧化碳氧化[29]、铃木反应[30]、烯烃还原[31-33]等多个反应中都展现出较好的反应活性和选择性.Pd、Cu单金属催化剂的催化性能有较大区别.在炔烃加氢反应中[34],单Pd催化剂往往具有较好的反应活性和较差的烯烃选择性[18, 19];而单Cu催化剂具有很好的烯烃选择性,但活性较低,且反应所需温度较高[35, 36].Pd-Cu双金属催化剂同时具有较好的反应活性和选择性,在催化炔烃加氢反应时,氢分子主要吸附在钯原子并解离成氢原子,随后在金属表面上游离并发生反应[37-40].
本文利用HET-PHIP技术详细研究了Pd-Cu双金属催化剂的催化性能以及PHIP极化效率.通过浸渍法,合成了一系列Pd-Cu/SiO2双金属催化剂.通过对催化剂金属比例及形貌的调控,研究了催化剂结构对丙炔选择性加氢反应PHIP极化效率的影响,并讨论其原因.
1 实验部分 1.1 催化剂材料准备Pd(NO3)2溶液(Pd质量分数为18.09 wt.%,溶解于10 wt.%硝酸溶液)和Cu(NO3)2·3H2O购买于Sigma-Aldrich Inc.气相SiO2(亲水,比表面积为400 m2/g)购买于上海麦克林生化科技有限公司.试剂未经纯化直接使用.
1.2 Cu-Pd /SiO2双金属催化剂制备利用等体积浸渍法合成了一系列不同Pd/Cu原子比的合金Cu-Pd/SiO2催化剂[41].通过配置特定浓度的Pd(NO3)2与Cu(NO3)2·3H2O混合水溶液来控制Pd/Cu原子比:保证Cu负载量为5 wt.%不变,变化Pd含量.所制得催化剂标记为Cu-Pdx/SiO2,其中x为Pd/Cu原子比,包括:Cu-Pd0.003、Cu-Pd0.006、Cu-Pd0.015、Cu-Pd0.025和Cu-Pd0.2.以Cu-Pd0.006/SiO2催化剂为例,催化剂中Cu质量分数占5%,Pd/Cu原子比为0.006.1 g载体上,Cu负载量为50 mg,0.786 8 mmol;Pd负载量为0.502 mg,0.004 72 mmol.具体合成方法如下:首先取0.19 g Cu(NO3)2·3H2O粉末,配置成1.9 mL的1 g/10 mL的Cu(NO3)2溶液.取2.78 mg Pd(NO3)2溶液,与Cu(NO3)2溶液混合.再向混合液中加入一定量的去离子水,配成3.3 mL的双金属盐溶液,随后等体积浸渍在1 g SiO2载体上.浸渍液的总体积需先通过实验测定,不同载体所需的体积不同.所得产物在80 ℃下干燥10 h,随后空气中以5 ℃/min升温至400 ℃,煅烧2 h.作为对比,利用相同的合成方式制备了单金属Pd0.2/SiO2和Cu/SiO2催化剂,Pd0.2/SiO2催化剂金属负载量与Cu-Pd0.2/SiO2中Pd含量相同,Cu/SiO2中Cu负载量仍为5%.
在保持负载金属比例不变的前提下,利用连续浸渍法制备了Pd/Cu原子比为0.006以及0.2的催化剂,分别标记为step-Cu-Pd0.006/SiO2、step-Cu-Pd0.2/SiO2[42].具体合成方式如下,首先通过浸渍法得到Cu/SiO2催化剂,Cu含量为5 wt.%.在得到Cu/SiO2催化剂后,使用特定浓度的Pd(NO3)2溶液对催化剂再次浸渍,随后同样在80 ℃干燥10 h,随后空气中以5 ℃/min升温至400 ℃,煅烧2 h.
所有催化剂在反应前需进行还原处理,使用氢气含量为20%的氮氢混合气,80 sccm流速,250 ℃下还原2 h.
1.3 丙炔加氢PHIP实验在液氮温度(77 K)下,将纯度为99.99%的氢气持续通过FeO(OH)催化剂,制得含量约为50%的仲氢气流,以此作为PHIP反应极化源.
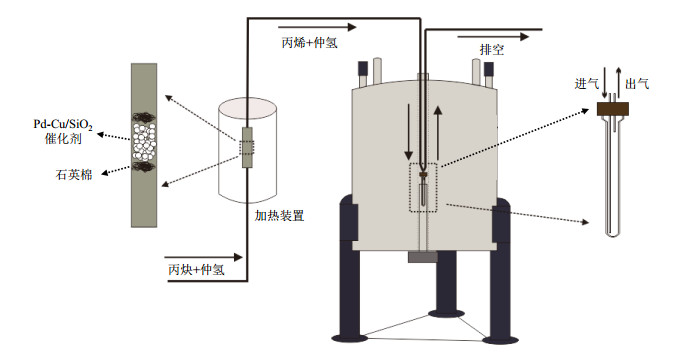
PHIP实验以ALTADENA(Adiabatic Longitudinal Transport After Dissociation Engenders Net Alignment)[43]方法进行,即反应在磁体外部发生,随后绝热转移到磁体内部进行观测.图 1为实验装置示意图.首先,取50 mg所制备的催化剂装入加热炉中反应器内.随后,往反应器内以300 sccm的速率通入氮气,并将加热炉在15 min内升温至250 ℃.到达指定温度后,停止通入氮气,然后同时向反应器通入300 sccm的仲氢气体和200 sccm的丙烯气体,开始催化反应.反应气体通过一根尺寸为0.159 cm i.d.(0.317 5 cm o.d.)的聚四氟乙烯(Poly Tetra Fluoro Ethylene, PTFE)软管通入反应器内.随后,气体产物再通入放置在磁体内部的10 mm自制NMR样品管内进行观察,NMR样品管预先加热至300 K.随后,气体通过一根尺寸为0.159 cm i.d.(0.317 5 cm o.d.)PTFE软管排空到室外.实验中,一般采集反应初期(0.5 min)的ALTADENA极化信号,在通气过程中采集信号.热平衡信号待停止通气后再进行采集.
|
图 1 Pd-Cu催化剂催化丙炔加氢反应的ALTADENA实验装置示意图 Figure 1 ALTADENA experimental schematic diagram of propyne hydrogenation catalyzed by Pd-Cu bimetallic catalysts |
1H PHIP NMR实验是在配备10 mm PABBO探头的Bruker Avance Ⅲ 600 NMR谱仪上进行的. 1H核的共振频率为599.94 MHz.为了更好地采集极化信号,选择π/2脉冲,脉冲宽度为26 μs.极化信号累加次数为8次,热平衡信号累加次数为256次.谱图增宽因子为3 Hz,通过基线校正[23].
1.5 丙炔选择性加氢生成丙烯产率计算反应流出物的热平衡1H NMR谱图可以用来确定丙烯的产率(PE Yield),通过统计丙炔分子链端的≡CH基团的积分面积(SPY)以及丙烯分子中部-CH=基团的积分面积(SPE),可得出反应后两者实际的量,以此计算反应产率.公式如下:
| $ PE\, \, Yield = {S_{PE}}/({S_{PY}} + {S_{PE}})*100\% $ | (1) |
PHIP技术可以极化产物分子中新加成的氢原子,由于丙烯分子中部-CH=基团上氢原子是反应中新加成上去的,且化学位移与其它信号不重叠,所以可以选择该信号进行信号增强倍数计算.通过对比同一信号在极化谱图与热平衡谱图中的实际信号强度,就可以得出信号增强倍数.由于极化谱图和热平衡谱图的采样次数不同,需要对信号谱峰积分面积归一化处理.公式如下:
| $ EF = ({S^{phip}}/8)/({S^{therm}}/256) $ | (2) |
其中EF为信号增强倍数;
XRD实验是在Panalytical X’Pert3粉末X射线衍射仪上完成的,X光源使用的是Cu Kα辐射(λ = 0. 154 04 nm, 40 kV, 40 mA),扫描步长为0.02˚.
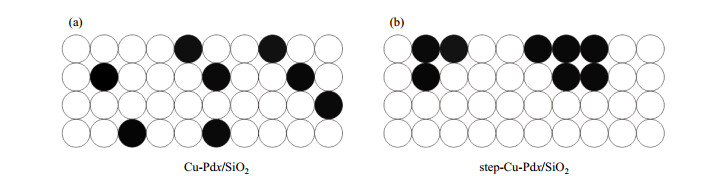
2 结果与讨论 2.1 XRD实验结果我们对所合成的催化剂进行了XRD表征,以确定其晶体结构.图 2所示为催化剂Cu/SiO2、Cu-Pd0.006/SiO2、Cu-Pd0.2/SiO2、step-Cu-Pd0.2/SiO2以及Pd0.2/SiO2的XRD谱图.由于使用的SiO2作为载体不具备长程有序结构,谱图中未观测到明显的载体信号.在催化剂Cu/SiO2与Pd0.2/SiO2上可分别观测到43.3˚和40.1˚衍射峰,分别属于金属Cu(111)晶面信号和Pd(111)晶面信号. Cu-Pd0.006/SiO2催化剂XRD信号与单金属Cu/SiO2催化剂一致,并未观测到明显的单金属Pd或Pd-Cu合金信号,这是由于Pd含量过低的缘故[41].同样,step-Cu-Pd0.006/SiO2催化剂XRD信号也与单金属Cu/SiO2催化剂基本一致(图中未列出).为了确定合成催化剂中负载纳米颗粒状态,我们提高了金属Pd的含量比例,使Pd/Cu原子比达到0.2.从图中可以看到,催化剂Cu-Pd0.2/SiO2在金属Cu衍射峰(43.3˚)与金属Pd衍射峰(40.1˚)之间出现了新的衍射峰(42.4˚),这代表着生成了均匀混合形貌的Pd-Cu合金颗粒[44].合成过程中,前驱体Cu(NO3)2溶液与Pd(NO3)2溶液均匀混合,促使生成的Pd-Cu合金中钯原子与铜原子均匀混合分布在整个纳米颗粒中.结构模型如图 3(a)所示.

|
图 2 Cu/SiO2、Cu-Pd0.006/SiO2、Cu-Pd0.2/SiO2、step-Cu-Pd0.2/SiO2、Pd0.2/SiO2催化剂XRD谱图.Cu-Pd0.2/SiO2与step-Cu- Pd0.2/SiO2催化剂在金属Cu衍射峰(43.3˚)与金属Pd衍射峰(40.1˚)之间出现的宽峰信号代表着Pd-Cu合金的生成 Figure 2 XRD patterns of Cu/SiO2, Cu-Pd0.006/SiO2, Cu-Pd0.2/SiO2, step-Cu-Pd0.2/SiO2, Pd0.2/SiO2 catalysts. The presence of a broad peak centered between the peaks of metallic copper (43.3˚) and metallic palladium (40.1˚) provides the evidence on the formation of alloy particle of Cu and Pd on the Cu-Pd0.2/SiO2 and step-Cu-Pd0.2/SiO2 catalysts |
|
图 3 不同方法制备的Pd-Cu/SiO2双金属催化剂结构示意图. (a) Cu-Pdx/SiO2; (b) step-Cu-Pdx/SiO2.其中白色小球代表铜原子,黑色小球代表钯原子 Figure 3 Structure model of Pd-Cu/SiO2 catalysts synthesized by different methods. (a) Cu-Pdx/SiO2; (b) step-Cu-Pdx/SiO2. The white circles represent the copper atoms, and the black circles represent the palladium atoms |
通过连续浸渍法合成的催化剂step-Cu-Pd0.2/SiO2与Cu-Pd0.2/SiO2具有相同的金属比例,但合成方式不同,最终负载金属结构也不同.合成的纳米颗粒具有如图 3(b)所示的层叠形貌[42].这一结果与XRD表征结果一致.如图 2所示,step-Cu-Pd0.2/SiO2虽也有Pd-Cu合金颗粒衍射峰,但仍有较多的单金属Cu衍射峰存在.这是因为在催化剂合成过程中,首先生成了单金属Cu的纳米颗粒,再引入金属Pd.引入的Pd原子会分布在Cu纳米颗粒表面上形成双金属催化剂,颗粒体相仍主要为Cu,所以XRD中有较强的单金属Cu衍射峰[42].
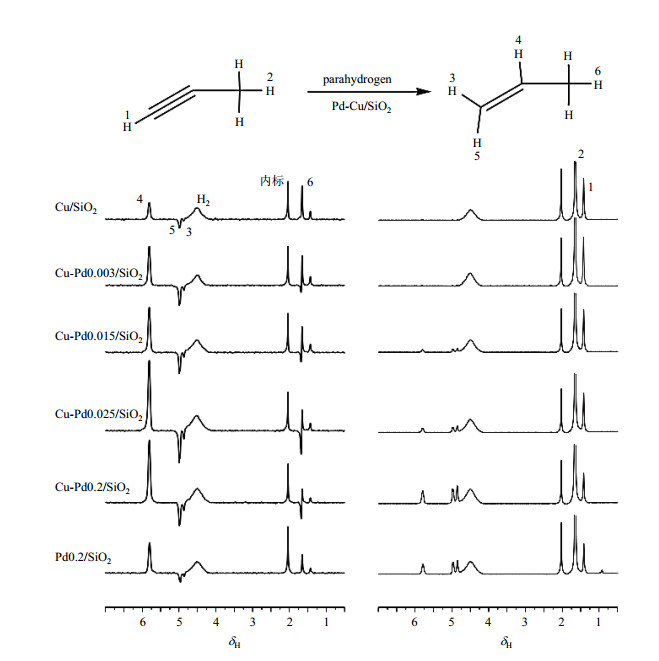
2.2 不同Pd/Cu比例的催化剂上丙炔加氢反应的PHIP极化效率我们利用ALTADENA方法研究了不同Pd/Cu比例催化剂对PHIP极化效率的影响.选择丙炔加氢反应作为研究对象,反应中丙炔可与氢分子发生加成反应选择性的生成产物丙烯,也可以进一步反应生成过还原产物丙烷.图 4所示为不同Pd/Cu比例催化剂催化的反应流出物极化1H NMR谱图(左)和热平衡1H NMR谱图(右).从热平衡1H NMR谱图中,我们可以看到,不同金属比例的催化剂都体现出较好的丙烯选择性,仅单金属Pd0.2/SiO2催化剂观测到少量过还原产物丙烷(δH 0.9)生成.随着催化剂中Pd含量的增大,产物丙烯信号增强,说明反应转化率提高,催化剂活性增强.极化1HNMR谱图谱中,不同催化剂上都展现出一定的极化性能,丙烯分子上氢原子都得到一定的极化.从催化剂Cu-Pd0.003/SiO2开始,随着Pd含量的增加,观测到的极化信号强度先随之增强,在Cu-Pd0.025/SiO2上观测到了极化信号强度的极大值,随后信号强度随着Pd含量的增长反而减弱.
|
图 4 等体积浸渍法制得的不同Pd/Cu比例催化剂催化丙炔加氢反应极化(左)和热平衡(右)1H NMR谱图 Figure 4 Hyperpolarized (left) and thermal polarized (right) 1H NMR spectra of the reaction effluent obtained on catalysts with different Pd/Cu ratios synthesized by isometric impregnation method |
但是,由于观测到的PHIP极化信号强度同时受到信号增强倍数与反应速率两种因素的影响,所以无法直接通过谱图信号强弱来判断不同催化剂的PHIP极化效率大小,只有根据(2)式通过计算处理才能进行判断.丙烯分子中部-CH=基团上氢原子(图 4中的H-4)与其它质子NMR信号不重叠,且极化信号为正向加强,利于统计,所以我们选择该信号来进行信号增强倍数计算.
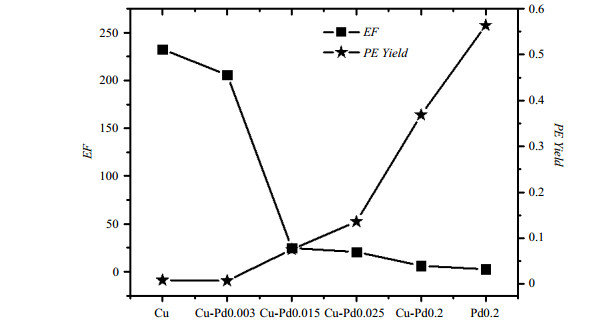
图 5为不同催化剂上丙烯信号增强倍数(EF)和丙烯产率(PE Yield).单金属Cu/SiO2催化剂丙炔产率很低,说明反应活性很差.这是因为金属Cu在吸附解离氢分子时能垒较高,反应难以发生[35, 36].只有极少的氢分子发生了解离,导致参与加氢反应的游离氢原子数量极少,催化剂活性极低.另一方面,正是由于游离氢原子数量极少,使得参与反应的氢原子有更大的概率来自于同一氢分子,从而使得单金属Cu/SiO2催化剂极化效率较高.与此相反,Pd0.2/SiO2催化剂具有很高的催化活性和较低的极化效率.这是因为金属Pd能够有效地解离氢分子,催化加氢反应,从而具有很高的反应活性[18, 19].但氢原子在Pd金属表面容易游离、扩散,使得最终参与加氢反应的氢原子很难保证来自于同一氢分子,不能有效的产生PHIP极化效应.
|
图 5 等体积浸渍法制得的不同Pd/Cu金属比例催化剂催化丙炔加氢反应的信号增强倍数(EF)与丙烯产率(PE Yield),催化剂载体为SiO2 Figure 5 Signal enhancement factor (EF) and yield of propyne (PE Yield) to propene on catalysts with different Pd/Cu ratios synthesized by isometric impregnation method, using SiO2 as support |
图 5中可以看到在等体积浸渍法制得的Pd-Cu双金属催化剂中,随着Pd含量比例增高,催化剂活性增强,PHIP极化效率减弱,催化剂负载金属的组成对极化效率有明显的调节作用.以往的报道中提到,Pd-Cu[42, 45-49],Pd-Ag[50]以及Pd-Au[51, 52]等多种含Pd双金属催化剂中,氢分子都是在孤立的钯原子上解离吸附,随后向四周溢流.通过以上XRD表征可知,在我们用等体积浸渍法制备的双金属催化剂中,纳米颗粒具有均匀混合的合金结构,即Pd组分被Cu组分完全分隔开来[53],形成许多小的Pd集簇. 反应中氢分子首先在Pd表面上裂解活化,生成的氢原子除了少部分能够溢流至金属Cu上,大部分仍停留在金属Pd表面,形成氢池.Pd含量的增多使得裂解的氢分子增多,从而导致催化剂活性增加;同时由于更少的Cu组分参与分割,使得催化剂表面上暴露出来的Pd集簇面积增大[54],形成的氢池面积也更大,氢原子拥有更广泛的活动区域,因此更容易分散,导致丙炔成对加氢的几率变小,PHIP极化效率降低.
2.3 不同形貌催化剂上催化丙炔加氢反应的PHIP极化效率不同制备方法能改变催化剂纳米颗粒的形貌.我们研究了不同合成方式催化剂对PHIP极化效率的影响.已有文献[42]报道,通过连续浸渍法可以合成层叠形貌的Cu-Pd双金属颗粒,形貌如图 3(b)所示.通过等体积浸渍法和连续浸渍法我们分别合成了Cu-Pd比例相同的Cu-Pd0.006/SiO2和step-Cu-Pd0.006/SiO2两种催化剂:Cu-Pd0.006/SiO2催化剂负载纳米颗粒具有均匀混合形貌,钯原子分散在纳米颗粒当中;step-Cu-Pd0.006/SiO2催化剂负载纳米颗粒则为层叠形貌,钯原子主要分布在金属表面.层叠形貌催化剂具有更高的原子利用率,能够极大节省贵金属的使用量.
我们仍然选择丙炔加氢反应来比较两种不同催化剂上PHIP极化效率.图 6(a)为催化剂Cu-Pd0.006/SiO2和step-Cu-Pd0.006/SiO2催化的丙炔加氢反应1H NMR极化图(左)和热平衡图(右),可以看到两种催化剂虽然Cu/Pd金属比例完全一致,但观测到的谱图信号有较大的区别.两种催化剂都表现出较好的丙烯选择性,其中step-Cu-Pd0.006/SiO2在热平衡1H NMR谱中观测到了更强的丙烯产物,说明丙烯产率更高,催化剂活性更强;同时极化1H NMR谱图中也观测到了更强的极化信号.我们计算了两种催化剂上丙烯信号增强倍数和丙烯产率.如图 6(b)所示,催化剂Cu-Pd0.006/SiO2具有较强的PHIP极化效率,但由于催化活性非常低,观测到的信号强度较弱;催化剂step-Cu-Pd0.006/SiO2具有较弱的极化效率,但其催化活性很高,观测到的PHIP极化信号强度更高.我们知道,金属纳米颗粒在催化加氢反应时,往往起到催化作用的是表面的金属原子.Cu-Pd双金属催化剂中,金属Pd起到了解离氢分子的关键作用,直接决定催化剂的活性.step-Cu- Pd0.006/SiO2催化剂中钯原子主要分布在催化剂表面,导致有更多的钯原子参与反应,因而展现出更高的催化活性,使得丙炔产率相比于Cu-Pd0.006/SiO2催化剂提高了16.2倍[图 6(b)],1.5%到24.3%).层叠形貌也导致了催化剂较弱的PHIP极化效率.相较于均匀混合形貌催化剂,同样的Cu-Pd金属比例下层叠形貌催化剂表面钯原子更多,暴露的单个钯面积更大,氢原子游离范围也更广,从而降低了成对加成的概率,导致催化剂极化效率的降低.

|
图 6 (a) 催化剂Cu-Pd0.006/SiO2和step-Cu-Pd0.006/SiO2催化的丙炔加氢反应极化(左)和热平衡(右)1H NMR谱图;(b)不同催化剂催化反应的信号增强倍数(EF)与丙烯产率(PE Yield).质子编号与图 4相同 Figure 6 (a) Hyperpolarized (left) and thermal polarized (right) 1H NMR spectra of the reaction effluent obtained on Cu-Pd0.006/SiO2 and step-Cu-Pd0.006/SiO2 catalysts; (b) Signal enhancement factor (EF) and propene yield (PE Yield) of the reactions. The proton numbers are the same with Fig. 4 |
本文通过HET-PHIP技术研究了Pd-Cu/SiO2双金属催化剂组成与形貌对其催化丙炔加氢反应极化效率的影响.通过等体积浸渍法和连续浸渍法分别合成了一系列不同金属比例、不同形貌的Pd-Cu/SiO2催化剂.发现Pd含量的增加会导致催化剂活性增强,PHIP极化效率减弱;在同Pd/Cu比例下,层叠形貌催化剂具有相对较弱的极化效率以及更强的催化性能.导致这些现象的主要原因都与催化剂负载金属所形成活性位有关,催化剂表面上独立的Pd集簇面积越大,氢原子的成对加成机率越小,极化效率越弱.因此通过合成方法减小Pd集簇的面积将有利于催化剂PHIP极化效率的提高.本工作中对Pd-Cu/SiO2双金属催化剂的研究结果为发展更高效的HET-PHIP催化剂提供了依据.
| [1] | BOWERS C R, WEITEKAMP D P. Transformation of symmetrization order to nuclear-spin magnetization by chemical-reaction and nuclear-magnetic-resonance[J]. Phys Rev Lett, 1986, 57(21): 2645-2648. DOI: 10.1103/PhysRevLett.57.2645. |
| [2] | BOWERS C R, WEITEKAMP D P. Para-hydrogen and synthesis allow dramatically enhanced nuclear alignment[J]. J Am Chem Soc, 1987, 109(18): 5541-5542. DOI: 10.1021/ja00252a049. |
| [3] | EISENSCHMID T C, KIRSS R U, DEUTSCH P P, et al. Para hydrogen induced polarization in hydrogenation reactions[J]. J Am Chem Soc, 1987, 109(26): 8089-8091. DOI: 10.1021/ja00260a026. |
| [4] | REINERI F, AIME S, GOBETTO R, et al. Role of the reaction intermediates in determining PHIP (parahydrogen induced polarization) effect in the hydrogenation of acetylene dicarboxylic acid with the complex[Rh (dppb)](+) (dppb:1, 4-bis(diphenylphosphino) butane)[J]. J Chem Phys, 2014, 140(9): 094307. DOI: 10.1063/1.4867269. |
| [5] | BARSKIY D A, SHCHEPIN R V, COFFEY A M, et al. Over 20% 15N Hyperpolarization in under one minute for metronidazole, an antibiotic and hypoxia probe[J]. J Am Chem Soc, 2016, 138(26): 8080-8083. DOI: 10.1021/jacs.6b04784. |
| [6] | GLOGGLER S, GRUNFELD A M, ERTAS Y N, et al. A nanoparticle catalyst for heterogeneous phase para-hydrogen-induced polarization in water[J]. Angew Chem Int Edit, 2015, 54(8): 2452-2456. DOI: 10.1002/anie.201409027. |
| [7] | WANG W Y, XU J, ZHAO Y X, et al. Facet dependent pairwise addition of hydrogen over Pd nanocrystal catalysts revealed via NMR using para-hydrogen-induced polarization[J]. Phys Chem Chem Phys, 2017, 19(14): 9349-9353. DOI: 10.1039/C7CP00352H. |
| [8] | KOVTUNOV K V, BECK I E, BUKHTIYAROV V I, et al. Observation of parahydrogen-induced polarization in heterogeneous hydrogenation on supported metal catalysts[J]. Angew Chem Int Edit, 2008, 47(8): 1492-1495. DOI: 10.1002/(ISSN)1521-3773. |
| [9] | ZHOU R H, ZHAO E W, CHENG W, et al. Parahydrogen-induced polarization by pairwise replacement catalysis on Pt and Ir nanoparticles[J]. J Am Chem Soc, 2015, 137(5): 1938-1946. DOI: 10.1021/ja511476n. |
| [10] | BARSKIY D A, COFFEY A M, NIKOLAOU P, et al. NMR hyperpolarization techniques of gases[J]. Chem Eur J, 2017, 23(4): 725-751. DOI: 10.1002/chem.201603884. |
| [11] | ZHIVONITKO V V, KOVTUNOV K V, BECK I E, et al. Role of different active sites in heterogeneous alkene hydrogenation on platinum catalysts revealed by means of parahydrogen-induced polarization[J]. J Phys Chem C, 2011, 115(27): 13386-13391. DOI: 10.1021/jp203398j. |
| [12] | ZHOU R H, CHENG W, NEAL L M, et al. Parahydrogen enhanced NMR reveals correlations in selective hydrogenation of triple bonds over supported Pt catalyst[J]. Phys Chem Chem Phys, 2015, 17(39): 26121-26129. DOI: 10.1039/C5CP04223B. |
| [13] | SALNIKOV O G, BURUEVA D B, GERASIMOV E Y, et al. The effect of oxidative and reductive treatments of titania-supported metal catalysts on the pairwise hydrogen addition to unsaturated hydrocarbons[J]. Catal Today, 2017, 283: 82-88. DOI: 10.1016/j.cattod.2016.02.030. |
| [14] | ZHAO E W, ZHENG H B, LUDDEN K, et al. Strong metal-support interactions enhance the pairwise selectivity of parahydrogen addition over Ir/TiO2[J]. ACS Catal, 2016, 6(2): 974-978. DOI: 10.1021/acscatal.5b02632. |
| [15] | KOVTUNOV K V, TRUONG M L, BARSKIY D A, et al. Propane-d(6) heterogeneously hyperpolarized by parahydrogen[J]. J Phys Chem C, 2014, 118(48): 28234-28243. DOI: 10.1021/jp508719n. |
| [16] | KOPTYUG I V, KOVTUNOV K V, BURT S R, et al. Para-hydrogen-induced polarization in heterogeneous hydrogenation reactions[J]. J Am Chem Soc, 2007, 129(17): 5580-5586. DOI: 10.1021/ja068653o. |
| [17] | WANG W Y, HU H, XU J, et al. Tuning Pd-Au bimetallic catalysts for heterogeneous parahydrogen-induced polarization[J]. J Phys Chem C, 2018, 122(2): 1248-1257. DOI: 10.1021/acs.jpcc.7b11801. |
| [18] | KOVTUNOV K V, BECK I E, ZHIVONITKO V V, et al. Heterogeneous addition of H-2 to double and triple bonds over supported Pd catalysts:a parahydrogen-induced polarization technique study[J]. Phys Chem Chem Phys, 2012, 14(31): 11008-11014. DOI: 10.1039/c2cp40690j. |
| [19] | BURUEVA D B, SALNIKOV O G, KOVTUNOV K V, et al. Hydrogenation of unsaturated six-membered cyclic hydrocarbons studied by the parahydrogen-induced polarization technique[J]. J Phys Chem C, 2016, 120(25): 13541-13548. DOI: 10.1021/acs.jpcc.6b03267. |
| [20] | SALNIKOV O G, KOVTUNOV K V, BARSKIY D A, et al. Evaluation of the mechanism of heterogeneous hydrogenation of alpha, beta-unsaturated carbonyl compounds via pairwise hydrogen addition[J]. ACS Catal, 2014, 4(6): 2022-2028. DOI: 10.1021/cs500426a. |
| [21] | HORIUTI I, POLANYI M. Exchange reactions of hydrogen on metallic catalysts[J]. Trans Faraday Soc, 1934, 30: 1164-1172. DOI: 10.1039/tf9343001164. |
| [22] | ZHIVONITKO V V, SKOVPIN I V, CRESPO-QUESADA M, et al. Acetylene oligomerization over Pd nanoparticles with controlled shape:a parahydrogen-induced polarization study[J]. J Phys Chem C, 2016, 120(9): 4945-4953. DOI: 10.1021/acs.jpcc.5b12391. |
| [23] | ZHAO E W, MALIGAL-GANESH R, XIAO C, et al. Silica-encapsulated Pt-Sn intermetallic nanoparticles:a robust catalytic platform for parahydrogen-induced polarization of gases and liquids[J]. Angew Chem Int Edit, 2017, 129(14): 3983-3987. DOI: 10.1002/ange.201701314. |
| [24] | BURUEVA D B, KOVTUNOV K V, BUKHTIYAROV A V, et al. Selective single-site Pd-In hydrogenation catalyst for production of enhanced magnetic resonance signals using parahydrogen[J]. Chem Eur J, 2018, 24(11): 2547-2553. DOI: 10.1002/chem.v24.11. |
| [25] | ZHANG H, JIN M S, XIA Y N. Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd[J]. Chem Soc Rev, 2012, 41(24): 8035-8049. DOI: 10.1039/c2cs35173k. |
| [26] | SHAN S Y, PETKOV V, PRASAI B, et al. Catalytic activity of bimetallic catalysts highly sensitive to the atomic composition and phase structure at the nanoscale[J]. Nanoscale, 2015, 7(45): 18936-18948. DOI: 10.1039/C5NR04535E. |
| [27] | WU J F, SHAN S Y, LUO J, et al. PdCu nanoalloy electrocatalysts in oxygen reduction reaction:role of composition and phase state in catalytic synergy[J]. Acs Appl Mater Inter, 2015, 7(46): 25906-25913. DOI: 10.1021/acsami.5b08478. |
| [28] | LOUKRAKPAM R, SHAN S Y, PETKOV V, et al. Atomic ordering enhanced electrocatalytic activity of nanoalloys for oxygen reduction reaction[J]. J Phys Chem C, 2013, 117(40): 20715-20721. DOI: 10.1021/jp4067444. |
| [29] | CASTEGNARO M V, GORGESKI A, BALKE B, et al. Charge transfer effects on the chemical reactivity of PdxCu1-x nanoalloys[J]. Nanoscale, 2016, 8(1): 641-647. DOI: 10.1039/C5NR06685A. |
| [30] | SHI W W, CHEN X Q, XU S Y, et al. Highly efficient PdCu3 nanocatalysts for Suzuki-Miyaura reaction[J]. Nano Res, 2016, 9(10): 2912-2920. DOI: 10.1007/s12274-016-1176-9. |
| [31] | LIU J, ZHU Y N, LIU C, et al. Excellent selectivity with high conversion in the semihydrogenation of alkynes using palladium-based bimetallic catalysts[J]. Chemcatchem, 2017, 9(21): 4053-4057. DOI: 10.1002/cctc.v9.21. |
| [32] | MARKOV P V, BRAGINA G O, BAEVA G N, et al. Pd-Cu catalysts from acetate complexes in liquid-phase diphenylacetylene hydrogenation[J]. Kinet Catal, 2015, 56(5): 591-597. DOI: 10.1134/S0023158415050122. |
| [33] | LIU Y A, HE Y F, ZHOU D R, et al. Catalytic performance of Pd-promoted Cu hydrotalcite-derived catalysts in partial hydrogenation of acetylene:effect of Pd-Cu alloy formation[J]. Catal Sci Technol, 2016, 6(9): 3027-3037. DOI: 10.1039/C5CY01516B. |
| [34] | RAVANCHI M T, SAHEBDELFAR S, KOMEILI S. Acetylene selective hydrogenation:a technical review on catalytic aspects[J]. Rev Chem Eng, 2018, 34(2): 215-237. DOI: 10.1515/revce-2016-0036. |
| [35] | BRIDIER B, LOPEZ N, PEREZ-RAMIREZ J. Partial hydrogenation of propyne over copper-based catalysts and comparison with nickel-based analogues[J]. J Catal, 2010, 269(1): 80-92. DOI: 10.1016/j.jcat.2009.10.019. |
| [36] | WEHRLI J T, THOMAS D J, WAINWRIGHT M S, et al. Selective hydrogenation of propyne over an ion-exchanged copper on silica catalyst[J]. Appl Catal, 1990, 66(1): 199-208. DOI: 10.1016/S0166-9834(00)81638-3. |
| [37] | BOND G C. Catalysis by metals[M]. Academic Press, 1962. |
| [38] | JOICE B J, ROONEY J J, WELLS P B, et al. Nature and reactivity of intermediates in hydrogenation of buta-13-diene catalyzed by cobalt and palladium-gold alloys[J]. Discuss Faraday Soc, 1966(41): 223-236. |
| [39] | RUSHFORD H G, WHAN D A. Catalytic hydrogenation of but-2-yne on palladium-gold alloys[J]. Trans Faraday Soc, 1971, 67(588): 3577-3584. |
| [40] | BOND G C, RAWLE A F. Catalytic hydrogenation in the liquid phase. Part 1. Hydrogenation of isoprene catalysed by palladium, palladium-gold and palladium-silver catalysts[J]. J Mol Cata A-Chem, 1996, 109(3): 261-271. DOI: 10.1016/1381-1169(96)00027-1. |
| [41] | PEI G X, LIU X Y, YANG X F, et al. Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions[J]. ACS Catal, 2017, 7(2): 1491-1500. DOI: 10.1021/acscatal.6b03293. |
| [42] | MCCUE A J, GIBSON A, ANDERSON J A. Palladium assisted copper/alumina catalysts for the selective hydrogenation of propyne, propadiene and propene mixed feeds[J]. Chem Eng J, 2016, 285: 384-391. DOI: 10.1016/j.cej.2015.09.118. |
| [43] | PRAVICA M G, WEITEKAMP D P. Net NMR alignment by adiabatic transport of parahydrogen addition products to high magnetic field[J]. Chem Phys Lett, 1988, 145(4): 255-258. DOI: 10.1016/0009-2614(88)80002-2. |
| [44] | ZHANG J Y, FENG A N, BAI J, et al. One-pot synthesis of hierarchical flower-like Pd-Cu alloy support on graphene towards ethanol oxidation[J]. Nanoscale Res Lett, 2017, 12(1): 521. DOI: 10.1186/s11671-017-2290-7. |
| [45] | BOUCHER M B, ZUGIC B, CLADARAS G, et al. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions[J]. Phys Chem Chem Phys, 2013, 15(29): 12187-12196. DOI: 10.1039/c3cp51538a. |
| [46] | MCCUE A J, ANDERSON J A. CO induced surface segregation as a means of improving surface composition and enhancing performance of CuPd bimetallic catalysts[J]. J Catal, 2015, 329: 538-546. DOI: 10.1016/j.jcat.2015.06.002. |
| [47] | MCCUE A J, MCRITCHIE C J, SHEPHERD A M, et al. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation[J]. J Catal, 2014, 319: 127-135. DOI: 10.1016/j.jcat.2014.08.016. |
| [48] | MCCUE A J, SHEPHERD A M, ANDERSON J A. Optimisation of preparation method for Pd doped Cu/Al2O3 catalysts for selective acetylene hydrogenation[J]. Catal Sci Technol, 2015, 5(5): 2880-2890. DOI: 10.1039/C5CY00253B. |
| [49] | TIERNEY H L, BABER A E, KITCHIN J R, et al. Hydrogen dissociation and spillover on individual isolated palladium atoms[J]. Phys Rev Lett, 2009, 103(24): 246102. DOI: 10.1103/PhysRevLett.103.246102. |
| [50] | PEI G X, LIU X Y, WANG A Q, et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene[J]. ACS Catal, 2015, 5(6): 3717-3725. DOI: 10.1021/acscatal.5b00700. |
| [51] | MCCUE A J, BAKER R T, ANDERSON J A. Acetylene hydrogenation over structured Au-Pd catalysts[J]. Faraday Discuss, 2016, 188: 499-523. DOI: 10.1039/C5FD00188A. |
| [52] | PEI G X, LIU X Y, WANG A Q, et al. Promotional effect of Pd single atoms on Au nanoparticles supported on silica for the selective hydrogenation of acetylene in excess ethylene[J]. New J Chem, 2014, 38(5): 2043-2051. DOI: 10.1039/c3nj01136d. |
| [53] | TIRUVALAM R C, PRITCHARD J C, DIMITRATOS N, et al. Aberration corrected analytical electron microscopy studies of sol-immobilized Au plus Pd, Au{Pd} and Pd{Au} catalysts used for benzyl alcohol oxidation and hydrogen peroxide production[J]. Faraday Discuss, 2011, 152: 63-86. DOI: 10.1039/c1fd00020a. |
| [54] | GAO F, GOODMAN D W. Pd-Au bimetallic catalysts:understanding alloy effects from planar models and (supported) nanoparticles[J]. Chem Soc Rev, 2012, 41(24): 8009-8020. DOI: 10.1039/c2cs35160a. |
 2018, Vol. 35
2018, Vol. 35 
