2. Functional Molecules Analysis and Biotransformation Key Laboratory of Universities in Yunnan Province, School of Chemical Science and Technology, Yunnan University, Kunming 650091, China
2. 云南大学 化学科学与工程学院, 云南省高校功能分子分析与生物转化重点实验室, 云南 昆明 650091
Aconitum handelianum Comber (Ranunculaceae), a perennial herb, is endemic to Jianchuan County in Yunnan Province of China. The roots of A. handelianum have long been utilized to treat various pains by the native[1, 2]. Diterpenoid alkaloids, which possess complex structures and varied biological activities, have been reported to be the characteristic bioactive ingredients in this plant[3, 4]. In recent years, the research of diterpenoid alkaloids has grown markedly due to the development and application of the nuclear magnetic resonance (NMR) techniques[5]. This paper deals with the structural elucidation and NMR spectral assignments of two C19-diterpenoid alkaloids isolated from the roots of A. handelianum (Fig. 1). Homochasmanine (compound 1) is an aconitine-type C19-diterpenoid alkaloid with a methoxy group rarely substituted at C-8. To our knowledge, no complete NMR spectral assignment of compound 1 has been undertaken[6-8]. 14-debenzoylfranchetine (compound 2) is a franchetine-type C19-diterpenoid alkaloid, whose 13C NMR data had been assigned previously but the complete 1H NMR data were not reported in literatures[9, 10]. To provide more important NMR spectroscopic data for structure determination of diterpenoid alkaloids, the NMR signals of compounds 1 and 2 have been full assigned accurately by the extensive NMR spectroscopic analyses (i.e. 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HSQC, HMBC and NOESY)[11, 12].
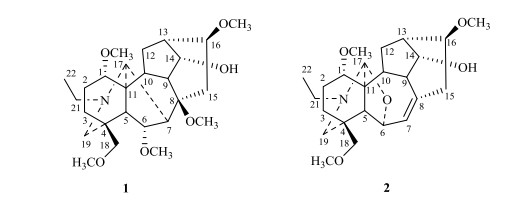
|
Figure 1 Structures of homochasmanine (compound 1) and 14-debenzoylfranchetine (compound 2) |
1H NMR (400.13 MHz, CDCl3) and 13C NMR (100.06 MHz, CDCl3) spectra were acquired with a Bruker AM-400 spectrometer using tetramethylsilane (TMS) as the internal reference, and 2D NMR (400.13 MHz, CDCl3) spectra were recorded at standard conditions.
High resolution mass spectrum with electronic spray ionizer (HR-ESI-MS) were recorded with Agilent G3250AA (Agilent, Santa Clara, USA) and Auto Spec Premier P776 spectrometer (Waters, Milford, USA). Silica gel (300~400 mesh; Qingdao Haiyang, Qingdao, China) and Sephadex LH-20 (GE Healthcare, Fairfield, USA) were used for column chromatography (CC).
Fractions were monitored by thin layer chromatography (TLC) and visualized by spraying with modified Dragendorff's reagent.
A Nicolet Magna-IR 550 spectrometer was used for scanning IR spectroscopy with KBr pellets.
1.2 Plant materialRoots of A. handelianum were collected from Jianchuan County in Yunnan Province of China in September 2013 and identified by professor Lu Shu-gang, School of Life Sciences, Yunnan University. A voucher specimen (2013-hcw-1) is deposited in the Key Laboratory of Medicinal Chemistry for Natural Resources, Ministry of Education, School of Chemical Science and Technology, Yunan University.
1.3 Extraction and isolationAir-dried and powdered roots (2.0 kg) of A. handelianum were percolated with 0.5% HCl. The aqueous acidic solution was basified with 10% ammonia to pH 9.0 and then extracted with ethyl acetate. Removal of the solvent under reduced pressure afforded the total crude alkaloids (15.0 g) as yellowish amorphous powder. The total alkaloids were subjected to silica gel CC eluted with CHCl3/CH3OH gradient system [V(CHCl3):V(CH3OH)=100:1~1:1] to give eight fractions (FrA~FrH). FrA (0.4 g) was further subjected to silica gel CC [V(petroleum ether):V(acetone):V(diethylamine)=100:2:1~100:10:1) to yield compound 1 (12 mg). FrG (1.3 g) was subjected to silica gel CC [V(CHCl3):V(CH3OH)=20:1~5:1) to yield compound 2 (23 mg).
2 Results and discussion 2.1 Structural elucidation and NMR spectral assignment of homochasmanine (compound 1)Compound 1 was isolated as a white powder and its molecular formula was deduced to be C26H43NO6 by HR-ESI-MS at m/z of 466.316 3 [M+H]+ (calculated for C26H43NO6, 466.316 9) with an unsaturation degree of six. IR (KBr, cm−1): νmax 3 454, 2 972, 2 818, 1 621, 1 454, 1 377, 1 098.
The NMR spectra displayed signals of an N-ethyl group [δH 1.05 (3H, t, J = 7.2 Hz); δC 13.7 (q), 49.2 (t)] and five methoxy groups [δH 3.22 (3H, s), 3.24 (3H, s), 3.29 (3H, s), 3.31 (3H, s), 3.35 (3H, s); δC 48.8 (q), 56.3 (q), 56.5 (q), 58.9 (q), 59.2 (q)]. The 13C NMR, DEPT and HSQC spectra revealed compound 1 contains nineteen carbons except for the N-ethyl and methoxyl groups, including six methylenes, ten methines and three quaternary carbons. The data summarized above, in combination with biogenetic consideration, suggested that compound 1 might be an aconitine-type C19-diterpenoid alkaloid[13, 14].
The 13C NMR spectrum revealed six oxygenated carbons [δC 75.2 (d), 78.6 (s), 80.4(t), 82.5 (d), 83.1 (d), 85.8 (d)], corresponding to the molecular formula, which indicated the presence of one hydroxyl and five methoxy groups. The characteristic methylene [δH 3.67, 3.09 (ABq, J = 8.0 Hz)] was undoubtedly attributed to H-18, indicating a methoxy group substituted at C-18, which was confirmed by HMBC correlation of OCH3-18/C-18 (Fig. 2). The HMBC correlations of OCH3-1/C-1, H-1/C-5, H-1/C-10, H-1/C-11 suggested a methoxy group was placed at C-1 (Fig. 3). Besides, the chemical shift of C-1 [δC 85.8 (d)] was in accordance with the typical OCH3-1α compounds chasmanine [δC 86.2 (d)] or talatisamine [δC 86.1 (d)]. Another two methoxy groups were placed at oxygenated methines C-6 and C-16 on the basis of HMBC correlations of OCH3-6/C-6 and OCH3-16/C-16, respectively. In addition, NOESY correlations of H-1β/H-5β, H-6β/H-9β, and H-13β/OCH3-16 demonstrated the α-orientation of OCH3-1, α-orientation of OCH3-6, and β-orientation of OCH3-16, respectively. A methoxy group substituted at oxygenated quaternary carbon C-8 was confirmed by the HMBC correlations of OCH3-8/C-8 and H-10/C-8, which was supported by the chemical shift of OCH3-8 [δC 48.8 (q)], which was upfield approximately δ 8~10 when compared with methoxy groups substituted at methines. Finally, the signal at δH 4.12 (m) was attributed to H-14β, suggesting the presence of α-orientation of OH [15], which was further supported by the NOESY correlation of H-10β/H-14β. Therefore, the structure of compound 1 was determined.

|
Figure 2 Key 1H-1H COSY (   |
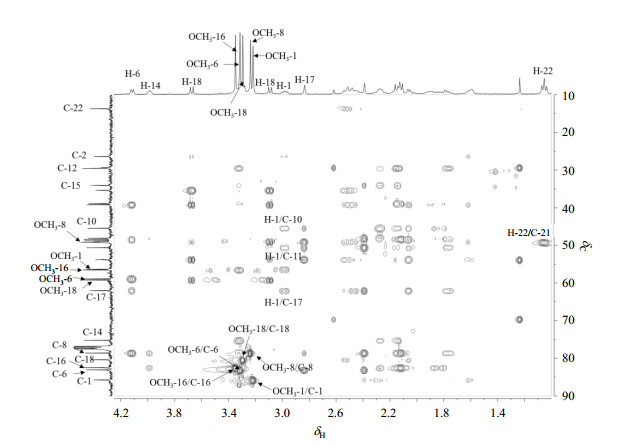
|
Figure 3 HMBC spectrum of compound 1 |
All 1H and 13C NMR signals of compound 1 were assigned based on its 2D NMR spectra (Table 1).
| Table 1 1H NMR, 13C NMR, HSQC, 1H-1H COSY, HMBC and NOESY data of compound 1 in CDCl3 |
Compound 2 was isolated as a white powder, whose molecular formula was deduced to be C24H37NO5 by HR-ESI-MS at m/z of 420.274 4 [M+H]+ (calculated for C24H37NO5, 420.275 0) with an unsaturation degree of seven. IR (KBr, cm−1): νmax 3 432, 2 927, 2 316, 1 723, 1 454, 1 371, 1 097.
The NMR spectra displayed signals of an N-ethyl group [δH 1.00 (3H, t, J = 7.2 Hz); δC 13.3 (q), 49.3 (t)], three methoxy groups [δH 3.29 (3H, s), 3.32 (3H, s), 3.33 (3H, s); δC 56.4 (q), 57.4 (q), 59.7 (q)], a characteristic trisubstituted double bond [δH 5.69 (1H, brs); δC 128.6 (d), 137.6 (s)] and an N, O-mixed ketal moiety [δH 4.33 (1H, s); δC 92.6 (d)]. The double bond accounts for one degree of unsaturation, indicating the presence of six rings in compound 2. The 13C NMR, DEPT and HSQC spectra revealed compound 2 contains nineteen carbons except for the N-ethyl and methoxyl groups, including six methylenes, ten methines and three quaternary carbons. The data summarized above, in combination with biogenetic consideration, suggested that compound 2 might be a franchetine-type C19-diterpenoid alkaloid[16].
The HMBC correlations of H-17/C-1 and H-6/C-17 further confirmed the presence of N, O-mixed ketal moiety (Fig. 2). The trisubstituted double bond was placed at C-7 and C-8 on the basis of HMBC correlations of H-5/C-7, H-6/C-7, H-10/C-8 and H-15/C-8 (Fig. 4). The 13C NMR spectrum revealed six oxygenated carbons [δC 85.0 (d), 75.1 (d), 77.5 (d), 85.0 (d), 92.6 (d), 79.4 (t)], which indicated the presence of one hydroxyl group except for the methoxy groups and N, O-mixed ketal moiety. Three methoxy groups were located at C-1, C-16 and C-18 on the basis of HMBC correlations of OCH3-1/C-1, OCH3-16/C-16 and OCH3-18/C-18, respectively. The NOESY correlations of H-1β/H-5β and H-13β/OCH3-16 demonstrated the α-orientation of OCH3-1 and β-orientation of OCH3-16, respectively. Finally, the broad singlet at δH 4.17 was attributed to H-14β, suggesting the presence of α-orientation of OH-14, which was further supported by the NOESY correlation of H-10β/H-14β[17, 18]. Therefore, the structure of compound 2 was determined.
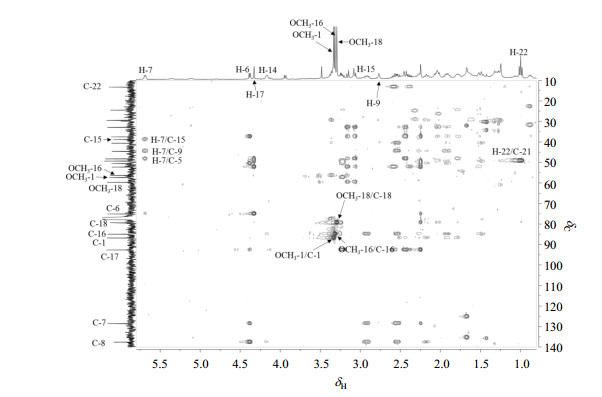
|
Figure 4 HMBC spectrum of compound 2 |
All 1H and 13C NMR signals of compound 2 were assigned based on its 2D NMR spectra (Table 2).
| Table 2 1H NMR, 13C NMR, HSQC, 1H-1H COSY, HMBC and NOESY data of compound 2 in CDCl3 |
The structural elucidation and full NMR spectral assignments of two C19-diterpenoid alkaloids homochasmanine (1) and 14-debenzoylfranchetine (2), which were isolated from the roots of A. handelianum, were accomplished by extensive spectroscopic analyses (1H NMR, 13C NMR, DEPT, 1H-1H COSY, HSQC, HMBC and NOESY). The present study provides important references for the structure determination of diterpenoid alkaloids.
| [1] | 王文采. 中国植物志(27卷)[M]. 北京: 科学出版社, 1979. |
| [2] | 大理白族自治州人民政府. 大理中药资源志[M]. 昆明: 云南民族出版社, 1991. |
| [3] | YIN T P, CAI L, XING Y, et al. Alkaloids with antioxidant activities from Aconitum handelianum[J]. J Asian Nat Prod Res, 2016, 18(6): 603-610. DOI: 10.1080/10286020.2015.1114473. |
| [4] |
YANG J, LIU W, YANG X D, et al. Diterpenoid alkaloids from Aconitum handelianum[J].
China J Chin Mater Med, 2009, 15: 1927-1929.
杨金, 刘卫, 羊晓东, 等. 剑川乌头中的二萜生物碱[J]. 中国中药杂志, 2009, 15: 1927-1929. DOI: 10.3321/j.issn:1001-5302.2009.15.012. |
| [5] | WANG F P, CHEN Q H, LIU X Y. Diterpenoid alkaloids[J]. Nat Prod Rep, 2010, 27(4): 529-570. DOI: 10.1039/b916679c. |
| [6] | ACHMATOWICZ J O, MARION L. The structure of homochasmanine[J]. Can J Chem, 1965, 43(5): 1093-1095. DOI: 10.1139/v65-145. |
| [7] | PELLETIER S W, DIARMATI Z, LAJSIC S. Structure of neoline, chasmanine, and homochasmanine[J]. J Am Chem Soc, 1974, 96(25): 7817-7818. DOI: 10.1021/ja00832a038. |
| [8] | PELLETIER S W, DIARMATI Z, LAJSIC S, et al. Alkaloids of Delphinium staphisagria. The structure and stereochemistry of delphisine, neoline, chasmanine, and homochasmanine[J]. J Am Chem Soc, 1976, 98(9): 2617-2625.A. DOI: 10.1021/ja00425a035. |
| [9] |
PENG C S, DAI X P, CHEN D L, et al. Chemical study on the alkaloids of Aconitum hemslevanum Pritz var. pengshinese[J].
Nat Prod Res Dev, 1999, 11(3): 23-26.
彭崇胜, 代雪平, 陈东林, 等. 彭什藤乌中生物碱的化学研究[J]. 天然产物研究与开发, 1999, 11(3): 23-26. |
| [10] |
XIE G B, WANG F P. Chemical study on the norditerpenoid alkaloids of Aconitum iljestrandii[J].
Nat Prod Res Dev, 2002, 14(1): 32-34.
谢光波, 王锋鹏. 贡嘎乌头中生物碱成分研究[J]. 天然产物研究与开发, 2002, 14(1): 32-34. |
| [11] |
SUN W, GAO X, GUO J, et al. An NMR study on lomustine[J].
Chinese J Magn Reson, 2016, 33(2): 353-359.
孙伟, 高翔, 郭娟, 等. 洛莫司汀的1H, 13C NMR全归属[J]. 波谱学杂志, 2016, 33(2): 353-359. DOI: 10.11938/cjmr20160217. |
| [12] |
LIU Y Q, HE L, YU M X. An NMR spectroscopy study on peptidomimetics cyclo[-RGD-ψ(triazole)-GD-][J].
Chinese J Magn Reson, 2016, 33(2): 337-344.
刘雅琴, 何玲, 余明新. 新型拟肽cyclo[-RGD-ψ(triazole)-GD-]波谱学数据与结构确证[J]. 波谱学杂志, 2016, 33(2): 337-344. DOI: 10.11938/cjmr20160215. |
| [13] |
WANG F P. 13C nuclear magnetic resonance of diterpenoid alkaloids[J].
Chinese J Org Chem, 1982, 3: 161-169.
王锋鹏. 二萜生物碱的13C核磁共振谱[J]. 有机化学, 1982, 3: 161-169. |
| [14] | YIN T P, CAI L, FANG H X, et al. Diterpenoid alkaloids from Aconitum vilmorinianum[J]. Phytochem, 2015, 116: 314-319. DOI: 10.1016/j.phytochem.2015.05.002. |
| [15] | YIN T P, CAI L, HE J M, et al. Three new diterpenoid alkaloids from the roots of Aconitum duclouxii[J]. J Asian Nat Prod Res, 2014, 16(4): 345-350. DOI: 10.1080/10286020.2014.881802. |
| [16] | WANG F P, LI Z B, DAI X P, et al. Structural revision of franchetine and vilmorisine, two norditerpenoid alkaloids from the roots of Aconitum spp.[J]. Phytochem, 1997, 45(7): 1539-1542. DOI: 10.1016/S0031-9422(97)00164-7. |
| [17] | WANG D P, LOU H Y, HUANG L, et al. A novel franchetine type norditerpenoid isolated from the roots of Aconitum carmichaeli Debx. with potential analgesic activity and less toxicity[J]. Bioorg Med Chem Lett, 2012, 22(13): 4444-4446. DOI: 10.1016/j.bmcl.2012.04.132. |
| [18] |
WEI W, LI X W, YANG R J, et al. Structural elucidation of three monoester-diterpenoid aconines by NMR spectroscopy[J].
Chinese J Magn Reson, 2010, 27(2): 238-248.
魏巍, 李绪文, 杨瑞杰, 等. 3种单酯型乌头碱的NMR研究[J]. 波谱学杂志, 2010, 27(2): 238-248. |
 2018, Vol. 35
2018, Vol. 35 
