2. University of Chinese Academy of Sciences, Beijing 100049, China
2. 中国科学院大学, 北京 100049
The B1 immunoglobulin-binding domain of staphylococcal protein G (GB1) is a small, 56-residues, stable globular protein. It consists of a four-stranded β-sheets spanned by an α-helix[1]. It has been widely used as a research model for developing protein structural determination methods[2-11] and studying protein stability, dynamics and folding[12-18].
Taking GB1 as the research model, various paramagnetic metal-tags have been developed to help obtain significant distance and orientation restraints for in vitro and in vivo protein structural determination[2-11]. For instance, paramagnetic EDTA-Mn2+, EDTA-Cu2+ and TETAC-Cu2+ were introduced to GB1 to help obtain longitudinal nuclear paramagnetic relaxation enhancement (PRE)[2-6, 8, 9], which can provide long-range distance restraints for structural determination. The paramagnetic lanthanide-tag DOTA-M7Py and 4PhSO2-PyMTA were introduced to GB1 to help obtain pseudocontact chemical shifts (PCSs) and residual dipolar couplings (RDCs) data for in-cell protein structural determination based on PCS and RDC, which can provide nuclear distance and orientation restraints[10, 11].
Recently, it has been reported that there exist intrinsic weak Cu2+ binding sites in GB1, which resulted in anomalous increase of the longitudinal 15N PREs of residues involved[4]. It implies that it is necessary to investigate the binding of GB1 with other ions, especially lanthanide ions, in order to ensure reliability of paramagnetic nuclear magnetic resonance (NMR) data such as PCSs, PREs and RDCs based on paramagnetic metal-tags. Herein, we characterized the interaction of GB1 with lanthanide as well as divalent metal ions by NMR spectroscopy.
1 Materials and methods 1.1 Protein expression and purificationPlasmids containing the coding sequence of GB1 were transformed into Escherichia coli strain BL21 (DE3) competent cells. Expression and purification of 15N labeled GB1 were performed as previously described[19]. The purified protein was passed through a HiPrep 26/10 desalting column (GE Healthcare) equilibrated and eluted with Milli-Q water. The eluted protein was lyophilized and stored as aliquots at -20 ℃. The concentration of protein was determined by A280 nm (using a molar extinction coefficient of 9 970 L·mol-1·cm-1.
1.2 Chemical reagentsTmCl3·6H2O and LuCl3·6H2O from Sigma-Aldrich Co. or Alfa Aesar Co., Ltd. were used without further purification. CuSO4, MnCl2, MgSO4, CoSO4, CaCl2 and ZnSO4 salts of the highest purity available were purchased from Merck Co., Ltd. or Sigma-Aldrich Co. All the other chemicals of analytical grade were purchased from Sinopharm Chemical Reagent Co., Ltd.
1.3 NMR experimentsThe lyophilized protein was dissolved in NMR buffer [containing 20 mmol/L MES, V(H2O)/V(D2O) = 9/1, pH 6.5)] with final concentration of 0.20 mmol/L. 1H-15N HSQC spectra were acquired on Bruker 850 MHz or 600 MHz spectrometers equipped with a triple-resonance cryoprobe at 298 K. The HSQC spectra were acquired with sweep widths of 11 904.7 Hz (1H) and 3 446.8 Hz (15N), sampling points of t2 (1H)×t1 (15N) = 1 024×128, 4 scans per t1 point, and recycle delay of 1.0 s.
1.4 Dissociation constant determinationDissociation constant (KD) was determined from the titration curve according to equation (1) as follows[20], where Δδ is the measured chemical shift perturbation at each ion concentration, Δδmax is the maximum chemical shift perturbation, [P]t is the total protein concentration, and [L]t is the total ion concentration.
| $ \begin{array}{l} \mathit{\Delta }\delta = \mathit{\Delta }{\delta _{\max }}\left\{ {{K_D} + {{[P]}_t} + {{[L]}_t} - [{{({K_D} + {{[P]}_t} + {{[L]}_t})}^2} - {{(4 \cdot {{[P]}_t} \cdot {{[L]}_t})}^{1/2\;}}]} \right\}\\ /(2\;{[P]_t}) \end{array} $ | (1) |
In the backbone HN chemical shift perturbation versus residue plots, the combined HN perturbation (ΔδHN) is calculated as follows, where ΔδH and ΔδN in equation (2)[21-23] are the chemical shift differences of proton and nitrogen nuclei, respectively.
| $ \mathit{\Delta }{{\delta }_{\rm{HN}}}=\sqrt{\frac{1}{2}(\mathit{\Delta } \delta _{\rm{H}}^{2}+\frac{\mathit{\Delta } \delta _{\rm{N}}^{2}}{25})} $ | (2) |
The 1H-15N HSQC spectra were used to examine the binding sites of metal ions to GB1. Fig. 1(a) shows the HSQC spectra of GB1 titrated with diamagnetic Lu3+. Cross peaks shifting with Lu3+ addition was observed, suggesting that Lu3+ can bind to GB1. KD values were obtained by individually fitting the titration curve from backbone amide resonances with significant chemical shifts perturbations (CSP) according to equation (1). Fig. 1(b) shows the fitting curves of representative 6 residues. Fig. 1(c) shows KD values for 14 residues of which chemical shift changes in titration are more than 0.04. The mean KD value is about 0.18 mmol/L.
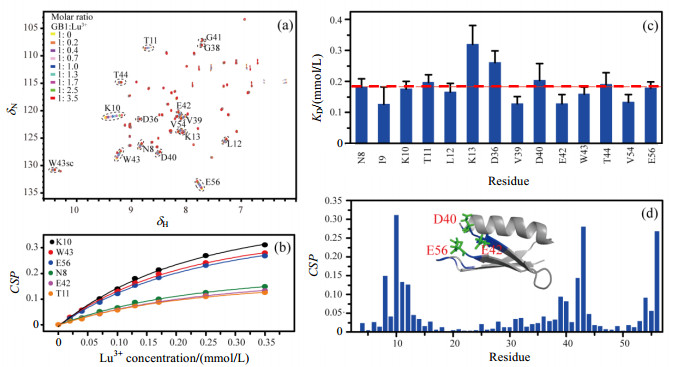
|
Figure 1 The binding of Lu3+ to GB1. (a) 1H-15N HSQC spectra of GB1 titrated with different molar ratio Lu3+; (b) Fitting curves of 6 residues to determine KD for GB1 and Lu3+; (c) KD values for 14 residues of which chemical shift changes in titration are more than 0.04. The red dash line indicates the mean KD value for all the 14 residues; (d) Backbone amide CSP of GB1 when titrated with 3.5 equivalents of Lu3+. The residues with large CSP are colored blue in the structure of GB1 (PDB 3GB1). The possible binding sites of Lu3+ in GB1 are shown using green sticks |
Fig. 1(d) shows the CSP of GB1 titrated with 3.5 equivalents of Lu3+. The larger chemical shift perturbations mainly come from residues N8-K13, V39-W43 and V54-E56. We speculate the possible binding sites of Lu3+ in GB1 are negatively charged residues D40, E42 and E56.
We also acquired HSQC spectra of GB1 titrated with lanthanide paramagnetic Tm3+ (Fig. 2). Fig. 2(a) shows the HSQC spectra of GB1 in the presence of Tm3+ (red) and Lu3+ (blue), respectively. Tm3+ has anisotropic magnetic susceptibility tensor and thus can cause both PCSs and PRE[24]. Therefore, in the presence of Tm3+, it was observed that the cross peaks of some residues shifted or disappeared in HSQC spectrum. Taking Lu3+ as a diamagnetic reference[24], PCSs of backbone amide 1H induced by Tm3+ were obtained [Fig. 2(b)]. The residues with larger PCSs are mainly located in the first (residues N8-L12), the third (residues N37-G41) and the fifth loop (residues T53-E56) of GB1. Magnetic susceptibility anisotropy values were obtained by fitting PCSs to the structure of GB1 (PDB 3GB1[25]) by using Numbat software[26]. Fig. 2(c) shows the correlations between experimental and back-calculated PCSs from which the consistency of PCSs can be observed, implying that the binding with lanthanide metal ions Lu3+ or Tm3+ don't greatly disturb the structure of GB1.
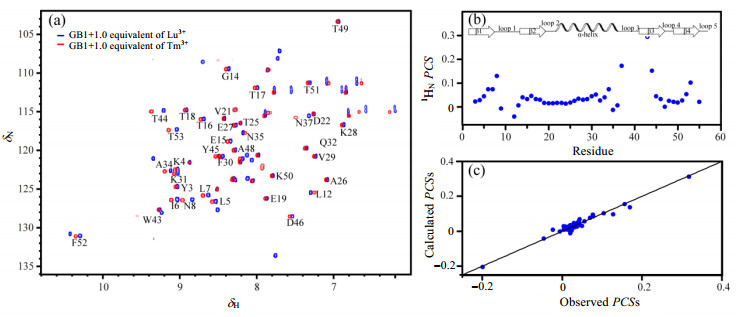
|
Figure 2 The binding of Tm3+ to GB1. (a) 1H-15N HSQC spectra of GB1 in the presence of Tm3+ (red) and Lu3+ (blue), respectively; (b) PCSs of GB1 induced by Tm3+; (c) Observed vs. back-calculated PCSs for GB1. The back-calculated PCSs was obtained using PDB 3GB1 structure |
In all, the lanthanide metal ions Lu3+ and Tm3+ have weak binding affinity with GB1. The dissociation constant for GB1 and Lu3+ is about 0.18 mmol/L. The possible binding sites are residues D40, E42 and E56. Therefore, for GB1, the paramagnetic metal-tags with high binding affinity are needed to avoid possible interference to paramagnetic NMR data.
2.2 Divalent metal ions binding sites determinationBeside lanthanide metal ions, the binding of divalent metal ions with GB1 were also investigated. Fig. 3(a) shows the HSQC spectra of GB1 in the presence of Ca2+ (green), Mg2+ (blue) and Zn2+ (purple) at equal molar ratio, respectively. Compared to GB1, no chemical shifts changes were observed in the spectra after adding these ions, suggesting that GB1 probably can not bind with these three ions.

|
Figure 3 The binding of divalent metal ions to GB1. (a) 1H-15N HSQC spectra of GB1 in the absence and presence of Ca2+, Mg2+ and Zn2+, respectively; (b) 1H-15N HSQC spectra of GB1 titrated with Mn2+; (c) 1H-15N HSQC spectra of GB1 of titrated with Mg2+ in the presence of Mn2+; (d) 1H-15N HSQC spectra of GB1 of titrated with Zn2+ in the presence of Mn2+; (e) 1H-15N HSQC spectra of GB1 titrated with Co2+; (f) 1H-15N HSQC spectra of GB1 titrated with Cu2+ |
Fig. 3(b) shows the HSQC spectra of GB1 in the presence of paramagnetic Mn2+ at 0.2/1, 0.6/1 and 1/1 molar ratio of Mn2+/GB1, respectively. Compared to GB1, in the presence of Mn2+, the cross peaks of some residues, such as K10, G38, V39, D40, G41, E42, W43 and E56, severely broadened and even disappeared due to PRE effects. The resonance from D40 disappears at 0.2/1 molar ratio of Mn2+/GB1, suggesting that Mn2+ binding site in GB1 may be in the region near D40 residue. Chemical shifts induced by Mn2+ binding have not been observed, suggesting that the binding of Mn2+ with GB1 is weak and have little impact on GB1 structure.
We also performed NMR titration of Mg2+ and Zn2+ in the presence of Mn2+, respectively [Fig. 3(c) and 3(d)]. No changes were observed in the spectra with Mg2+ and Zn2+ addition, suggesting that neither Mg2+ nor Zn2+ can substitute for Mn2+ to bind with GB1, which is consistent with the observation on Mg2+ and Zn2+ titration.
Fig. 3(e) shows the HSQC spectra of GB1 titrated with paramagnetic Co2+. Compared to GB1, the cross peaks of residues G38, V39, D40, G41 and E56 shifted a little due to PCSs and binding. Only the cross peak of residue V39 exhibited a decrease in peak intensity, probably due to the PRE effect. The observations suggest that Co2+ has a very low binding affinity with GB1.
Fig. 3(f) shows the HSQC spectra of GB1 titrated with paramagnetic Cu2+. Compared to GB1, the cross peaks of some residues, such as Y3, E19, A20, V21, D22, A26, D40, G41, E42 and E56, severely broadened or disappeared due to PRE effects with Cu2+ addition. The cross peaks of some residues, such as K4, K10, T18 and E56, shifted a little due to PCS effects. By analyzing the distribution of these residues in GB1 structure, we speculate that Cu2+ has two binding sites in GB1, among which one is D40, E42 and E56, and the other is E19 and D22 (Fig. 4). Furthermore, the binding affinities of these two binding sites are different. At 0.6/1 molar ratio of Cu2+/GB1, the cross peaks of D40 and E42 have disappeared while E19 and D22 residues still exist, suggesting that Cu2+ has a higher binding affinity with the former.

|
Figure 4 Possible binding sites for Mn2+, Co2+ and Cu2+ in GB1. (a) The structure of GB1 (PDB 3GB1); (b) Possible binding sites for Mn2+ and Co2+, D40/E42/E56 (red); (c) Two possible binding sites for Cu2+, D40/E42/E56 (red) and E19/D22 (blue) |
In all, for divalent metal ions, GB1 can bind with paramagnetic ions such as Cu2+, Mn2+ and Co2+, but not with diamagnetic ions such as Ca2+, Mg2+ and Zn2+. The possible binding sites of Mn2+, Co2+ and Cu2+ in GB1 were shown in Fig. 4(b) and 4(c), respectively. For Mn2+ and Co2+, the possible binding sites are residues D40/E42/E56. For Cu2+, there exist two binding sites, one is residues D40/E42/E56, and the other one is E19/D22. The former has relative higher binding affinity than the latter. According to the Irving-Williams series[27] which ranks the relative stability of complexes formed by divalent metal ions, the stability of the complex with Cu2+ is higher than that with Mn2+ and Co2+, which is the possible reason that the E19/D22 sites weakly bind with Cu2+, but not with Mn2+ and Co2+.
3 ConclusionIn this research, we determined the binding of GB1 with various divalent cations and lanthanide ions, respectively, using 2D 1H-15N HSQC spectroscopy. GB1 weakly binds with paramagnetic divalent cations Cu2+, Mn2+ and Co2+, and lanthanide ions Lu3+ and Tm3+, but not with diamagnetic divalent cations Ca2+, Mg2+ and Zn2+. Our studies demonstrate that NMR spectroscopy is a powerful tool to study weak binding between protein and metal ions, and also hint that care must be taken to avoid possible interference to paramagnetic NMR data when using GB1 as a model protein.
| [1] | GRONENBORN A M, FILPULA D R, ESSIG N Z, et al. A novel, highly stable fold of the immunoglobulin binding domain of streptococcal protein G[J]. Science, 1991, 253(5020): 657-661. DOI: 10.1126/science.1871600. |
| [2] | NADAUD P S, HELMUS J J, HOFER N, et al. Long-range structural restraints in spin-labeled proteins probed by solid-state nuclear magnetic resonance spectroscopy[J]. J Am Chem Soc, 2007, 129(24): 7502-7503. DOI: 10.1021/ja072349t. |
| [3] | NADAUD P S, HELMUS J J, KALL S L, et al. Paramagnetic ions enable tuning of nuclear relaxation rates and provide long-range structural restraints in solid-state NMR of proteins[J]. J Am Chem Soc, 2009, 131(23): 8108-8120. DOI: 10.1021/ja900224z. |
| [4] | NADAUD P S, SENGUPTA I, HELMUS J J, et al. Evaluation of the influence of intermolecular electron-nucleus couplings and intrinsic metal binding sites on the measurement of 15N longitudinal paramagnetic relaxation enhancements in proteins by solid-state NMR[J]. J Biomol NMR, 2011, 51(3): 293-302. DOI: 10.1007/s10858-011-9536-y. |
| [5] | SENGUPTA I, NADAUD P S, HELMUS J J, et al. Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy[J]. Nat Chem, 2012, 4(5): 410-417. DOI: 10.1038/nchem.1299. |
| [6] | SENGUPTA I, NADAUD P S, JARONIEC C P. Protein structure determination with paramagnetic solid-state NMR spectroscopy[J]. Acc Chem Res, 2013, 46(9): 2117-2126. DOI: 10.1021/ar300360q. |
| [7] | LI J P, PILLA K B, LI Q F, et al. Magic angle spinning NMR structure determination of proteins from pseudocontact shifts[J]. J Am Chem Soc, 2013, 135(22): 8294-8303. DOI: 10.1021/ja4021149. |
| [8] | SENGUPTA I, GAO M, ARACHCHIGE R J, et al. Protein structural studies by paramagnetic solid-state NMR spectroscopy aided by a compact cyclen-type Cu(Ⅱ) binding tag[J]. J Biomol NMR, 2015, 61(1): 1-6. DOI: 10.1007/s10858-014-9880-9. |
| [9] | TAMAKI H, EGAWA A, KIDO K, et al. Structure determination of uniformly 13C, 15N labeled protein using qualitative distance restraints from MAS solid-state 13C-NMR observed paramagnetic relaxation enhancement[J]. J Biomol NMR, 2016, 64(1): 87-101. DOI: 10.1007/s10858-015-0010-0. |
| [10] | MUNTENER T, HAUSSINGER D, SELENKO P, et al. In-cell protein structures from 2D NMR experiments[J]. J Phys Chem Lett, 2016, 7(14): 2821-2825. DOI: 10.1021/acs.jpclett.6b01074. |
| [11] | PAN B B, YANG F, YE Y S, et al. 3D structure determination of a protein in living cells using paramagnetic NMR spectroscopy[J]. Chem Commun, 2016, 52(67): 10237-10240. DOI: 10.1039/C6CC05490K. |
| [12] | SEEWALD M J, PICHUMANI K, STOWELL C, et al. The role of backbone conformational heat capacity in protein stability:temperature dependent dynamics of the B1 domain of Streptococcal protein G[J]. Protein Sci, 2000, 9(6): 1177-1193. DOI: 10.1110/ps.9.6.1177. |
| [13] | BARCHI JR J J, GRASBERGER B, GRONENBORN A M, et al. Investigation of the backbone dynamics of the IgG-binding domain of streptococcal protein G by heteronuclear two-dimensional 1H-15N nuclear magnetic resonance spectroscopy[J]. Protein Sci, 1994, 3(1): 15-21. |
| [14] | TUNNICLIFFE R B, WABY J L, WILLIAMS R J, et al. An experimental investigation of conformational fluctuations in proteins G and L[J]. Structure, 2005, 13(11): 1677-1684. DOI: 10.1016/j.str.2005.08.006. |
| [15] | JEE J, BYEON I J, LOUIS J M, et al. The point mutation A34F causes dimerization of GB1[J]. Proteins, 2008, 71(3): 1420-1431. |
| [16] | MCCALLISTER E L, ALM E, BAKER D. Critical role of beta-hairpin formation in protein G folding[J]. Nat Struct Biol, 2000, 7(8): 669-673. DOI: 10.1038/77971. |
| [17] | KARANICOLAS J, BROOKS C L. The origins of asymmetry in the folding transition states of protein L and protein G[J]. Protein Sci, 2002, 11(10): 2351-2361. |
| [18] | WILTON D J, TUNNICLIFFE R B, KAMATARI Y O, et al. Pressure-induced changes in the solution structure of the GB1 domain of protein G[J]. Proteins, 2008, 71(3): 1432-1440. |
| [19] | GRONENBORN A M, FILPULA D R, ESSIG N Z, et al. A novel, highly stable fold of the immunoglobulin binding domain of streptococcal protein-G[J]. Science, 1991, 253(5020): 657-661. DOI: 10.1126/science.1871600. |
| [20] | THORDARSON P. Determining association constants from titration experiments in supramolecular chemistry[J]. Chem Soc Rev, 2011, 40(3): 1305-1323. DOI: 10.1039/C0CS00062K. |
| [21] | GRZESIEK S, BAX A, CLORE G M, et al. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase[J]. Nat Struct Biol, 1996, 3(4): 340-345. DOI: 10.1038/nsb0496-340. |
| [22] | CHEEVER M L, SATO T K, DE BEER T, et al. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes[J]. Nat Cell Biol, 2001, 3(7): 613-618. DOI: 10.1038/35083000. |
| [23] | FISHER R D, WANG B, ALAM S L, et al. Structure and ubiquitin binding of the ubiquitin-interacting motif[J]. J Biol Chem, 2003, 278(31): 28976-82894. DOI: 10.1074/jbc.M302596200. |
| [24] | OTTING G. Protein NMR using paramagnetic ions[J]. Annu Rev Biophys, 2010, 39: 387-405. DOI: 10.1146/annurev.biophys.093008.131321. |
| [25] | KUSZEWSKI J, GRONENBORN A M, CLORE G M. Improving the packing and accuracy of NMR structures with a pseudopotential for the radius of gyration[J]. J Am Chem Soc, 1999, 121(10): 2337-2338. DOI: 10.1021/ja9843730. |
| [26] | SCHMITZ C, STANTON-COOK M J, SU X C, et al. Numbat:an interactive software tool for fitting Delta chi-tensors to molecular coordinates using pseudocontact shifts[J]. J Biomol NMR, 2008, 41(3): 179-189. DOI: 10.1007/s10858-008-9249-z. |
| [27] | WILLIAMS R J P. Chemical selection of elements by cells[J]. Coordin Chem Rev, 2001, 216, 217: 583-595. DOI: 10.1016/S0010-8545(00)00398-2. |
 2018, Vol. 35
2018, Vol. 35 
