2. Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China;
3. State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuhan 430071, China
2. 中国科学院 青岛生物能源与过程研究所, 山东 青岛 266101;
3. 波谱与原子分子物理国家重点实验室, 中国科学院 武汉物理与数学研究所, 湖北 武汉 430071
An accurate, reliable, and convenient way for the determination of enantiomeric excess (e.e.) and absolute configurations of chiral compounds is in demand with the increasing development of enantioselective syntheses[1-6]. Nuclear magnetic resonance (NMR) with chiral solvating agent or shift reagent has been the prevailing tool for this purpose due to its good potential[7-11]. Enantiomeric discrimination occurs when (R)- and (S)-enantiomers associate with the chiral solvating agent to form two different diastereomeric complexes that exhibit different chemical shifts in the NMR spectrum on the NMR time scale[12, 13]. So far, various types of NMR-based chiral solvating agents or shift reagents have been investigated, such as lanthanide complexes[14, 15], cyclodextrins[8, 16, 17], ethers[18, 19], and others[20-23]. Unfortunately, these reagents have inherent drawbacks. For examples, the chiral lanthanide shift reagents broaden the NMR signals, and the cyclodextrin can only be dissolved in D2O or in D2O/organic solvent mixtures. High-performance chiral solvating agents or shift reagents of versatility, signal sharpness, high resolution, high sensitivity, and so forth, are therefore required.
Understanding the mechanism of enantiomeric discrimination is very important to the development of high-performance practical chiral solvating agents or shift reagents. Generally, hydrogen-bonding (HB) interaction, π-π stacking, van de Waal’s force, and steric hindrance effect etc. are considered as the essential factors for chiral recognition between the hosted and guested enantiomers[24-26]. Various chiral solvating agents or shift reagents[27, 28], in particular the HB type ones[26, 29], were thus designed and synthesized to study the mechanism of chiral recognition. Nevertheless, the effects of the above factors on chiral discrimination are still not completely unobscured. For example, we do not really understand the effect of HB interaction between host and guest on chiral recognition. Therefore,great effort needs to be put into this work so that we can thoroughly predict the nature of enantiomeric discrimination.
It is also known that some chiral compounds have the ability to recognize themselves[30, 31]. That means a chiral guest may also be discriminated by a structurally similar chiral host. The aims of the present work are to investigate the chiral recognition of a diphenylethylenediamine (DPEDA) derivative from its structural analogues using high-resolution NMR, and to investigate the effect of inter-molecular HB interaction on chiral recognition. It was previously reported that the DPEDA is a good NMR chiral shift reagent for chiral carboxylic acids[32, 33]. DPEDA was then modified in order to recognize other kind of chiral compounds[34-36]. For instance, a chiral A2B2 macrocyclic minireceptor derived from DPEDA displayed good recognition ability to various amino acid derivatives in the 1H NMR spectrum[34]. In this paper, the urea derivative of (1R, 2R)-(+)-1, 2-DPEDA was prepared and employed as a host 1 bearing both HB donor and acceptor sites. The α-phenylethylamine, α-(p-methoxyphenyl) ethylamine and their derivatives (2~13), which are the structural analogues of host 1 were selected as the guested enantiomers (Fig. 1).
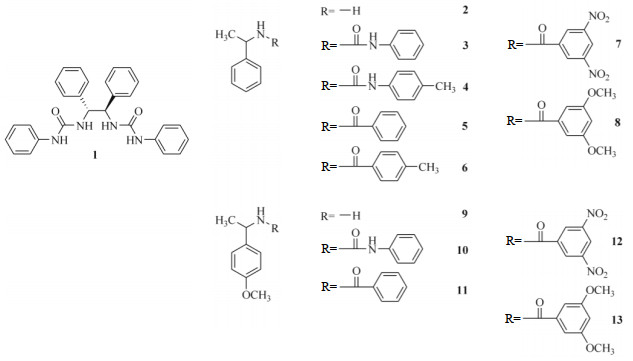
|
Fig. 1 Structures of host 1 and analytes 2~13 |
(1R, 2R)-(+)-1, 2-DPEDA, α-phenylethylamine (guest 2), and α-(p-methoxyphenyl)ethylamine (guest 9) were purchased from China National Medicines Corporation Ltd. and used as received. Phenylisocyanate was obtained from Sigma-Aldrich and used as received. Deuterated chloroform (CDCl3) and deuterated trifluoroacetic acid were purchased from Cambridge Isotope Laboratories, Inc. CDCl3 was well-dried with molecular sieve before use. All other chemicals used for the syntheses of host and guests were of analytical grade and used as received.
1.2 Syntheses of host 1 and guests (3~8, 10~13)To a solution of (1R, 2R)-(+)-1, 2-DPEDA (2.12 g, 0.010 mol) in dried pyridine (30 mL), phenylisocyanate (2.98 g, 0.025 mol) was added dropwise under an N2 atmosphere at 5 ℃. The resulting solution was then standing for 8 h at 65 ℃. After the removal of pyridine, the residue was recrystallized using acetone to obtain host 1 (4.02 g, 0.009 mol). m.p.: 219~221 ℃.
(R)- and (S)-guests (3~8 and 10~13) were obtained by the derivation of the corresponding primary amines 2 and 9 with phenylisocyanate or aromatic acyl chloride.
1.3 NMR sample preparation(a) General experiment. For each of the 12 guested enantiomers, NMR sample was prepared by dissolving proper amount of 1 and analytes with CDCl3 to achieve the desired concentrations (typically 5.0 mmol/L for each, except those in the indicated conditions).
(b) Titrations of guest enantiomers with host 1. The titration experiments were accomplished according to the following procedure: (1) Stock solutions (10 mmol/L in CDCl3) of host 1 and guested enantiomers were prepared before and after; (2) the NMR solutions were prepared by taking the proper amounts of the above host and analyte solutions into the NMR tubes to achieve the desired concentrations. The concentration of guest was kept constant at 1.0 mmol/L and molar ratio of host to guest was changed from 0:1 to 9:1.
1.4 NMR experimentCDCl3 was used as the solvent for all 1H NMR measurements at 25 ℃ (except for those in the indicated conditions) performed on Bruker Avance Ⅲ 500 or 600 MHz spectrometers with tetramethylsilane as an internal reference. When necessary, assignments were confirmed using 2D DQF-COSY or NOESY spectra.
2 Results and discussion 2.1 NMR studies of chiral discriminationThe ability of the host in recognizing the enantiomers of guests was evaluated by NMR spectra of 1:1 mixtures. As a typical example, the 1H NMR spectra characterizing the enantiomeric discrimination of guest 3 by host 1 are shown in Fig. 2. In absence of host 1, the signal of the CHCH3 and PhNH group of guest 3 appear at δH 1.500 (d) and δ 6.193 (s), separately [Fig. 2(d)]. When host 1 was added into the solution, the signal of the CHCH3 group of 3 shifts upfield obviously and splits into two doublets [δH 1.482 for (R)-3 and δH 1.471 for (S)-3, Fig. 2(a)]. Meanwhile, the signal of the PhNH group of 3 shifts downfield and also splits into two singlets [δ 6.267 for (R)-3 and δ 6.287 for (S)-3, Fig. 2(a)]. The above result indicates that nonequivalent CHCH3 or PhNH signals of (S)-3 and (R)-3 can be well resolved by host 1. In this sense, host 1 in NMR spectra.

|
Fig. 2 Partial 1H NMR spectra (600 MHz) of (R and/or S)-3 and with or without addition of 1 in CDCl3. (a) (R and S)-3 with 1; (b) (S)-3 with 1; (c) (R)-3 with 1; (d) (R and S)-3 without 1. □PhNH of 1, ○CHNH of 1, ■PhNH of 3, and ●CHCH3 of 3 |
Table 1 summarizes the chemical shift differences (∆∆δ =∆δR ﹣∆δS) between the (R)- and (S)-analytes 2~13 for four different groups CHCH3, CHCH3, CHNH, and PhNH, when these analytes are interacted with the host 1 and detected at 5 ℃ and 20 ℃. As seen in Table 1, host 1 exhibits different recognition abilities to different types of enantiomers, which have identical chiral center but different substituent groups. Two primary amines, 2 and 9, are not discriminated by 1 in the 1H NMR spectra. This may be partly due to the fewer effective HB sites of 2 and 9, and/or partly due to the absence of steric hindrance near their chiral centers. Therefore, the urea and amide derivatives of 2 and 9, which have more HB acceptors or donor sites and larger steric hindrance groups, were selected as guests. The host 1 can distinguish both urea derivatives (3, 4, and 10) and amide derivatives (5, 6, 8, 11, and 13) lacking of NO2 group very well, but unexpectedly not the amide derivatives 7 and 12 containing NO2 group. Generally, π-acidic hosts can well recognize π-basic enantiomers and vice versa[37-39]. Herein, however, 7 and 12, showing strongly π-acidic in nature due to the existence of NO2 group, cannot be discriminated by the weakly π-basic host 1. While, guests (3~6, 8, 10, 11 and 13) with π-basic groups are all chirally recognized by π-basic host 1. Similar phenomenons have been observed by other researchers, including π-acid-acid[40] or π-base-base[41] interaction presented in the chiral recognition and led to good enantiomer selectivity. But both of them were investigated through high-performance liquid chromatography (HPLC) without excluding the non-chiral factors.
| Table 1 Enantiomeric discrimination of 1 to chiral analytes 2~13 in CDCl3 obtained by 1H NMR spectra (500 MHz) |
It is shown that 10, 11, and 13, the derivatives of 9 containing methoxyl-group exhibit larger ∆∆δ values of the CHCH3 or CHCH3 group than 3, 5, and 8, the corresponding derivatives of 2 lacking of OCH3 group. Thus, it seems that the appearance of OCH3 group is favorable to the discrimination of host. More interestingly, for the (R)- and (S)-isomeric urea derivatives (3, 4 or 10) the ∆∆δ values of the CHCH3 groups approximately equal to those of CHCH3 groups at both 5 ℃ and 20 ℃, while for the (R)- and (S)-isomeric amide derivatives (5, 6, 8, 11 or 13), the ∆∆δ values of the CHCH3 groups are only about half of those of CHCH3 groups. Additionally, the ∆∆δ values of the CHCH3 groups for the NO2-excluded derivatives (3~6, and 8) at 5 ℃ and 20 ℃ are almost the same and independent of the substituent groups. And it is observed that the PhNH groups of (R)- and (S)-isomeric analytes (3, 4, and 10) can be obviously recognized due to their large ∆∆δ values, but the CHNH groups of all (R)- and (S)-isomeric analytes cannot be recognized by host 1.
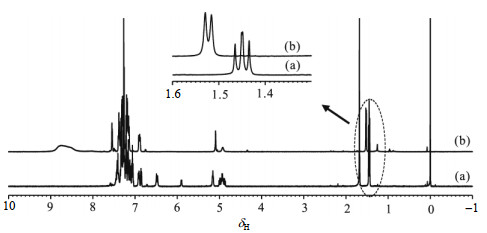
2.2 HB interaction and its effect on enantiomeric discriminationIt is believed that HB interaction is involved in the enantiomeric discrimination. In this research, the effect of HB on discrimination was investigated by NMR titration experiment (Table 2). The data in Table 2 show that an increase in the concentration of host 1 causes the downfield shifts of the signals of two amide groups of 3 and enlarges the PhNH signal separation between (R)-3 and (S)-3. This indicates that both host-guest HB interaction and discrimination ability of the host to the guest increase with the increasing concentration of host 1. To further investigate the effect of HB interaction on discrimination, a little of deuterated trifluoroacetic acid was added into the solutions of 1 and 3, and it then turned out that the enantiomers of 3 cannot be discriminated by the host (Fig. 3). This ‘acid-treatment’ experimental result implies that no HB interaction results in non-discrimination between the host and the guest, confirming that HB interaction is essential to the chiral discrimination of the host to the guests.
| Table 2 Titration experimental data (600 MHz) for the amide group signals of 1 and 3 in CDCl3 |
|
Fig. 3 1H NMR spectra (500 MHz) of (R and S)-3 with addition of 1 (a) in CDCl3, and (b) in CDCl3 with little deuterated trifluoroacetic acid |
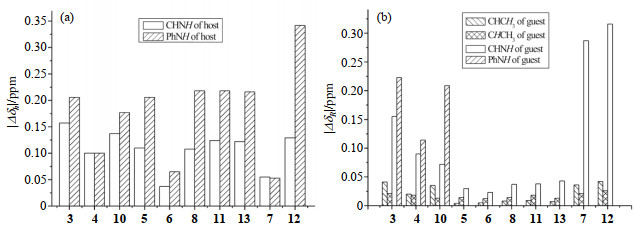
Fig. 4(a) and 4(b) show the chemical shifts changes (|∆δ |) of host 1, and (R)-guests 3~8 and 10~13, before and after host-guest mixing. It is obviously that when host 1 mixing with various (R)-guests (3~6, 8, 10, 11, and 13) lacking NO2 group, the |∆δh| values (0.1~0.2 ppm) of CHNH (or PhNH) group of 1 are not very sensitive to the substituent groups of the guests except guest 6. On the contrary, the corresponding |ΔδR| values of (R)-guests strongly depend on the substituent groups of the guests, as shown in Fig. 4(b). Moreover, the ∆δR values of the CHNH and PhNH groups of urea derivatives 3, 4, and 10 are much larger than those of amide derivatives 5, 6, 8, 11, and 13. The same trend of ∆δS was also observed in the mixtures of host 1 and (S)-guests (Table S1, available in the online edition).
|
Fig. 4 Chemical shift changes induced by host-(R)-analyte complexation of (a) CHNH and PhNH groups of 1, and (b) CHCH3, CHCH3, CHNH and PhNH groups of R-analytes. If not specifically stated, the molar ratio of host to guest was 1:1 |
The above results could be interpreted in terms of the different HB interactions between host and different guests. In the present case, the hydrogen-bonding NH--O=C between host and guest is dominant. Both host 1 and urea derivative 3, 4, and 10 contain urea groups, and thus two protons of a urea group of the host (or guests 3, 4 and 10) form double-HB simultaneously with a carbonyl oxygen of the urea derivatives (or host) [see Fig. 5(a)]. On the other hand, guests 5, 6, 8, 11, and 13 contain only one amide group, and one proton of an amide group can only form single-HB with a carbonyl oxygen of the host as shown in Fig. 5(b). Generally, the single HB interaction between the host and the amide guest is expected to be weaker than the double-HB interaction between the host and the urea guest. Therefore, the two amide protons of host 1 and guests 3, 4 and 10 have larger |∆δ | values than those of guests 5, 6, 8, 11, and 13. Furthermore, guests 3, 4 and 10 are fixed to the host via the double-HB, and thus have less ability to re-orientate, compared to those guests 5, 6, 8, 11, and 13 fixed to the host via single HB. The relatively fixed orientation together with larger binding constants between the host and the urea guest may be responsible for the larger |∆δR| values of CHCH3 groups of guests 3, 4 and 10 and for the higher enantiomeric discrimination ability of host toward CHCH3 groups of the (R)- and (S)-guests.

|
Fig. 5 Schematic representation of the HB interaction (a) between host and urea derivatives, and (b) between host and amide derivatives |
From Fig. 4(a) and 4(b), one sees that the |∆δR| values of the CHNH groups of amide guests 7 and 12 are much larger than those of other guests, indicating strong HB interaction occurring between host’s carbonyl and guest 7’ (or 12’) amide groups. Although the guests 7 and 12 with NO2 group have strong HB interaction with host 1, they cannot be recognized by host 1. This unexpected result cannot be interpreted at present.
3 ConclusionsIn summary, high-resolution 1H NMR studies reveal that host 1 has different chiral recognition abilities to its structurally similar guests 2~13, depending on the types of HB bonds, substituent groups and the steric hindrance. Host 1 cannot discriminate two primary amines 2 and 9, but can well recognize their urea and amide derivatives, except for 7 and 12 containing two NO2 groups. Compared to the derivatives of primary amine 2, the corresponding derivatives of primary amine 9 with the methoxyl-group are better chirally recognized by host 1. Host 1 not only has stronger HB interaction with the urea derivatives than with the amide derivatives, but also has higher enantiomeric discrimination ability to the CHCH3 groups (but not the CHCH3 groups) of the (R)- and (S)-urea-derivatives than those of the (R)- and (S)-amide-derivatives.
| [1] | WONG Y T A, BRYCE D L. Recent advances in B-11 solid-state nuclear magnetic resonance spectroscopy of crystalline solids[M]. WEBB G A Ed. Annual reports on NMR spectroscopy. 2018, 93: 213-279. |
| [2] | MOLDOVAN R C, BODOKI E, SERVAIS A C, et al. (+) or (-)-1-(9-fluorenyl)ethyl chloroformate as chiral derivatizing agent:A review[J]. J Chromatogr A, 2017, 1513: 1-17. DOI: 10.1016/j.chroma.2017.07.045. |
| [3] | LAZZERETTI P. Chiral discrimination in nuclear magnetic resonance spectroscopy[J]. J Physics Condens Matter, 2017, 29(44): 443001. DOI: 10.1088/1361-648X/aa84d5. |
| [4] | YIP Y C, WONG S K, CHOI S M. Assessment of the chemical and enantiomeric purity of organic reference materials[J]. Trac-Trend Anal Chem, 2011, 30(4): 628-640. |
| [5] |
WANG W G, SHEN X M, ZHANG C. Progress on application of NMR chiral solvating agents to determine enantiomeric ratio and absolute configuration[J].
Chinese Journal of Organic Chemistry, 2010, 30(8): 1126-1141.
王文革, 申秀民, 张聪. 手性溶解剂在NMR法测定对映体比率和绝对构型的研究进展[J]. 有机化学, 2010, 30(8): 1126-1141. |
| [6] | WENZEL T J, WILCOX J D. Chiral reagents for the determination of enantiomeric excess and absolute configuration using NMR spectroscopy[J]. Chirality, 2003, 15(3): 256-270. DOI: 10.1002/chir.10190. |
| [7] | SEO M S, JANG S, KIM H. A chiral aluminum solvating agent (CASA) for H-1 NMR chiral analysis of alcohols at low temperature[J]. Chem Commun, 2018, 54(50): 6804-6807. DOI: 10.1039/C8CC00574E. |
| [8] | DALVANO B E, WENZEL T J. Sulfated cyclodextrins as water-soluble chiral NMR solvating agents for cationic compounds[J]. Tetrahedron-Asymmetr, 2017, 28(8): 1061-1069. DOI: 10.1016/j.tetasy.2017.07.003. |
| [9] | LI G W, CAO J M, ZONG W, et al. Enantiodiscrimination of carboxylic acids using the diphenylprolinol NMR chiral solvating agents[J]. Org Chem Front, 2016, 3(1): 96-102. |
| [10] | STORCH G, HAAS M, TRAPP O. Attracting enantiomers:chiral analytes that are simultaneously shift reagents allow rapid screening of enantiomeric ratios by NMR spectroscopy[J]. Chem-Eur J, 2017, 23(23): 5414-5418. DOI: 10.1002/chem.201700461. |
| [11] | BEEREN S R, MEIER S. Supramolecular chemical shift reagents inducing conformational transitions:NMR analysis of carbohydrate homooligomer mixtures[J]. Chem Commun, 2015, 51(15): 3073-3076. DOI: 10.1039/C4CC09710F. |
| [12] | BESLI S, COLES S J, DAVIES D B, et al. Anomalous NMR behavior of meso compounds with remote stereogenic centers on addition of chiral shift reagent or chiral solvating agent[J]. J Am Chem Soc, 2003, 125(16): 4943-4950. DOI: 10.1021/ja028871r. |
| [13] | NGOC H P, WENZEL T J. A Water-soluble calix 4 resoreinarene with alpha-methyl-l-prolinylmethyl groups as a chiral NMR solvating agent[J]. J Org Chem, 2011, 76(3): 986-989. DOI: 10.1021/jo102197w. |
| [14] | MARIE C, HISCOX B, NASH K L. Characterization of HDEHP-lanthanide complexes formed in a non-polar organic phase using P-31 NMR and ESI-MS[J]. Dalton T, 2012, 41(3): 1054-1064. DOI: 10.1039/C1DT11534K. |
| [15] | PROVENCHER K A, WENZEL T J. Carboxymethylated cyclodextrins and their paramagnetic lanthanide complexes as water-soluble chiral NMR solvating agents[J]. Tetrahedron-Asymmetr, 2008, 19(15): 1797-1803. DOI: 10.1016/j.tetasy.2008.07.024. |
| [16] | RECIO R, ELHALEM E, BENITO J M, et al. NMR study on the stabilization and chiral discrimination of sulforaphane enantiomers and analogues by cyclodextrins[J]. Carbohyd Polym, 2018, 187: 118-125. DOI: 10.1016/j.carbpol.2017.12.022. |
| [17] | DOWEY A E, PUENTES C M, CAREY-HATCH M, et al. Synthesis and utilization of trialkylammonium-substituted cyclodextrins as water-soluble chiral NMR solvating agents for anionic compounds[J]. Chirality, 2016, 28(4): 299-305. DOI: 10.1002/chir.22582. |
| [18] | DUDDECK H, GOMEZ E D. Chiral recognition of ethers by NMR spectroscopy[J]. Chirality, 2009, 21(1): 51-68. DOI: 10.1002/chir.20605. |
| [19] | RODRIGUEZ Y C, DUARTE T M, SZAKONYI Z, et al. Utilization of (18-Crown-6)-2, 3, 11, 12-tetracarboxylic acid as a chiral NMR solvating agent for diamines and-amino acids[J]. Chirality, 2015, 27(10): 708-715. DOI: 10.1002/chir.22491. |
| [20] | ZHOU L L, YUE J L, FAN Y X, et al. Self-assembly and chiral recognition of chiral cationic gemini surfactants[J]. Langmuir, 2018, 34(43): 12924-12933. DOI: 10.1021/acs.langmuir.8b02599. |
| [21] | POSPISIL M J, SAHA P, ABDULQUDDOS S, et al. Orientation relaxation dynamics in cellulose nanocrystal dispersions in the chiral liquid crystalline phase[J]. Langmuir, 2018, 34(44): 13274-13282. DOI: 10.1021/acs.langmuir.8b02350. |
| [22] | POLYANCEV F M, METLUSHKA K E, SADKOVA D N, et al. The isomeric structure of pentacoordinate chiral spirophosphoranes in solution by the combined use of NMR experiments and GIAO DFT calculations of NMR parameters[J]. Dalton T, 2017, 46(25): 8146-8156. DOI: 10.1039/C7DT01605K. |
| [23] | MONTEAGUDO E, VIRGILI A, PARELLA T, et al. Chiral recognition by dissolution DNP NMR spectroscopy of C-13-labeled DL-methionine[J]. Anal Chem, 2017, 89(9): 4939-4944. DOI: 10.1021/acs.analchem.7b00156. |
| [24] | WOLF C, COOK A M, DANNATT J E. Enantiodifferentiation of multifunctional tertiary alcohols by NMR spectroscopy with a Whelk-O type chiral solvating agent[J]. Tetrahedron-Asymmetr, 2014, 25(2): 163-169. DOI: 10.1016/j.tetasy.2013.11.014. |
| [25] | LI X J, HOPMANN K H, HUDECOVA J, et al. Determination of absolute configuration and conformation of a cyclic dipeptide by NMR and chiral spectroscopic methods[J]. J Phys Chemistry A, 2013, 117(8): 1721-1736. DOI: 10.1021/jp311151h. |
| [26] | JAIN N, PATEL R B, BEDEKAR A V. Modified Kagan's amide:synthesis and application as a chiral solvating agent for hydrogen-bonding based chiral discrimination in NMR[J]. RSC Adv, 2015, 5(57): 45943-45955. DOI: 10.1039/C5RA06959A. |
| [27] | KIM S M, CHOI K. A practical solvating agent for the chiral NMR discrimination of carboxylic acids[J]. Eur J Org Chem, 2011, 25: 4747-4750. |
| [28] | NATH N, KUMARI D, SURYAPRAKASH N. Application of selective F-1 decoupled H-1 NMR for enantiomer resolution and accurate measurement of enantiomeric excess at low chiral substrate/auxiliary concentration[J]. Chem Phys Lett, 2011, 508(1-3): 149-154. DOI: 10.1016/j.cplett.2011.04.012. |
| [29] | RAO R N, RAMACHANDRA B, SANTHAKUMAR K. Evaluation of (R)-(-)-a-methoxy phenyl acetic acid as a chiral shift reagent for resolution and determination of R and S enantiomers of modafinil in bulk drugs and formulations by 1H NMR spectroscopy[J]. Chirality, 2012, 24(4): 339-344. DOI: 10.1002/chir.22002. |
| [30] | URAY G, NIEDERREITER K S, MAIER N M, et al. Diphenylethanediamine (DPEDA) as chiral selector Ⅸ:Self recognition of chiral selectors-efficient HPLC-separation of the enantiomers of 3, 5, -dinitrobenzoylated diphenylalkaneamides on the immobilized analogue[J]. Chirality, 1999: 11404-408. |
| [31] | HUANG S H, BAI Z W, FENG J W. Chiral self-discrimination of the enantiomers of alpha-phenylethylamine derivatives in proton NMR[J]. Magn Reson Chem, 2009, 47(5): 423-427. DOI: 10.1002/mrc.2406. |
| [32] | FULWOOD R, PARKER D. 1, 2-Diphenylethane-1, 2-diamine:an effective NMR chiral solvating agent for chiral carboxylic acids[J]. J Chem Soc-Perk T Ⅱ, 1994, 1: 57-64. |
| [33] | RAJAMOORTHI K, STOCKTON B W. Direct NMR determination of optical purity of nicotinic and quinolinic carboxylic acid compounds using 1, 2-diphenylethane-1, 2-diamine as a chiral solvating agent[J]. Spectros Lett, 2001, 34(3): 255-266. DOI: 10.1081/SL-100002280. |
| [34] | GASPARRINI F, MISITI D, PIERINI M, et al. A chiral A2B2 macrocyclic minireceptor with extreme enantioselectivity[J]. Org Lett, 2002, 4(23): 3993-3996. DOI: 10.1021/ol026363g. |
| [35] | CHAUVIN A-S, BERNARDINELLI G, ALEXAKIS A. Determination of the absolute configuration of chiral aryl-alkyl carbinols using organophosphorus diamine derivatizing agents by 31P NMR spectroscopy[J]. Tetrahedron:Asymmetry, 2004, 15(12): 1857-1879. DOI: 10.1016/j.tetasy.2004.04.031. |
| [36] | CUCCIOLITO M E, FLORES G, VITAGLIANO A. Chiral recognition in silver(Ⅰ) olefin complexes with chiral diamines. resolution of racemic alkenes and NMR discrimination of enantiomers[J]. Organometallics, 2004, 23(1): 15-17. |
| [37] | LINDNER W, URAY G, STEINER U. (S, S)-diphenylethylethanediamine derivatives as chiral selectors Ⅱ. Gasparrini-type bound chiral stationary phase with high enantioselectivity for naphthylamides[J]. J Chromatogr A, 1991, 553: 373-381. DOI: 10.1016/S0021-9673(01)88507-1. |
| [38] | URAY G, MAIER N M, LINDNER W. Diphenylethanediamine derivatives as chiral selectors Ⅲ. Comparison of 4 new diastereomeric chiral stationary phases prepared by addition of mono-3, 5-dinitrobenzoyldiphenylethanediamine derivatives to optically pure epoxy silica[J]. J Chromatogr A, 1994, 666(1/2): 41-53. |
| [39] | MALYSHEV O R, VINOGRADOV M G. Convenient synthesis of π-acceptor chiral stationary phases for high-performance liquid chromatography from halogen-substituted 3, 5-dinitrobenzoylamides[J]. J Chromatogr A, 1999, 859: 143-151. DOI: 10.1016/S0021-9673(99)00871-7. |
| [40] | WELCH C J. Evolution of chiral stationary phase design in the pirkle laboratories[J]. J Chromatogr A, 1994, 666(1/2): 3-26. |
| [41] | SCHLEIMER M, PIRKLE W H, SCHURIG V. Enantiomer separation by high-performance liquid chromatography on polysiloxane-based chiral stationary phases[J]. J Chromatogr A, 1994, 679: 23-34. DOI: 10.1016/0021-9673(94)80308-0. |
 2019, Vol. 36
2019, Vol. 36 
