2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
帕金森症(PD)是常见的神经退行性疾病,主要病理学特征为患者脑部出现路易小体,路易小体的主要蛋白成分则是异常聚集的α-synuclein[1].α-synuclein是1个含有140个氨基酸的天然无结构蛋白.根据α-synuclein各个部分的特点,可以分为3个区域:N端的1~60位残基,含有4个不完全重复的保守片段(KTKEGV)[2];NAC区域61~95位残基,含有3个重复的保守片段(KTKEGV);C端96~140位残基,含有无结构蛋白特有的大量酸性氨基酸和脯氨酸.N端和NAC端是与生物膜相互作用的主要区域[3],而酸性C端则被认为是与其他蛋白质相互作用的主要位置[4].研究[5, 6]表明α-synuclein在细胞内质网中的浓度大大高于细胞质,高浓度的α-synuclein可损害内质网的正常生理功能,导致内质网压力变化.
在细胞内质网的压力应对机制中,蛋白质二硫键异构酶(PDI)发挥着重要作用[7].PDI主要存在于细胞内质网中,是一种多功能蛋白.PDI负责氧化还原内质网中新生肽链二硫键的形成和断裂,酶催化分子中错误链接的二硫键重排形成正确的二硫键[8];而且通过分子伴侣功能防止无结构蛋白发生聚集,并最终使无结构蛋白被运输出内质网[9].PDI通过控制内质网中的无结构蛋白应答来调节内质网的压力平衡[10].PDI的结构整体呈马蹄型[11],含有4个类似硫氧还原蛋白的结构域ABB´A´:A和A´分别含有1个二硫键酶活性位点CXXC,A和A´两个活性结构域由结构相对刚性的B和B´连接,B´上有1个疏水口袋,是主要的底物结合位点[12];在A´和B´之间有一段灵活性很强的X linker,强酸性的C末端含有29个残基,带有1个KDEL内质网定位信号[13].然而,在PD患者的脑细胞中,大量PDI酶活性位点的半胱氨酸被亚硝基化,从而失去了调控α-synuclein等无结构蛋白聚集和运输的功能[14, 15].Cheng等人[16]的研究表明,野生型PDI能够抑制α-synuclein的纤维化聚集.但是目前关于PDI与α-synuclein的相互作用,及其对α-synuclein纤维化聚集的调节机制尚不清楚,这些机制的揭示将有助于了解α-synuclein的纤维化聚集过程.
核磁共振(NMR)方法是研究不同蛋白之间相互作用的有力手段,NMR在α-synuclein的结构及相互作用研究中已经取得了许多研究成果[17].本文通过NMR方法研究了α-synuclein与PDI的相互作用,发现α-synuclein与PDI的结合位点主要位于α-synuclein的N端,但是与半胱氨酸突变为丝氨酸的突变体——PDI C-S的结合位点则位于α-synuclein的C末端.硫磺素T(ThT)是能与淀粉样纤维特异性结合的荧光染料,可以利用ThT荧光实验来检测纤维结构的形成,蛋白纤维化聚集程度越大,测得的荧光强度越强[18].通过ThT荧光实验我们发现,相对于野生型PDI,PDI C-S突变体抑制α-synuclein的纤维化聚集作用明显减弱.结合NMR和ThT荧光实验结果可知,PDI通过与α-synuclein的N端残基结合抑制其纤维化聚集,并且PDI的半胱氨酸残基在这个过程中起到重要的作用.
1 实验部分 1.1 样品制备 1.1.1 野生型PDI及其突变体PDI C-S的表达与纯化首先挑取已转入野生型或突变型PDI重组质粒(His6-PDI-pET-28a)的大肠杆菌BL21(DE3)单克隆于LB培养基中,37 ℃条件下培养至在600 nm处的光密度值(OD600)为0.8,然后加入异丙基硫代半乳糖苷(IPTG)至终浓度为1.0 mmol/L,37 ℃继续诱导表达5 h;6 000 rpm离心10 min,去上清收集细胞;将细胞重悬后超声裂解至透亮,20 000 rpm离心收集上清.收集的上清液首先用Ni亲和层析柱(GE Healthcare Life Sciences China,北京)纯化,然后洗脱液再用Superdex S-75(GE Healthcare Life Sciences China,北京)分子筛纯化,最后经阴离子交换柱Source Q(GE Healthcare Life Sciences China,北京)纯化,得到纯度达标的样品,冻干封存备用.
1.1.2 α-synuclein的表达与纯化α-synuclein的表达和纯化方法参考戴晨晔等人[19]文章.
1.2 NMR实验 1.2.1 NMR样品制备将冻存的α-synuclein蛋白样品溶解于450 μL NMR缓冲液(含20 mmol/L Tris和100 mmol/L NaCl,pH 7.0)中,加入50 μL D2O,α-synuclein的最终浓度为0.125 mmol/L.
1.2.2 1H-15N HSQC实验1H-15N HSQC实验均在Bruker 600 MHz谱仪上完成,实验温度为298 K.谱宽为7 240 Hz(1H)×1 580 Hz(15N),采样数据点阵为t2×t1 = 2 048×256,累加次数(ns)为20,弛豫等待时间(d1)为1.0 s.谱图化学位移归属根据BioMagResBank(BMRB entry number 16543).
1.3 ThT荧光实验70 μmol/L的α-synuclein溶于1 mL的缓冲溶液中(含20 mmol/L Tris-Hcl和100 mmol/L NaCl,pH 7.0),加入PDI或突变体PDI C-S使其终浓度为35 μmol/L(对照组不加PDI或突变体PDI C-S),在37 ℃、220 rpm的条件下进行纤维化诱导.间隔一定时间,取样10 μL加入1 mL的ThT(浓度为50 μmol/L)混合均匀.使用FS5荧光仪(Edinburgh Instruments Ltd.,英国)进行荧光测定,选择5 nm的狭缝带宽,在450 nm处进行激发,发射光谱记录范围为470~600 nm,记录步长为1 nm.纤维生长情况在482 nm下进行评估.
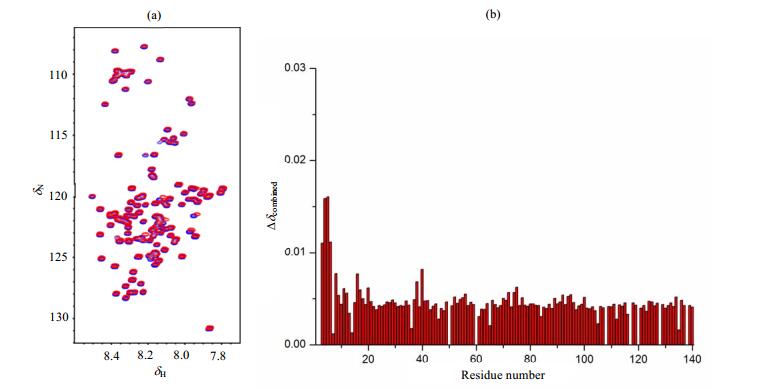
2 结果与讨论 2.1 α-synuclein与PDI及其突变体PDI C-S结合位点的研究PDI抑制α-synuclein纤维化聚集的功能已有报道[16],但具体的作用机制并不清楚.为了研究α-synuclein与PDI相互作用的结合位点,我们采集了15N标记的α-synuclein的1H-15N HSQC谱图[图 1(a)中的蓝色谱图所示],谱峰集中出现在δH 7.5~8.5之间,表明α-synuclein呈现无结构状态;向α-synuclein中加入终浓度为0.2 mmol/L PDI,在同样的实验条件下再次采集α-synuclein的1H-15N HSQC谱图[图 1(a)中的红色谱图所示],发现α-synuclein的部分氨基酸残基出现化学位移变化.通过(1) 式计算各个残基的化学位移变化,得到滴定后各个残基的化学位移变化柱状图[图 1(b)].
|
图 1 (a) 0.125 mmol/L 15N标记的α-synuclein加入终浓度为0.2 mmol/L野生型PDI前(蓝色)后(红色)的1H-15N HSQC谱图;(b)加入PDI后,α-synuclein各个氨基酸残基的化学位移变化柱状图 Figure 1 (a) 1H-15N HSQC spectra of 15N-labeled α-synuclein (0.125 mmol/L) in the absence (blue) and presence (red) of wild type PDI (0.2 mmol/L); (b) Chemical shift changes of the amino acid residues in α-synuclein upon interaction with PDI |
| $ \Delta {\delta _{{\rm{combined}}}} = 0.25\sqrt {{{\left[ {\delta \left( {^1{{\rm{H}}_{{\rm{free}}}}} \right) - \delta \left( {^1{{\rm{H}}_{{\rm{bound}}}}} \right)} \right]}^2} + {{\left\{ {1/5\left[ {\delta \left( {^{15}{{\rm{N}}_{{\rm{free}}}}} \right) - \delta \left( {^{15}{{\rm{N}}_{{\rm{bound}}}}} \right)} \right]} \right\}}^2}} $ | (1) |
图 1显示发生化学位移变化的残基主要位于α-synuclein的N端,特别是V3、F4、M5、K6等残基[图 1(b)],表明α-synuclein主要是通过这些位点与PDI相互作用.
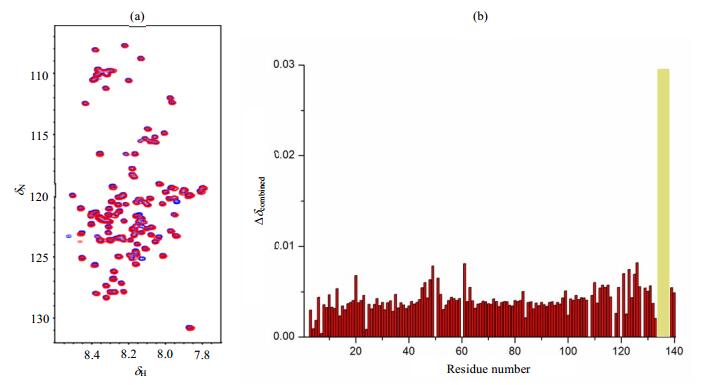
为了研究PDI的半胱氨酸对α-synuclein与其结合的影响,我们将PDI的6个半胱氨酸突变为丝氨酸,得到突变体PDI C-S,并分别采集加入突变体PDI C-S前后α-synuclein的1H-15N HSQC谱图[图 2(a)],发现加入突变体PDI C-S后α-synuclein C末端的Q134、D135、Y136、E137等信号消失[图 2(b)],表明C端残基是α-synuclein与PDI C-S结合的主要位点.
|
图 2 (a) 0.125 mmol/L 15N标记的α-synuclein加入终浓度为0.2 mmol/L PDI C-S前(蓝色)后(红色)的1H-15N HSQC谱图;(b)加入PDI C-S后,α-synuclein各个残基的化学位移变化柱状图,黄色部分表示消失的峰 Figure 2 (a) 1H-15N HSQC spectra of 15N-labeled α-synuclein in the absence (blue) and presence (red) of PDI C-S (0.2 mmol/L); (b) Chemical shift changes of amino acid residues in α-synuclein upon interaction with PDI C-S, yellow part represents the NMR peaks disappeared |
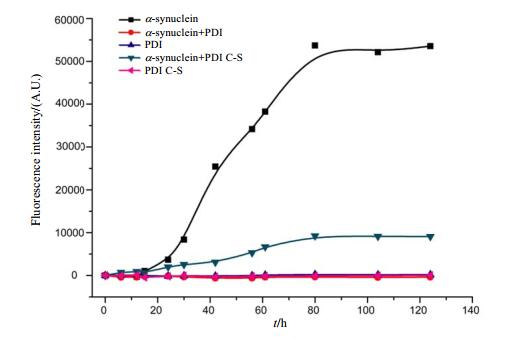
从NMR实验结果得到野生型PDI与α-synuclein的N端残基结合,而突变体PDI C-S与α-synuclein的C端结合.为了比较野生型PDI和突变体PDI C-S对α-synuclein纤维化聚集过程是否也会有不同影响,我们采用ThT荧光实验进行研究.从图 3可以看到,野生型PDI几乎完全抑制了α-synuclein的纤维化聚集,而突变体PDI C-S的抑制作用减弱,这可能是由于α-synuclein的N端和C端在纤维化聚集的过程中起着不同的作用.
|
图 3 硫磺素T(ThT)荧光实验监测PDI及其突变体PDI C-S对α-synuclein纤维化聚集的影响 Figure 3 Influence of PDI or PDI C-S to the fibrillation of α-synuclein monitoring by thioflavin T(ThT) fluorescence |
Rao等人[20]发现许多抑制α-synuclein聚集的小分子与α-synuclein的接触位点主要位于N端的3~18和38~51之间,与C端基本没有相互作用;同时突触前蛋白synphilin-1与α-synuclein的N端结合,抑制了α-synuclein聚集[21];α-synuclein的N端乙酰化破坏了其N端与其他部位的相互作用,使得α-synuclein的聚集减缓[22];此外,α-synuclein主要通过N端接触形成二聚体,而二聚体比单体α-synuclein更稳定,因而N端在α-synuclein纤维化聚集过程中起到重要作用[23].Cheng等人[16]的研究表明,PDI的A´结构域在抑制α-synuclein纤维化聚集的过程中发挥着重要作用.A´结构域对于PDI与无结构蛋白的结合至关重要[12],所以PDI可能通过A´结构域与α-synuclein的N端结合,破坏α-synuclein分子间相互作用,阻碍α-synuclein二聚的形成,从而进一步抑制了α-synuclein的纤维化聚集.
α-synuclein的C端有分子伴侣的功能,能够与α-synuclein的N端和NAC端接触,防止α-synuclein的纤维化聚集[24];同时负电荷富集的C端结合钙离子或者被截去,都会使α-synuclein的纤维化聚集大大加快[25, 26].因此,突变体PDI C-S与α-synuclein的C端结合,可能使得α-synuclein的疏水区暴露,导致PDI C-S抑制α-synuclein聚集的功能减弱,这表明PDI上的半胱氨酸对PDI抑制α-synuclein纤维化聚集至关重要,半胱氨酸在这个过程中的具体作用有待进一步研究.对于PD患者,影响PDI与α-synuclein N端相互作用,可能是由于患者脑细胞中PDI的二硫键位点半胱氨酸被亚硝基化从而失去抑制α-synuclein纤维化聚集功能的作用机制[14].
3 结论通过结合NMR和ThT荧光实验研究了PDI抑制α-synuclein纤维化的作用机制,发现PDI主要是通过结合α-synuclein的N端残基,抑制α-synuclein纤维化聚集,并且半胱氨酸对于PDI抑制α-synuclein纤维化聚集的功能至关重要.
| [1] | SPILLANTINI M G, CROWTHER R A, JAKES R, et al. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies[J]. P Natl Acad Sci USA, 1998, 95(11): 6469-6473. DOI: 10.1073/pnas.95.11.6469. |
| [2] | KESSLER J C, ROCHET J C, LANSBURY P T. The N-terminal repeat domain of alpha-synuclein inhibits beta-sheet and amyloid fibril formation[J]. Biochemistry, 2003, 42(3): 672-678. DOI: 10.1021/bi020429y. |
| [3] | ELIEZER D, KUTLUAY E, BUSSELL R, et al. Conformational properties of alpha-synuclein in its free and lipid-associated states[J]. J Mol Biol, 2001, 307(4): 1061-1073. DOI: 10.1006/jmbi.2001.4538. |
| [4] | UVERSKY V N, FINK A. Amino acid determinants of alpha-synuclein aggregation:putting together pieces of the puzzle[J]. Febs Lett, 2002, 522(1-3): 9-13. DOI: 10.1016/S0014-5793(02)02883-1. |
| [5] | ZHANG L, ZHANG C Y, ZHU Y Y, et al. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons:An immunogold electron microscopic study using a C-terminal specific monoclonal antibody[J]. Brain Res, 2008, 1244: 40-52. DOI: 10.1016/j.brainres.2008.08.067. |
| [6] | SMITH W W, JIANG H B, PEI Z, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity[J]. Hum Mol Genet, 2005, 14(24): 3801-3811. DOI: 10.1093/hmg/ddi396. |
| [7] | LYLES M M, GILBERT H F. Catalysis of the oxidative folding of ribonuclease-a by protein disulfide isomerase-dependence of the rate on the composition of the redox buffer[J]. Biochemistry, 1991, 30(3): 613-619. DOI: 10.1021/bi00217a004. |
| [8] | FREEDMAN R B, DUNN A D, RUDDOCK L W. Protein folding:A missing redox link in the endoplasmic reticulum[J]. Curr Biol, 1998, 8(13): 468-470. DOI: 10.1016/S0960-9822(98)70295-7. |
| [9] | CAI H, WANG C C, TSOU C L. Chaperone-like activity of protein disulfide-isomerase in the refolding of a protein with no disulfide bonds[J]. J Biol Chem, 1994, 269(40): 24550-24552. |
| [10] | MAATTANEN P, GEHRING K, BERGERON J J M, et al. Protein quality control in the ER:The recognition of misfolded proteins[J]. Semin Cell Dev Biol, 2010, 21(5): 500-511. DOI: 10.1016/j.semcdb.2010.03.006. |
| [11] | WANG C, LI W, REN J Q, et al. Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase[J]. Antioxid Redox Sign, 2013, 19(1): 44-53. |
| [12] | KLAPPA P, RUDDOCK L W, DARBY N J, et al. The b' domain provides the principal peptide-binding site of protein disulfide isomerase but all domains contribute to binding of misfolded proteins[J]. Embo J, 1998, 17(4): 927-935. DOI: 10.1093/emboj/17.4.927. |
| [13] | FREEDMAN R B, GANE P J, HAWKINS H C, et al. Experimental and theoretical analyses of the domain architecture of mammalian protein disulphide-isomerase[J]. Biol Chem, 1998, 379(3): 321-328. |
| [14] | UEHARA T, NAKAMURA T, YAO D D, et al. S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration[J]. Nature, 2006, 441(7092): 513-517. DOI: 10.1038/nature04782. |
| [15] | NAKAMURA T, TU S C, AKHTAR M W, et al. Aberrant protein S-nitrosylation in neurodegenerative diseases[J]. Neuron, 2013, 78(4): 596-614. DOI: 10.1016/j.neuron.2013.05.005. |
| [16] | CHENG H, WANG L, WANG C C. Domain a' of protein disulfide isomerase plays key role in inhibiting alpha-synuclein fibril formation[J]. Cell Stress Chaperon, 2010, 15(4): 415-421. DOI: 10.1007/s12192-009-0157-2. |
| [17] |
DAI C Y, ZHANG Z T, LIU M L, et al. Application of NMR in the studies of structure and interactions of α-synuclein[J].
Chinese J Magn Reson, 2016, 33(1): 154-167.
戴晨晔, 张则婷, 刘买利, 等. NMR在α-synuclein的结构及相互作用研究中的应用[J]. 波谱学杂志, 2016, 33(1): 154-167. |
| [18] | KHURANA R, COLEMAN C, IONESCU-ZANETTI C, et al. Mechanism of thioflavin T binding to amyloid fibrils[J]. J Struct Biol, 2005, 151(3): 229-238. DOI: 10.1016/j.jsb.2005.06.006. |
| [19] |
DAI C Y, LIU M L, LI C G. Salt content-dependent conformational changes of α-synuclein studied by 19F NMR[J].
Chinese J Magn Reson, 2015, 32(1): 33-40.
戴晨晔, 刘买利, 李从刚. 低盐和高盐环境下α-synuclein构象的19F NMR研究[J]. 波谱学杂志, 2015, 32(1): 33-40. DOI: 10.11938/cjmr20150104. |
| [20] | RAO J N, DUA V, ULMER T S. Characterization of alpha-synuclein interactions with selected aggregation-inhibiting small molecules[J]. Biochemistry, 2008, 47(16): 4651-4656. DOI: 10.1021/bi8002378. |
| [21] | XIE Y Y, ZHOU C J, ZHOU Z R, et al. Interaction with synphilin-1 promotes inclusion formation of alpha-synuclein:mechanistic insights and pathological implication[J]. Faseb J, 2010, 24(1): 196-205. DOI: 10.1096/fj.09-133082. |
| [22] | ZABROCKI P, BASTIAENS I, DELAY C, et al. Phosphorylation, lipid raft interaction and traffic of alpha-synuclein in a yeast model for Parkinson[J]. BBA-Mol Cell Res, 2008, 1783(10): 1767-1780. |
| [23] | YU J P, MALKOVA S, LYUBCHENKO Y L. Alpha-synuclein misfolding:Single molecule AFM force spectroscopy study[J]. J Mol Biol, 2008, 384(4): 992-1001. DOI: 10.1016/j.jmb.2008.10.006. |
| [24] | HONG D P, XIONG W, CHANG J Y, et al. The role of the C-terminus of human alpha-synuclein:Intra-disulfide bonds between the C-terminus and other regions stabilize non-fibrillar monomeric isomers[J]. Febs Lett, 2011, 585(3): 561-566. DOI: 10.1016/j.febslet.2011.01.009. |
| [25] | LOWE R, POUNTNEY D L, JENSEN P H, et al. Calcium(Ⅱ) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain[J]. Protein Sci, 2004, 13(12): 3245-3252. |
| [26] | LI W X, WEST N, COLLA E, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations[J]. P Natl Acad Sci USA, 2005, 102(6): 2162-2167. DOI: 10.1073/pnas.0406976102. |
 2017, Vol. 34
2017, Vol. 34 
