文章信息
- 翟紫凝, 吴琼, 李从刚
- Zi-ning ZHAI, Qiong WU, Cong-gang LI
- 乙酰化修饰抑制α-synuclein的纤维化聚集
- Lysine Acetylation Inhibits α-Synuclein Fibrillation
- 波谱学杂志, 2016, 33(2): 179-187
- Chinese Journal of Magnetic Resonance, 2016, 33(2): 179-187
- http://dx.doi.org/10.11938/cjmr20160201
-
文章历史
收稿日期: 2015-05-13
修订日期: 2016-04-08


DOI:10.11938/cjmr20160201
2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
Parkinson’s disease (PD) is the second most common neurodegenerative disease[1]. The cause of the disease is complex and still unclear,but substantial evidence suggests that the aggregation of α -synuclein ( α -syn) is a critical step because characteristic fibrils of α -syn are observed in Lewy bodies,the hallmark of PD pathology[2, 3].> α -Syn is an abundant brain protein of 140 residues,a typical intrinsically disordered protein (IDP). The structure of > α-syn can be divided into three regions: N-terminal region (residues 1~60) with a highly conserved hexamer motif KTKEGV forms α-helical in association with membranes[4, 5]; A central hydrophobic region,known as non-amyloid- β component (NAC) (residues 61~95),is proposed to provide the hydrophobic core and play a key role in fibrillation[6]; The highly charged C-terminal region (residues 96~140) has been proposed to regulate the aggregation of full-length α-syn and determine the diameter of α-syn filaments[7].
α-Syn possesses a remarkable conformational plasticity modulated by its environment,being able to adopt multiple conformations including an unfolded native state,an fibrillation prone partially folded conformation,variousα-helical and β-sheet species and several types of aggregates like oligomers,amorphous aggregates and amyloid-like fibrils[8]. The aggregation of α-syn occurs via a nucleation-dependent mechanism during which there is an initial lag phase followed by a growth phase[9]. Many factors such as increased temperature,low pH,polyamines,metal ions or high salt will accelerate α-syn aggregation[10, 11]. While the proteins like chaperones and small molecules can inhibit α-syn aggregation[8].
To better understand the aggregation mechanism of α-syn,the detailed structure has been described by NMR and other spectroscopic approaches. At neutral pH,transient long-range interactions between the C- and N-terminal regions and also between the C-terminal and NAC regions have been demonstrated using paramagnetic relaxation enhancement (PRE) measurements[12-14]. The contacts between the C-terminal and NAC regions have been thought to shield the hydrophobic core and serve as autoinhibitory for aggregation[12, 14]. Release of these interactions and exposure of NAC region accelerate the aggregation rate[11, 12, 15]. At low pH,charge distribution of the three regions changes because of acid residues protonate,and especially C-terminal becomes more like NAC and locally collapsed[16].The comparison of neutral- and low-pH ensembles suggest that change in long-range interactions,the compaction of C-terminal region and the uneven charge distribution are all key to faster aggregation.
Acetylation of N-terminal and lysine residues is an abundant posttranslational modification within the eukaryotic cells[17]. Here we focus on generating highly negative charges by lysine acetylation,in contrast to highly positive charges by lowering pH,to assess the charge effect on α-syn aggregation from another way. By reaction with acetic anhydride at room temperature,a modified acetylated α-syn is yielded and identified. Our results show that lysine acetylated α-syn is still disordered and the acetylation inhibits α-syn fibrillation.
1 Materials and methods 1.1 Protein expression and purificationA single colony of Escherichia coli strain BL21 (DE3) harboring the pT7-7 plasmid was used to inoculate 5 mL LB media. The culture was shaken about 8~10 h at 37 ℃ and then 1 mL was used to inoculate 100 mL M9 media. After overnight incubation,the 100 mL M9 culture was transferred into 900 mL M9 media. For uniform 15N enrichment,15NH4Cl (CIL) was used as a substitution for NH4Cl. The culture was shaken at 37 ℃ until the OD600 reached 0.8 when the inducer isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mmol/L. Cells were grown for an additional 6 h at 37 ℃ and harvested by centrifugation.
Cell pellet was sonicated after being resuspended in lysis buffer [including 10 mmol/L tris,1 mmol/L EDTA and 1 mmol/L phenylmethanesulfonyl fluoride (PMSF,added fresh)]. Then cell lysate was transferred to centrifuge tube and boiled 20 min to precipitate globular protein. The supernatant was collected after centrifugation and 10 mg/mL streptomycin sulfate was added and stirred at 4 ℃ for 30 min. Then supernatant was retrieved after centrifugation and 360 mg/mL ammonium sulfate was added and stirred at 4 ℃ for 30 min to precipitate α-syn[18]. Pellet was resuspended and loaded onto a Hi-Trap Q Sepharose fast-flow column (GE Healthcare) in 20 mmol/L tris (pH 7.7) and eluted in a gradient of 0~1 mol/L NaCl. Fractions containing α-syn (analyzed by SDS-PAGE) were concentrated in an Ultrafree 10 k MW filter (Millipore),loaded onto a SephacrylTM S-100 HR column (GE Healthcare) and eluted in 20 mmol/L tris and 150 mmol/L NaCl (pH 7.7). Fractions containing α-syn were combined and lyophilized.
1.2 AcetylationLyophilized α-syn (1.5 mg/mL) was dissolved in 25 mmol/L HEPES buffer (pH 7.7) and the concentration was determined using UV-vis absorption spectroscopy with the extinction coefficient (ε) of 5 960 mol-1•L•cm-1 at 280 nm. 0.1 mmol/L acetic hydride (in dioxane) was added gradually with various molar ratios of acetic anhydride∶lysines. The pH value of reaction mixture was monitored with a pH electrode and kept at 7.7 by the addition of NaOH,then kept reaction for 1 h in ice bath[19]. Acetylated α-syn was dialyzed against HEPES buffer using an Ultrafree 10 k MW filter (Millipore) to remove the residual dioxane and acetic acid.
1.3 Native PAGEA series of acetylated reaction samples with different molar ratios were diluted and then loaded on 15% native polyacrylamide gel. No SDS existed in the gel,loading buffer and running buffer (pH 8.8). The electrophoresis chamber was in ice bath and the voltage was kept at 100 V until the end. The native gel was stained by Coomassie brilliant blue just as the same procedure of SDS-PAGE.
1.4 NMR detection1H-15N HSQC spectra were acquired at 298 K on a Bruker Avance 800 MHz spectrometer equipped with a 5 mm H/C/N triple resonance cryoprobe. Diffusion spectra were acquired at 298 K on a Varian Inova 600 MHz spectrometer equipped with a H/F/(C,N) triple resonance cryogenic probe. The samples (pH 6.0) contained 0.25 mmol/L wild-type or acetylated α -syn,10% (v/v) D2O and 20 mmol/L HEPES. 1H-15N HSQC spectra were collected with 2 048 (8 transients) and 256 complex points in the direct and indirect dimensions,respectively. Diffusion was measured using a standard stimulated echo sequence[20],with a PFG duration of 0.02 ms,diffusion time of 0.3 s and gradient strengths ranging of 3.78~37.8 10-4 T/cm.
1.5 FibrillationWild-type and acetylated α -syn (100 μmol/L,500μL),respectively,was incubated in 25 mmol/L HEPES,1 mmol/L EDTA (pH 7.4),and shaken at 37 ℃,220 rpm in glass vials. Samples (10μL) were removed and combined with 500μL aqueous 50 mol/L thioflavin T (ThT,Sigma Aldrich). Fluorescence was measured on a Horiba spectrofluorometer in 1 cm path length quartz cuvettes. Emission spectra (465~530 nm) were recorded for excitation at 450 nm,using a 5 nm band-pass for both excitation and emission. Fibril growth was assessed from the emission at 489 nm.
Fibrils were separated from smaller species by centrifugation at 4 000 rpm for 10 min. Fibrils (pellet) resuspended in H2O to a same volume as the supernatant,and smaller aggregates (supernatant) were combined with loading buffer and boiled for 10 min before SDS-PAGE analysis. Coomassie-stained gels were analyzed by using a Versa Doc MP imager (Bio-Rad).
2 Resultsα -Syn (MW: 14.46 k,pI value: 4.67) is 140 amino acids long with distinct regions,including positively charged N-terminal region,hydrophobic NAC region and negatively charged C-terminal region. α -Syn has 15 lysine residues and 24 aspartic acid or glutamic acid residues without disulfide bond [Fig. 1(a)]. Since α -syn is an IDP,all the lysine and N-terminus ammonium groups are exposed to solvent and can be acetylated totally.
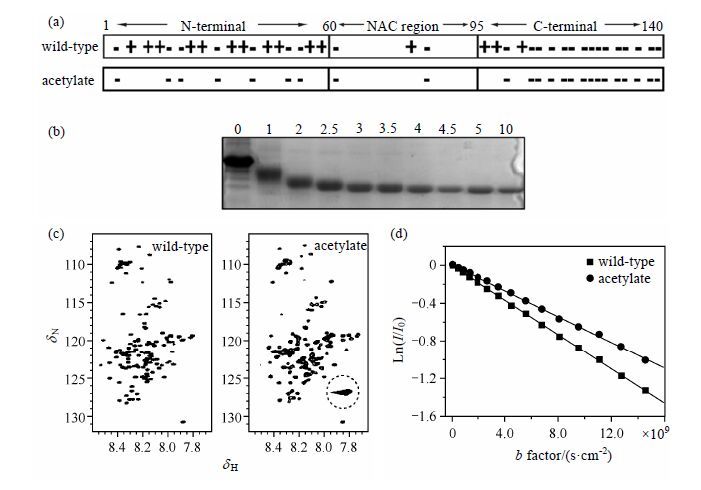
|
| Fig.1 Indentifying acetylation of α -syn. (a) The charge distribution in three regions of wild-type and complete acetylated α-syn. (b) The acetylation results by native-PAGE analysis. A series of molar ratios of acetic anhydride: lysine is respectively marked above the gel. (c) 1H-15N HSQC spectra of wild-type and acetylate. The dotted circle in the spectrum of acetylate indicates the characteristic resonance for side chain of acetylated lysines. (d) Diffusion results for wild-type and acetylate. b factor = (γ G δ )2Δ, where γ is the gyromagnetic ratio, G is the gradient strength, δ is the duration of gradient pulse, and Δ is the delay between gradient pluses, the slope is Dt. |
By adding different volumes of acetic anhydride (100 mmol/L) to a set of α -syn solutions,we obtained a group of reaction mixtures with various molar ratios of acetic anhydride:lysines. The acetylation of lysine or N-terminus ammonium groups (lysine ε-NH3+→lysine-ε-NHCOCH3) results in the decrease of positive charge and increase of net negative charge for α-syn. To assess the level of chemical modification,native polyacrylamide gel electrophoresis (native PAGE) was employed because of its easy use and sensitive to change of net charge. There is a positive correlation between electrophoretic mobility and net charge. As shown in Fig. 1(b),with an increae in molar ratio,electrophoretic mobility gradually increases until the molar ratio reaches 5,suggesting the most net negative charge due to a complete lysine acetylation. Therefore,the acetylated sample of 5-fold molar ratio was used for later research.
Then we acquired 1H-15N HSQC spectra of wild-type and totally acetylated α-syn [Fig. 1(c)]. The similar distribution with narrow chemical shift dispersion suggests that acetylated α-syn still exhibits a disordered conformation as wild-type. The strong cross-peak indicated by dotted circle is the characteristic resonance of lysine side chains,which only appears for acetylated α-syn. The reason is that the exchange rate of hydrogen and water is much slower for amide in acetylate than amino in wild-type.
To further understand the structural property after acetylation,the translational diffusion coefficient (Dt) is measured by using a stimulated echo sequence[20]. Dt is related to viscosity via the Stokes-Einstein law,Dt = κT/6πηr,where η is viscosity,κ is the Boltzmann constant,T is tempreture,and r is the protein’s radius. Although this relationship applies only to spherical proteins in homogeneous solution,the size change of nonspherical proteins like α-syn can also be qualified according to the reciprocal relationship between Dt and r. Fig. 1(d) shows plots of the logarithm of the relative intensity versus b factor. The slope is Dt. Remarkably,the Dt value of acetylate (6.849 × 10−11 m2/s) has decreased 25.7% than that of wild-type (9.216 × 10−11 m2/s),which suggesting that acetylated α-syn shows a larger hydrodynamic radius.
2.2 Acetylation inhibits the fibrillation of α-synTo assess the effect of fibrillation by acetylation,ThT fluorescence is used to monitor amyloid fibrils formation for wild-type and acetylated α-syn [Fig. 2(a)]. ThT is a benzothiazole dye that exhibits enhanced fluorescence upon binding to amyloid fibrils[21]. ThT fluorescence was very weak when the incubation was at the initial stage. For wild-type the intensity increased sharply after a lag time of about 15 h and reached a constant value after about 40 h. The fibril growth exhibits a sigmoidal time dependence as previously reported,which is consistent with a nucleation-dependent mechanism[22]. However,the intensity maintains weak even after 45 h incubation for acetylated α-syn,which indicating that no fibril forms.
Then we analyzed the sample component after fibrillation by SDS-PAGE. The soluble supernatant samples represent monomer and small oligomers,while the insoluble pellet samples represent high molecular mass fibrils. All the aggregates turned to monomers after boiling with denaturant SDS,aiming to quantify. As seen in Fig. 2(b),most of the wild-type α-syn is in the pellet,while most of the acetylate is in the supernatant. This result further verifies the data of ThT fluorescence,namely,no fibril forms in acetylated α-syn.
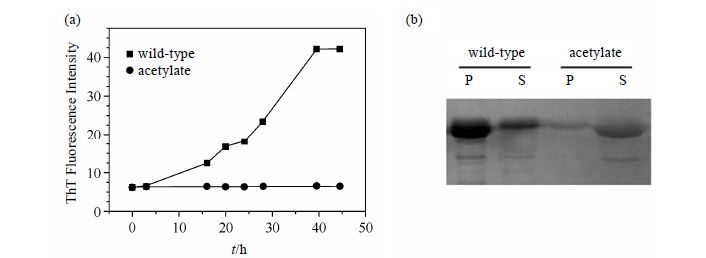
|
| Fig.2 Acetylation inhibits the fibrillation of α-syn. (a) Fibrillation of wild-type and acetylate monitored by ThT fluorescence. (b) SDS-PAGE analysis of supernatant (S) and pellet (P) for wild-type and acetylate after fibrillation. The bands of acetylate are lighter than that of wild-type because the acetylation of lysine ammonium groups reduces the efficiency of Coomassie brilliant blue staining |
In fact,some small particles were also observed on the flask wall for acetylate sample during the incubation. These particles can be dissolved after violently shaking the flask,so we speculate that acetylated,highly negative charged α-syn can form some small soluble oligomers,but can’t further develop into larger fibrils.
3 DiscussionLysine acetylation alters the charge distribution of the disordered α-syn chain,from net charge of 4 to -7 for N-terminus,and -12 to -15 for C-terminus individually. Transient long-range interactions between N- and C-terminal regions have been proposed for wild-type because of the electrostatic attraction[12],while electrostatic repulsion interaction replaces the attraction after acetylation. This leads to an extended structure verified by a larger hydrodynamic radius from NMR diffusion measurement,and a release of long-range interactions simultaneously.
Many factors like acid pH,high salt or polyamines induce a release of the long-range tertiary interactions and exposure of NAC hydrophobic core,potentiating the aggregation[8]. The charge change of C-terminal is the key in these previous researches. Here we focus on the charge change of N-terminal and charge of C-terminal remaining by acetylation. In contrast,the fibrillation of α-syn is severely inhibited although acetylation also destabilizes the long-range interactions and increases the exposure of NAC region. The reason can be considered that strong electrostatic force from highly negative charged N- and C-terminal regions impedes monomers or small oligomers close together for fibrils assembling. In this charge distribution,the electrostatic repulsion dominates the aggregation rather than hydrophobic effect.
Acetylation is one of the common post-translational modifications occurring in eukaryotic cells[17]. Simple and effective acetylated modification inhibits the fibrillation of α-syn. Our proposed mechanism provides a new way to inhibit α-syn fibrillation and to give insights into the role of electrostatic effect in α-syn aggregation.
| [1] | Goedert M. Alpha-synuclein and neurodegenerative diseases[J]. Nat Rev Neurosci, 2001, 2(7):492–501. |
| [2] | Trojanowski J Q, Lee V M Y. Parkinson's disease and related alpha-synucleinopathies are brain amyloidoses[J]. Ann NY Acad Sci, 2003, 991:107–110. |
| [3] | Spillantini M G, Crowther R A, Jakes R, et al. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies[J]. Proc Natl Acad Sci U S A, 1998, 95(11):6469–6473. |
| [4] | Ulmer T S, Bax A, Cole N B, et al. Structure and dynamics of micelle-bound human alpha-synuclein[J]. J Biol Chem, 2005, 280(10):9595–9603. |
| [5] | Bussell R J, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins[J]. J Mol Biol, 2003, 329(4):763–778. |
| [6] | El-Agnaf O M A, Bodles A M, Guthrie D J S, et al. The N-terminal region of non-A beta component of Alzheimer's Disease amyloid is responsible for its tendency to assume beta-sheet and aggregate to form fibrils[J]. Eur J Biochem, 1998, 258(1):157–163. |
| [7] | Murray I V J, Giasson B I, Quinn S M, et al. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro[J]. Biochemistry, 2003, 42(28):8530–8540. |
| [8] | Uversky V N. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation[J]. J Neurochem, 2007, 103(1):17–37. |
| [9] | Wood S J, Wypych J, Steavenson S, et al. Alpha-synuclein fibrillogenesis is nucleation-dependent:Implications for the pathogenesis of Parkinson's disease[J]. J Biol Chem, 1999, 274(28):19509–19512. |
| [10] | Uversky V N, Li J, Fink A L. Evidence for a partially folded intermediate in alpha-synuclein fibril formation[J]. J Biol Chem, 2001, 276:10737–10744. |
| [11] | Fernandez C O, Hoyer W, Zweckstetter M, et al. NMR of alpha-synuclein-polyamine complexes elucidates the mechanism and kinetics of induced aggregation[J]. Embo J, 2004, 23(10):2039–2046. |
| [12] | Bertoncini C W, Jung Y S, Fernandez C O, et al. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein[J]. Proc Natl Acad Sci U S A, 2005, 102(5):1430–1435. |
| [13] | Sung Y H, Eliezer D. Residual structure, backbone dynamics, and interactions within the synuclein family[J]. J Mol Biol, 2007, 372(3):689–707. |
| [14] | Dedmon M M, Lindorff-Larsen K, Christodoulou J, et al. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations[J]. J Am Chem Soc, 2005, 127(2):476–477. |
| [15] | Hoyer W, Cherny D, Subramaniam V, et al. Impact of the acidic C-terminal region comprising amino acids 109-140 on alpha-synuclein aggregation in vitro[J]. Biochemistry, 2004, 43(51):16233–16242. |
| [16] | Wu K P, Weinstock D S, Narayanan C, et al. Structural reorganization of alpha-synuclein at low pH observed by NMR and REMD simulations[J]. J Mol Biol, 2009, 391(4):784–796. |
| [17] | Arnesen T, Van Damme P, Polevoda B, et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans[J]. Proc Natl Acad Sci U S A, 2009, 106(20):8157–8162. |
| [18] | Hoyer W, Antony T, Cherny D, et al. Dependence of alpha-synuclein aggregate morphology on solution conditions[J]. J Mol Biol, 2002, 322(2):383–393. |
| [19] | Shaw B F, Arthanari H, Narovlyansky M, et al. Neutralizing positive charges at the surface of a protein lowers its rate of amide hydrogen exchange without altering its structure or increasing its thermostability[J]. J Am Chem Soc, 2010, 132(49):17411–17425. |
| [20] | Ferrage F, Zoonens M, Warschawski D E, et al. Slow diffusion of macromolecular assemblies by a new pulsed field gradient NMR method[J]. J Am Chem Soc, 2003, 125(9):2541–2545. |
| [21] | Khurana R, Coleman C, Ionescu-Zanetti C, et al. Mechanism of thioflavin T binding to amyloid fibrils[J]. J Struct Biol, 2005, 151(3):229–238. |
| [22] | Fink A L. The aggregation and fibrillation of alpha-synuclein[J]. Acc Chem Res, 2006, 39(9):628–634. |
 2016, Vol. 33
2016, Vol. 33



