文章信息
- 肖超妮, 王培, 马翠霞, 荣泽铭, 郑晓晖
- XIAO Chao-ni, WANG Pei, MA Cui-xia, RONG Ze-ming, ZHENG Xiao-hui
- 牡丹不同根部位的代谢物分布
- The Distribution of Metabolites in Different Root Parts from Tree Peony
- 波谱学杂志, 2015, 32(4): 648-660
- Chinese Journal of Magnetic Resonance, 2015, 32(4): 648-660
- http://dx.doi.org/10.11938/cjmr20150410
-
文章历史
- 收稿日期:2015-10-16
- 收修改稿日期:2015-11-09
植物药牡丹(Paeonia suffruticosa Andr.)属于芍药科芍药属的木本灌木.其干燥根皮(牡丹皮)为传统中药,具有清热凉血、活血化瘀和清肝泻火之功效[1].近年来,生物学研究发现牡丹皮还可以改善酒精性肝炎[2]、抑制脂多糖诱导的炎症[3, 4]、保护急性肝毒性[5]以及改善1-甲基-4苯基-1,2,3,6-四氢吡啶(MPTP)诱导的帕金森疾病[6].自1887年以来,牡丹皮的化学组成得到了深入细致的研究,主要包括单萜、苯乙酰类、没食子葡萄糖类和酚酸类等物质[7].这些次生代谢物在生物活性和药材品质评价方面发挥着重要作用.
植物根部是储存大量初生和次生代谢物的主要器官,然而这些代谢物积累情况随根部位的不同而发生变化,这导致不同根部位具有不同的治疗效应.正如文献所报道,芍药不同根部位的芍药苷含量是明显不同的[7].市场上牡丹皮的品质主要依据根的长度和尺寸等外形来评价,尽管丹皮酚和芍药苷等一些高含量的活性成分被用作高牡丹皮品质的评价标准[8, 9],但是牡丹皮不同根部位的代谢物分布,尤其是高活性物质的分布却鲜为人知,因此缺少足够的科学依据来评价牡丹皮的品质.
近年来,NMR与多变量分析相结合的代谢组学方法已经在中药品质评价和食物分析中得到广泛应用[10, 11].本文采用NMR和HPLC-MS方法系统分析了牡丹皮不同根部位的代谢物组成,应用基于NMR技术的代谢组学方法揭示了牡丹皮不同根部位代谢物的分布情况,为牡丹皮的等级划分提供了必要的科学依据.
1 实验部分 1.1 化学试剂分析级甲醇和乙酸铵购自于天津恒兴试剂公司,色谱级乙腈购于Fisher科技公司(USA),氘代甲醇(CD3OD,99.9%氘代)和2,2,3,3-氘代三甲基硅烷丙酸钠(C6D4H9NaO2Si,TSP,98%氘代)购于Cambridge Isotope Laboratories(MA,USA).
1.2 样品收集与制备一批牡丹植株(赵粉)于2012年10月采自山东菏泽信军园艺公司栽培基地(山东菏泽牡丹北路国花社区1 189号),经西北大学房敏峰教授鉴定,划分不同根部位为主根(直径为8~9 mm)、一级侧根(直径为6~7 mm)和二级侧根(直径为3~4 mm).将新鲜根洗涤后,进行抽芯扒皮[1],在烘箱中40 ℃恒温烘干24 h,在粉碎机上制成牡丹皮粉末(40目).
分别称取不同根部位的牡丹皮粉末约1 g,各平行制样5份,分别置入具塞锥形瓶中,精密加入甲醇25 mL,混合均匀,将锥形瓶放入超声波震荡仪(200 W,KQ5200DE型,昆山市超声仪器有限公司)中冰水浴(0 ℃)超声提取30 min.转移第一次提取液后,再加入甲醇25 mL,采用同样方法进行提取.最终将两次提取液合并,以8 000 rpm离心10 min,通过旋转蒸发仪除去大量溶剂后进行真空干燥,得到提取物粉末(平均提取率为21.3 %).
1.3 NMR和HPLC-MS实验精密称定提取物10 mg,溶解在含0.05 % (m/v) TSP的氘代甲醇(0.6 mL)中,混合均匀后,以10 000 rpm离心5 min,转移0.5 mL上清液至5 mm的NMR样品管中.所有NMR实验在Varian VNMRS 600型核磁共振谱仪(Agilent Technologies,USA)上完成.该谱仪的质子共振频率为599.90 MHz,所用探头为携带z方向梯度场的超低温探头,实验温度设置为298 K.1D 1H NMR实验所用的脉冲序列为NOESYPR1D,即RD-90°- t1-90°-tm-90°-acquisition,在等待时间(RD,2.0 s)和混合时间(tm,100 ms)内施加强度约为60 Hz的低功率连续波脉冲对水峰信号进行抑制.每个样品的90°脉冲宽度约为10 ms.其他采样参数为:累加次数为64,谱宽为12 000 Hz,采样点数为32 k,t1为6 ms.在进行傅立叶变换(FT)前,将所有自由感应衰减信号(FID)乘以线宽因子为1.0 Hz的指数窗函数并填零至128 k.此外,为了确认代谢物的归属,采集了选定样品的1H-1H J-Resolved、1H-1H COSY、 1H-1H TOCSY、1H-13C HSQC和1H-13C HMBC等一系列2D NMR谱,其采样和处理参数与研究报道的相同[13].
精密称取0.6 mg提取物,溶解于3 mL甲醇中,涡流混旋1 min,经0.45 μm有机滤膜过滤后用于HPLC-MS检测.HPLC-MS实验在G6520 LC/MS Q-TOF系统(Agilent Technologies,USA)上完成.色谱柱为Agilent Extend-C18 column (150 mm×4.6 mm,孔径为5 μm);流动相A为乙酸铵水溶液(2 mmol/L),流动相B为乙腈.梯度洗脱程序为0~13 min:15% B~28% B,13~22 min:28% B,22~37 min:28% B~58% B,37~45 min:58% B.流速为0.6 mL/min;进样量为20 μL;柱温为30 ℃.电喷雾离子源在负离子模式下检测样品,干燥气体温度为350 ℃,流速为10 L/min;喷雾器压力为40 psi;毛细管电压为4 000 V;溶剂化离子去簇电压为170 V;锥孔电压为65 V.一级质谱的离子采集范围为100~1 500 m/z,二级质谱的离子采集范围为50~1 500 m/z,采集频率均为 1张/s.选用m/z=112.985 5和1 033.988 1作为数据采集过程中的参比离子.
1.4 数据处理对所有1D 1H NMR谱进行手动调整相位并校正基线,以TSP信号(δ 0.00)进行化学位移定标.去除水信号所在区域(δ 4.80~4.96)、甲醇信号区域(δ 3.33~3.35)以及氘代甲醇信号区域(δ 3.29~3.33)旨在于排除水峰和外源性信号对数据分析的影响,再应用AMIX软件(V3.8,Bruker Biospin,Germany)以1.2 Hz积分区间对谱宽区间(δ 0.50~8.50)进行分段积分,所得数据SIMCA-P+软件(V.11,Umetrics,Sweden)进行主成分(PCA)分析.
根据内标物TSP的浓度和相应的积分面积,以及代谢物NMR信号的积分面积,计算得到牡丹皮提取物中代谢物的浓度[13],以平均值±标准偏差表示(mean ± SD,mg/g,n = 5).根据代谢物的浓度,使用软件SPSS (V.13.0,SPSS Inc,USA)进行统计分析(one- way ANOVA).
2 结果与讨论 2.1 牡丹皮代谢物的NMR分析图 1显示了牡丹皮主根甲醇提取物的1D 1H NMR谱,其信号峰被归属为不同的代谢物.与文献数据相比较,结合2D NMR和HPLC-MS数据,共鉴定了牡丹皮中的16个代谢物(表 1),其中1个初生代谢物为蔗糖[14, 15],15个次生代谢物包括了4种苯乙酰类(丹皮酚、丹皮酚原苷、丹皮酚苷和丹皮酚新苷)[16, 17, 18]、6种酚酸及没食子酰苷类物质(苯甲酸、4-羟基苯甲酸、没食子酸、没食子酸甲酯、没食子酸吡喃葡萄糖和五没食子酸酰葡萄糖)[19, 20, 21, 22, 23]、2种单萜苷类(芍药苷和氧化芍药苷)[20]、1种黄酮类(儿茶素[24])以及2种不饱和脂肪酸类代谢物.

|
| 图 1 牡丹皮主根甲醇提取物的600 MHz 1H NMR谱,化学位移区域(δ 4.90~8.10)相对于区域(δ 0.80~4.80)纵向放大2倍(数字编号为表 1中代谢物的编号) Fig. 1 The 600 MHz 1H NMR spectra of methanol extract from the axial root bark of tree peony. The region (δ 4.90~8.10) is magnified 2 times relative to the region (δ 0.80~4.80) (see Table 1 for the metabolite identification key) |
| Number | Metabolite | Group | δH(J/Hz) | δC | m/z |
| 1 | 苯甲酸 | 1-C | # | 121.026 8[M-H]- | |
| Benzoic acid | 2,6 -CH* | 8.05(m) | 130.6 | ||
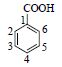
|
3,5 -CH | 7.49(m) | 129.8 | ||
| 4-CH | 7.61(m) | 134.5 | |||
| COOH | 168.1 | ||||
| 2 | 芍药苷 | 1-C | 89.3 | 479.157 2[M-H]- | |
| Paeoniflorin | 2-C | 87.3 | |||

|
3-CH2 | 1.81(d,12.0),2.19(d,12.0) | 44.6 | ||
| 4-C | 106.5 | ||||
| 5-CH | 1.96(m) | 23.7 | |||
| 6-CH2 | 2.49(m),2.58(m) | 44.3 | |||
| 7-C | 72.3 | ||||
| 8-CH2 | 4.75(d,12.0),4.72(d,12.0) | 61.3 | |||
| 9-CH | 5.42(s) | 102.4 | |||
| CH3 | 1.36(s) | 19.8 | |||
| C=O | 168.2 | ||||
| 1'-CH | 4.52(d,7.8) | 100.2 | |||
| 2'-CH | 3.22(#) | # | |||
| 3'-CH | 3.84(#) | # | |||
| 4'-CH | 3.31(#) | # | |||
| 5'-CH | 3.61(#) | # | |||
| 6'-CH2 | 4.46(#) | # | |||
| 1''-C | 111.4 | ||||
| 2'',6''-CH* | 8.03(m) | 130.6 | |||
| 3'',5''-CH | 7.47(m) | 129.8 | |||
| 4''-CH | 7.60(m) | 134.5 | |||
| 3 | 4-羟基苯甲酸 | 1-C | 122.8 | 137.023 3[M-H]- | |
| 4-Hydroxybenzoic acid | 2,6-CH* | 7.91(d,8.8) | 133.0 | 93.021 0[M-H-COO]- | |
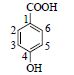
|
3,5-CH | 6.83(d,8.8) | 115.7 | 64.998 2[M-H-COO-H2O]- | |
| 4-C | 163.9 | ||||
| COOH | 168.1 | ||||
| 4 | 丹皮酚 | 1-C | 115.0 | 165.057 1[M-H]- | |
| Paeonol | 2-C | 166.4 | 150.034 4[M-H-CH3]- | ||
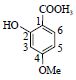
|
3-CH | 6.41(d,1.8) | 101.9 | 135.010 9[M-H-OCH2]- | |
| 4-C | 167.7 | 122.039 3[M-H-COCH3]- | |||
| 5-CH | 6.49(dd,1.8,7.8) | 108.4 | 91.492 3[M-H-COCH3-OCH3]- | ||
| 6-CH* | 7.79(d,7.8) | 134.0 | |||
| -OCH3 | 3.83(s) | 56.2 | |||
| CH3-C=O | 2.55(s) | 26.3 | |||
| C=O | 204.8 | ||||
| 5 | 丹皮酚新苷 | 1-C | 122.7 | 459.151 8[M-H]- | |
| Apiopaeonoside | 2-C | 160.7 | 293.082 2[M-H-C9H10O3]- | ||
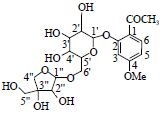
|
3-CH | 6.83(d,2.0) | 103.5 | 165.053 5[M-H-Glc-apiose]- | |
| 4-C | 166.4 | ||||
| 5-CH | 6.68(dd,2.0,7.8) | 109.1 | |||
| 6-CH | 7.75(d,7.8) | 133.3 | |||
| 1'-CH* | 5.02(d,7.8) | 102.5 | |||
| 2'-CH | 3.54(m) | 74.8 | |||
| 3'-CH | 3.48(m) | 78.3 | |||
| 4'-CH | 3.36(m) | 71.4 | |||
| 5'-CH | 3.63(m) | 77.2 | |||
| 6'-CH | 4.03(m) | 74.5 | |||
| -OCH3 | 3.87(s) | 56.4 | |||
| O=C-CH3 | 2.64(s) | 32.2 | |||
| O=C | 200.6 | ||||
| 1''-CH | 4.95(d,2.5) | 111.0 | |||
| 2''-CH | 3.88(d,2.5) | 78.1 | |||
| 3''-C | 80.0 | ||||
| 4''-CH2 | 4.05(m),4.03(m) | 74.9 | |||
| 5''-CH2 | 3.56(d,12.0),3.96(d,12.0) | 65.5 | |||
| 6 | 丹皮酚原苷 | 1-C | # | 459.151 1[M-H]- | |
| Paeonolide | 2-C | # | |||

|
3-CH | 6.85(d,2.0) | 102.5 | ||
| 4-C | 106.5 | ||||
| 5-CH | 6.67(dd,2.0,8.5) | 109.3 | |||
| 6-CH | 7.75(d,8.5) | 133.0 | |||
| -OCH3* | 3.88(s) | 56.1 | |||
| CH3-CO | 2.64(s) | 32.2 | |||
| C=O | 200.6 | ||||
| 1'-CH | 5.06(d,7.8) | 100.2 | |||
| 2'-CH | 3.55(#) | # | |||
| 3'-CH | 3.49(#) | # | |||
| 4'-CH | 3.36(#) | # | |||
| 5'-CH | 3.70(#) | # | |||
| 6'-CH2 | 3.79(#) | # | |||
| 1''-CH | 4.26(d,7.5) | # | |||
| 2''-CH2 | 3.56(#) | # | |||
| 3''-CH | 3.37(#) | # | |||
| 4''-CH | 3.48(#) | # | |||
| 5''-CH | 3.76(#) | # | |||
| 7 | 丹皮酚苷 | 1-C | # | 327.105 6[M-H]- | |
| Paeonoside | 2-C | # | 165.056 5[M-H-Glc]- | ||
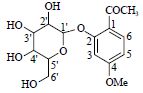
|
3-CH | 6.86(d,2.0) | 102.5 | ||
| 4-C | 106.5 | ||||
| 5-CH | 6.65(dd,2.0,8.7) | 109.3 | |||
| 6-CH | 7.74(d,8.7) | 133.0 | |||
| -OCH3* | 3.85(s) | 56.1 | |||
| CH3-CO | 2.60(s) | 32.2 | |||
| C=O | 200.6 | ||||
| 1'-CH | 5.05(d,7.8) | 100.2 | |||
| 2'-CH | 3.55(#) | # | |||
| 3'-CH | 3.49(#) | # | |||
| 4'-CH | 3.36(#) | # | |||
| 5'-CH | 3.70(#) | # | |||
| 6'-CH2 | # | # | |||
| 8 | 没食子吡喃葡萄糖 | 1-CH | 5.65(d) | 96.2 | 331.068 0[M-H]- |
| Glucogallin | 2,3,4,5-CH | 3.41~3.48 | # | 169.014 3[M-H-Glc]- | |
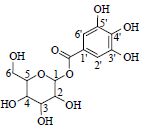
|
6-CH2 | 3.85~3.70 | # | 125.024 0[M-H-Glc-COO]- | |
| 1'-C | # | ||||
| 2',6'-CH* | 7.12(s) | 110.3 | |||
| 3',5'-C | 139.9 | ||||
| 4'-C | 146.6 | ||||
| 9 | 没食子酸甲酯 | C=O | 167.7 | ||
| Methyl gallate | 1-C | 121.4 | 183.030 2[M-H]- | ||
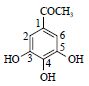
|
2,6-CH* | 7.08(s) | 110.1 | 168.004 7[M-H-CH3]- | |
|
3,5-C | 140.2 | 124.018 9[M-H-CH3-COO]- | ||
| 4-C | 146.4 | ||||
| C=O | 168.1 | ||||
| CH3 | 3.81(s) | 52.6 | |||
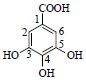
| 10 | 没食子酸 | 1-C | 122.8 | 169.013 3[M-H]- | |
| Gallic acid | 2,6-CH* | 7.03(s) | 110.1 | 125.022 9[M-H-COO]- | |
| 3,5-C | 139.8 | ||||
| 4-C | 146.4 | ||||
| COOH | 169.0 | ||||
| 11 | 五没食子酸酰苷 | 1-CH | 6.24(d,9.5 ) | 93.9 | 469.051 8[M-2H]2- |
| Pentagalloylglucose | 2-CH | 5.58(t,11.4) | 69.9 | 769.036 5[M-H-G]- | |
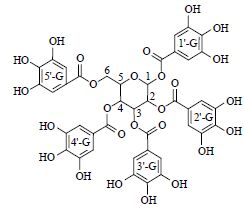
|
3-CH | 5.91(t,11.4) | 74.3 | 617.208 7[M-H-G-galloy]- | |
| 4-CH | 5.61(t,11.4) | 72.2 | |||
| 5-CH | 4.51(d,12.6) | 63.9 | |||
| 6-CH2 | 4.40(m),4.42(m) | 63.4 | |||
| 1'-G-CH | 7.05(s) | 110.4 | |||
| 2'-G-CH | 6.95(s) | 110.4 | |||
| 3'-G-CH* | 6.90(s) | 110.4 | |||
| 4'-G-CH | 6.98(s) | 110.4 | |||
| 6'-G-CH | 7.11(s) | 110.4 | |||
| 1'-G-C=O | 166.4 | ||||
| 2'-G-C=O | 167.0 | ||||
| 3'-G-C=O | 167.4 | ||||
| 4'-G-C=O | 167.0 | ||||
| 6'-G-C=O | 168.1 | ||||
| 12 | 儿茶素 | 2-CH | 4.56(d,7.8) | 83.0 | 289.072 4[M-H]- |
| Catechin | 3-CH | 3.97(ddd,5.4,8.2,7.8) | 68.7 | ||

|
4-CH2 | 2.84(dd,16.0,5.4) 2.50(dd,16.0,5.4) | 28.5 | ||
| 5-C | 157.1 | ||||
| 6-CH* | 5.84(d,2.0) | 95.6 | |||
| 7-C | 156.7 | ||||
| 8-CH | 5.91(d,2.0) | 96.3 | |||
| 9-C | 156.7 | ||||
| 10-C | 100.2 | ||||
| 1'-C | 132.1 | ||||
| 2'-CH | 6.83(d,2.0) | 115.5 | |||
| 3'-C | 145.1 | ||||
| 4'-C | 146.3 | ||||
| 5'-CH | 6.75(d,8.0) | 117.8 | |||
| 6'-CH | 6.71(dd,2.0,8.0) | 120.1 | |||
| 13 | 氧化芍药苷 | 1-C | 89.3 | 495.150 6[M-H]- | |
| Oxypaeoniflorin | 2-C | 87.3 | |||
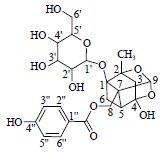
|
3-CH2 | 1.80(d,12.0),2.18(d,12.0) | 44.6 | ||
| 4-C | 106.5 | ||||
| 5-CH | 1.96 (m) | 23.7 | |||
| 6-CH2 | 2.48(m),2.57(m) | 44.3 | |||
| 7-C | 72.3 | ||||
| 8-CH2 | 4.76(d,12.0),4.73(d,12.0) | 61.3 | |||
| 9-CH | 5.39(s) | 102.4 | |||
| CH3 | 1.33(s) | 19.8 | |||
| C=O | 168.2 | ||||
| 1'-CH | 4.64(d,7.8) | 100.2 | |||
| 2'-CH | 3.26(#) | # | |||
| 3'-CH | # | # | |||
| 4'-CH | 3.36(#) | # | |||
| 5'-CH | 3.59(#) | # | |||
| 6'CH2 | 4.50(#) | # | |||
| 1''-C | 111.4 | ||||
| 2'',6''-CH* | 7.88(d,7.8) | 132.9 | |||
| 3'',5''-CH | 6.82(d,7.8) | 115.8 | |||
| 4''-C | 163.6 | ||||
| 14 | 蔗糖 | 1-CH* | 5.39(d,3.8) | 93.6 | 341.109 6[M-H]- |
| Sucrose | 2-CH | 3.43(m) | 73.3 | ||
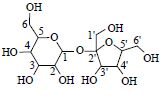
|
3-CH | 3.71(m) | 74.7 | ||
| 4-CH | 3.36(#) | 71.3 | |||
| 5-CH | 3.82(#) | 74.5 | |||
| 6-CH2 | 3.62(#) | 64.1 | |||
| 1'CH2 | # | 63.4 | |||
| 2'-C | 105.3 | ||||
| 3'-CH | 4.10(d,8.5) | 79.2 | |||
| 4'-CH | 4.04(#) | 75.7 | |||
| 5'-CH | 4.02(#) | 75.6 | |||
| 6'-CH2 | 3.78(#),3.76(#) | 63.9 | |||
| U1 | 不饱和脂肪酸 | CH3 | 0.90(t,7.8) | 14.5 | |
| Unsaturated fatty acid | (CH2)n | 1.32(m) | 23.7 | ||
| CH2-CH2-CO | 1.61(b) | 26.5 | |||
| CH2-CH=CH | 2.03(m),2.06(m) | 28.1 | |||
| CH2-COOH | 2.32(m) | 35.0 | |||
| CH2-OCOR | 4.15(m),4.36(m) | 63.4 | |||
| CH-OCOR | 5.35(m) | # | |||
| COOH | 174.5 | ||||
| U2 | 不饱和脂肪酸 | CH3 | 0.97(t,7.8) | 14.2 | |
| Unsaturated fatty acid | CH2 | 1.53(ddd,7.8,7.8,7.8) | 23.3 | ||
| CH2 | 2.36(q,7.8) | 29.2 | |||
| CH=CH | 5.43(t,7.8) | 114.7 | |||
| COOH | 150.0 | ||||
| 注:*表示用于定量计算的质子;s表示单峰,d表示双峰,t表示三重峰,q表示四重峰,dd表示双峰的双峰,ddd表示六重峰,m表示多重峰,b表示宽峰;U1和U2表示未归属的代谢物;#表示未被确定的信号或者峰裂分方式;Glc表示葡萄糖结构单元;apiose表示芹菜糖基;G表示没食子酸结构单元;galloy表示没食子酰基 | |||||
对主根、一级侧根和二级侧根甲醇提取物的1H NMR谱图进行比较(图 2)可以看出:不同根部位的代谢物类型大致相似,而苯乙酰类成分(Acetophenones)和蔗糖 (Sucrose)等代谢物信号强度具有明显差异.由于植物提取物成分的复杂性以及多组NMR数据比较的艰难性,直观分辨这些NMR谱的差异性具有一定的难度,而多变量数据分析更适合挖掘如此复杂的数据[13].
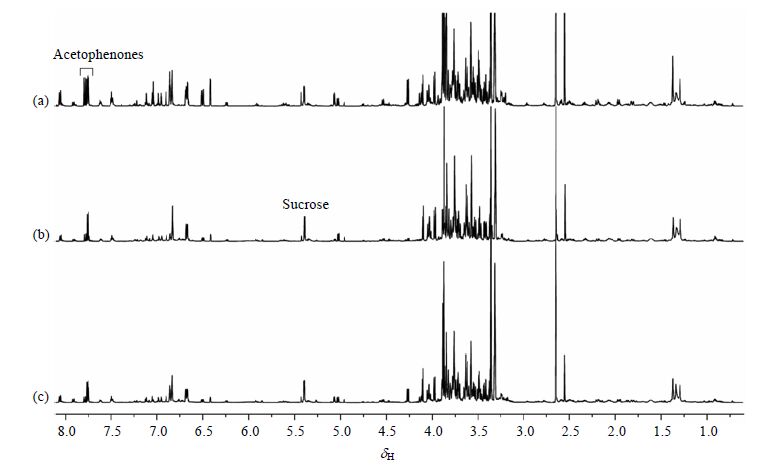
|
| 图 2 牡丹不同根部位甲醇提取物的1H NMR谱图. (a) 主根,(b) 一级侧根,(c) 二级侧根 Fig. 2 1H NMR spectra of tree peony methanol extracts from different root parts,including (a) axial root, (b) first lateral root and,(c) second lateral root |
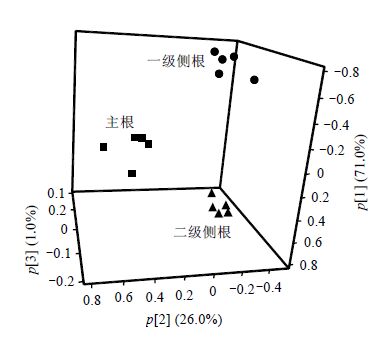
图 3显示了牡丹不同根部位提取物的3D主成分分析散点图.来自相同根部位的样本紧密地聚集,这表明它们代谢物组成相似以及样本提取过程和NMR测量具有重现性.此外,取自主根、一级侧根和二级侧根样本之间有着明显的分离,这表明牡丹不同根部位的代谢物组成具有一定的差异.为了明确牡丹不同根部位代谢组成的分布情况,对不同代谢物进行含量测定,并采用单尾-ANOVA进行统计分析.
|
| 图 3 牡丹不同根部位的3D PCA分析散点图 Fig. 3 The 3D PCA score plot of methanol extracts of tree peony different root parts |
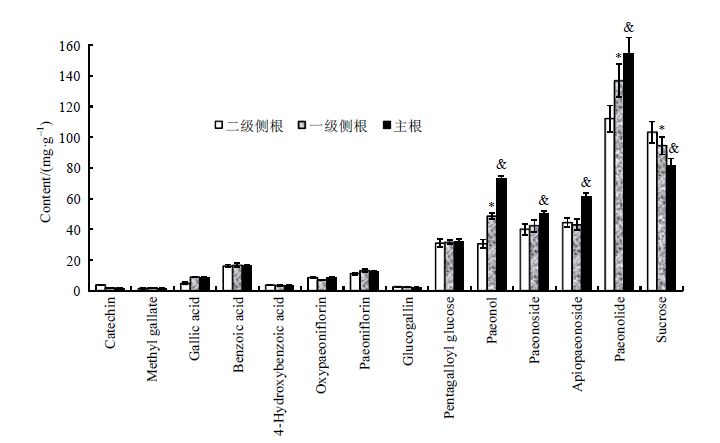
图 4描述了代谢物在牡丹干燥根皮中的含量变化.显而易见:丹皮酚原苷 (Paeonolide)在牡丹根部的含量最大(110~160 mg/g),丹皮酚(Paeonol)、丹皮酚苷(Paeonoside)、丹皮酚新苷(Apiopaeonoside)和蔗糖为牡丹根部含量中等的代谢物(30~100 mg/g),而苯甲酸(Benzoic acid)、4-羟基苯甲酸(4-Hydroxybenzoic acid)、没食子酸(Gallic acid)、没食子酸甲酯(Methyl gallate)、没食子酸吡喃葡萄糖(Glucogallin)、五没食子酸酰葡萄糖(Pentagalloyl glucose)、芍药苷(Paeoniflorin)、氧化芍药苷(Oxypaeoniflorin)以及儿茶素(Catechin)为含量较低的代谢物(2~30 mg/g).这表明苯乙酰类为牡丹根部甲醇提取物的主要代谢物,与报道的研究结果相一致[18].蔗糖为植物体内的内源性成分,为植物的生长发育提供营养物质,其含量较高是不难理解的[25].儿茶素在牡丹根中含量较低,这可能归结于黄酮类成分主要存在于牡丹的花中[26, 27].牡丹根部甲醇提取物中酚酸类和单萜类代谢物的含量较低,这可能是其在甲醇中溶解性较差之缘故.
|
| 图 4 牡丹皮不同根部位代谢物的含量变化(平均值±标准偏差,mg/g,n = 5),*与二级侧根相比,一级侧根具有显著性差异的代谢物(p < 0.05);&与二级侧根相比,主根中具有显著性差异的代谢物(p < 0.05) Fig. 4 The content variation (mean ± SD,mg/g,n = 5) of metabolites in different root parts of tree peony. * Metabolites with significant difference in the first lateral root compared with second lateral root (p < 0.05); & Metabolites with significant difference in the axial root compared with second lateral root (p < 0.05) |
不同根部位之间的代谢物含量相比较可以发现:苯乙酰类成分和蔗糖具有显著性差异(p < 0.05),而酚酸和单萜苷以及儿茶素等成分的含量几乎相同.丹皮酚(Paeonol)、丹皮酚新苷(Apiopaeonoside)、丹皮酚原苷(Paeonolide)和丹皮酚苷(Paeonoside)等苯乙酰类代谢物在二级侧根、一级侧根和主根的含量中依次升高.这些苯乙酰类代谢物为芍药属植物药的生物活性物质,具有抗糖尿病、保护肝损伤、抗氧化和清除自由基的作用,这些代谢物通常作为药用牡丹皮品质评价的关键代谢物.众所周知,植物根的不同部位在代谢物积累、合成和分泌等植物生理过程中发挥着不同的作用.植物根扎于土壤中,可储存必要的物质、完成水分和营养物的吸收及保护植物免受有害微生物的侵害等.为了抵抗感染,植物根部通常会释放大量的生物活性物质(次生代谢物)到植物根系区域的土壤中[28].牡丹主根比侧根的苯乙酰类代谢物含量更高,这可能归结于生物活性成分主要储存于主根,而从侧根中释放到土壤以保护自身免受病原真菌、细菌和病毒的侵害,同时,可初步推断牡丹主根的品质优于侧根.相反的是蔗糖的含量在二级侧根、一级侧根和主根的含量中依次降低,与根部的生长年限呈负相关.蔗糖为植物中的能量物质,也是生物合成丹皮酚苷类成分的必需原料.蔗糖在侧根的含量较高,表明植物在生长发育初期需要大量的能量物质,并为结构更复杂的次生代谢物的合成提供物质基础.
3 结论应用NMR和HPLC-MS方法对牡丹不同根部位进行了代谢物轮廓分析,共鉴定了16种代谢物.发现在牡丹根中蔗糖、丹皮酚以及丹皮酚苷等成分的含量较高,而酚酸、黄酮和单萜苷类成分的含量较低.在二级侧根、一级侧根和主根等不同根部位中,丹皮酚及丹皮酚苷类代谢物的含量依次较高,而蔗糖的含量依次降低,这与根部位的植物生理有关.由于主根对丹皮酚及丹皮酚苷等活性成分较高的储存量,推测主根的品质优于侧根,这些结果为市场上牡丹根的等级划分提供了重要的科学依据.
| [1] | Pharmacopoeia Commission of People’s Republic of China(国家药典委员会). Pharmacopoeia of People’s Republic of China (中华人民共和国药典)[M]. Beijing(北京): Chemical Industry Press(化学工业出版社), 2010. |
| [2] | Hu S L, Shen G, Zhao W G, et al. Paeonol, the main active principles of Paeonia moutan, ameliorates alcoholic steatohepatitis in mice[J]. J Ethnopharmacol, 2010, 128(1): 100-106. |
| [3] | Fu P K, Yang C Y, Tsai T H, et al. Moutan cortex radicis improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation[J]. Phytomedicine, 2012, 19(13): 1 206-1 215. |
| [4] | Yun C S, Choi Y G, Jeong M Y, et al. Moutan cortex radicis inhibits inflammatory changes of gene expression in lipopolysaccharide-stimulated gingival fibroblasts[J]. J Nat Med, 2013, 67(3): 576-589. |
| [5] | Park J, Kim H Y, Lee S M. Protective effects of Moutan cortex radicis against acute hepatotoxicity[J]. Afr J Tradit Complem Alterna Med, 2011, 8(5): 220-225. |
| [6] | Kim H G, Park G, Piao Y, et al. Effects of the root bark of Paeonia suffruticosa on mitochondria-mediated neuroprotection in an MPTP-induced model of Parkinson's disease[J]. Food Chem Toxicol, 2014, 65: 293-300. |
| [7] | He C N, Peng Y, Zhang Y C, et al. Phytochemical and biological studies of Paeoniaceae[J]. Chem Biodivers, 2010, 7: 805-838. |
| [8] | Zha L P, Cheng M E, Peng H S. Identification of ages and determination of paeoniflorin in roots of Paeonia lactiflora Pall. From four producing areas based on growth rings[J]. Microsc Res Techniq, 2012, 75(9): 1 191-1 196. |
| [9] | Ding Y, Wu E Q, Chen J B, et al. Quality evaluation of Moutan cortex radicis using multiple component analysis by high performance liquid chromatography[J]. B Kor Chem Soc, 2009, 30(10): 2 240-2 244. |
| [10] | Long Quan-jiang(龙全江), Wang Xiao-ge(王晓阁), Zhou Zhou(周宙), et al. Study on the influence of the contents of paeonol in the cutting process of fresh Cortex moutan(趁鲜切制法对牡丹皮饮片中丹皮酚含量的影响研究)[J]. Journal of Chinese Medicinal Materials(中药材), 2012, 35(6): 883-886. |
| [11] | Sun Qing-lei(孙庆雷), Zhao Hong-xia(赵红霞), Lin Yun-liang(林云良), et al. 1H NMR fringerprints of Scutellaria Baicalensis(黄芩的1H NMR指纹图谱研究)[J]. Chinese J Magn Reson(波谱学杂志), 2007, 24(2): 163-168. |
| [12] | Chen Bo(陈波), Kang Hai-ning(康海宁), Han Chao(韩超), et al. Applications of NMR spectroscopy and pattern recognition in food analysis (NMR指纹图谱与模式识别方法在食物分析中的应用)[J]. Chinese J Magn Reson(波谱学杂志), 2006, 23(3): 397-407. |
| [13] | Dai H, Xiao C N, Liu H B, et al. Combined NMR and LC-MS analysis reveals the metabonomic changes in Salvia miltiorrhiza Bunge induced by water depletion[J]. J Proteome Res, 2010, 9: 1 460-1 475. |
| [14] | Herve Du P C, Imberty A, Roques N, et al. Conformational behavior of sucrose and its deoxy analogue in water as determined by NMR and molecular modeling[J]. J Am Chem Soc, 1991, 113(10): 3 720-3 727. |
| [15] | Kwon D Y, Song H N, Yoon S H. Synthesis of medium-chain glycerides by lipase in organic solvent[J]. J Am Oil Chem Soc, 1996, 73(11): 1 521-1 525. |
| [16] | Kuwajima H, Shibano N, Baba T, et al. An acetophenone glycoside from Exacum affine[J]. Phytochemistry, 1996, 41(1): 289-292. |
| [17] | Yu J, Lang H Y, Xiao P G. A new compound, apiopaeonoside, isolated from the root of Paeonia suffruticosa[J]. Acta Pharm Sin, 1986, 21(3): 191-197. |
| [18] | Ha D T, Trung T N, HienT T, et al.Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesis via AMPK activation in human HepG2 cells[J]. J Ethnopharmacol, 2010, 131(2): 417-424. |
| [19] | Peungvicha P, Temsiririrkkul R, Prasain J K, et al. 4-Hydroxybenzoic acid: a hypoglycemic constituent of aqueous extract of Pandanus odorus root[J]. J Ethnopharmacol, 1998, 62(1): 79-84. |
| [20] | Lee S C, Kwon Y S, Son K H, et al. Antioxidative constituents from Paeonia lactiflora[J]. Arch Pharm Res, 2005, 28(7): 775-783. |
| [21] | Puppala M, Ponder J, Suryanarayana P, et al. The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis[J]. Plos One, 2012, 7(4): e31399. |
| [22] | Beretta G, Artali R, Caneva E, et al. Conformation of the tridimensional structure of 1, 2, 3, 4, 6-pentagalloyl-beta-D- glucopyranose (PGG) by H-1 NMR, NOESY and theoretical study and membrane interaction in a simulated phospholipid bilayer: a first insight[J]. Magn Reson Chem, 2010, 49(3): 132-136. |
| [23] | Piao X S, Piao X L, Kim H Y, et al. Antioxidative activity of geranium (Pelargonium inquinans Ait) and its active component, 1, 2, 3, 4, 6-penta-O-galloyl-beta-D-glucose[J]. Phytother Res, 2008, 22(4): 534-538. |
| [24] | Qi S H,Wu D G, Ma Y B, et al. A novel flavane carapa guianensis[J]. Acta Bot Sin, 2013, 45(9): 1 129-1 133. |
| [25] | Drew S W, Demain A L. Effect of primary metabolites on secondary metabolism[J]. Annu Rev Microbiol, 1977, 31: 343-356. |
| [26] | Wang X, Cheng C, Sun Q L, et al. Isolation and purification of four flavonoid constituents from the flowers of Paeonia suffruticosa by high-speed counter-current chromatography[J]. J Chromatogra A, 2005, 1 075(1-2): 127-131. |
| [27] | Li C, Du H, Wang L, et al. Flavonoid composition and antioxidant activity of tree peony (Paeonia Section Moutan) yellow flowers[J]. J Agric Food Chem, 2009, 57(18): 8 496-8 503. |
| [28] | Baetz U, Martinoia E. Root exudates: the hidden part of plant defense[J]. Trends Plant Sci, 2013, 19(2): 90-98. |
 2015, Vol. 32
2015, Vol. 32




