文章信息
- 王燕妮,金 芩,李慧丹,王朝杰
- WANG Yan-ni, JIN Qin, LI Hui-dan, WANG Chao-jie
- 吉非替尼谱学性质计算研究
- Theoretical Calculation of Spectral Characteristics of Gefitinib
- 波谱学杂志, 2015, 32(3): 528-541
- Chinese Journal of Magnetic Resonance, 2015, 32(3): 528-541
- http://dx.doi.org/10.11938/cjmr20150314
-
文章历史
- 收稿日期:2014-11-04
- 收修改稿日期:2015-07-30
肺癌作为一种常见的癌症,其发病率和死亡率均呈逐年上升趋势,并居我国十大恶性肿瘤排行榜榜首[1],其中非小细胞肺癌(NSCLC)约占肺癌的80%[2].吉非替尼(Gefitinib),又名易瑞沙(Iressa),是第一个被批准上市用于治疗晚期NSCLC的靶向药物[3, 4, 5],是一种合成的苯胺喹唑啉化合物[6],分子式为C22H24ClFN4O3,其结构式如图 1所示.吉非替尼对酪氨酸激酶受体具有很强的选择性抑制作用,是一种表皮生长因子受体(EGFR)酪氨酸酶抑制剂(TKI)[7].

|
| 图 1 吉非替尼分子结构及原子编号 Fig. 1 Schematic structure and atoms number of Gefitinib |
目前,国内外对吉非替尼的研究主要集中在两个方面.其一,基础研究[8, 9, 10, 11, 12].已有Chang等人[12]的研究结果表明吉非替尼通过氧化磷酸化作用来诱导人类胶质瘤细胞的凋亡,从而达到抗癌作用.其二,临床研究[13, 14, 15, 16, 17].Ochs等人[13]在美国第40届临床肿瘤年会上报道的21 064 例扩大临床试验(EAP)的结果证实了女性、东方人和疗效相关.在吉非替尼的II期试验中已经显示了其单药对化疗失败的NSCLC的疗效,同时揭示了一些与疗效相关的因素.近年来,理论计算得到了快速的发展和广泛的应用.采用杂化密度泛函理论B3LYP方法研究分子结构、IR、UV和NMR,是目前运用最为广泛的[18, 19, 20, 21, 22, 23, 24].
Gao等人[25]用不同的DFT方法研究抗癌的铂类化合物时发现,B3LYP/SDD是用于预测这种抗癌药13C NMR的最好方法.采用BHandHLYP,CAM-B3LYP,LC-ωPBE和M06- 2X方法用于描述晶体结构和吸收光谱也有相关报道[26, 27, 28, 29].本工作将采用上述方法计算研究吉非替尼的结构和谱学性质.
1 计算方法根据张瑞勤等人[30]提出的一种选择基组的有效方法,本文采用6-311++G**基组,运用Hybrid-GGA(B3LYP和BHandHLYP)、Hybrid-meta-GGA(M06-2X)和Range-separated hybrid(CAM-B3LYP和LC-ωPBE)三类5种密度泛函理论(DFT)方法对吉非替尼分子的结构进行优化并计算了IR,UV-Vis,1H NMR和13C NMR系列谱学性质,通过比较计算值和实验值得到最佳的计算条件.在计算吉非替尼的化学位移时,运用了Circularly Sy m metrical Gabor Transform(CSGT)[31]和Gauge-Including Atomic Orbital(GIAO)[32, 33]两种经典的方法,采用极化连续介质模型(PCM)考虑CHCl3,(CH3)2SO(DMSO),CH3OH三种溶剂的影响.为了便于和实验值进行比较,在化学位移的计算中,分别以四甲基硅烷(TMS)的氢和碳的化学位移为基准.全部计算工作均用Guassian 09[34]程序包完成.
2 结果与讨论 2.1 吉非替尼分子的平衡几何结构图 2为优化后吉非替尼分子的几何结构,原子编号序数示于图 1中.用5种方法计算得到吉非替尼在气相、氯仿、二甲亚砜和甲醇中的部分键长分别对应于图 2中的(a),(b),(c),(d).图的下方分别列出在不同环境下吉非替尼分子的部分键角和二面角.各种方法在同一溶剂中得到的键长比较接近.B3LYP方法的键长略长于其他4种方法;同一方法在不同介质中所得键长相差约0.001 nm,键角相差0.1°,二面角相差2°;吉非替尼在二甲亚砜和甲醇中的键长相比,一致度高达90%,这可能与二者均为极性溶剂有关.5种方法计算吉非替尼分子的A、B和C三环大致在一个平面上,5种方法计算得到的二面角D(C5C10N11C12)在179°~180°之间.由此我们可以看出,在计算分子结构时,对于刚性结构的部分,用不同类型的DFT方法结构大致相同.

|
| 图 2 吉非替尼在气相(a)、氯仿(b)、二甲亚砜(c)和甲醇(d)四中环境中的几何结构 Fig. 2 Geometrical structure of Gefitinib in gas phase (a),chloroform (b),dimethyl sulfoxide (c) and methanol (d) environments. The five values from top to bottom (from left to right) correspond to the results optimized at the B3LYP,BHandHLYP,M06-2X,CAM-B3LYP and LC-ωPBE levels in these environments,respectively; bond length in nm |
我们采用不同DFT方法在6-311++G**基组水平对吉非替尼进行红外光谱计算.用5种DFT方法计算红外光谱数值,Origin8.0作图,结果如图 3所示.从图 3中可以看出吉非替尼的红外特征峰集中在1 800~1 000 cm-1之间.以CAM-B3LYP方法进行讨论,1 707~1 687 cm-1之间的一组峰是由环上的C-C伸缩振动产生,而1 678~1 548 cm-1之间的一组峰是由环上的C-C和C-N伸缩振动引起的,其中1 678~1 637 cm-1之间的一组峰以C-N的伸缩振动为主,而1 597~1 548 cm-1之间的一组峰则主要是C-C的伸缩振动.位于1 535~1 456 cm-1的一组峰是由链上的C-H弯曲振动造成,其中1 498 cm-1处的峰是由-CH3的不对称变形振动引起,而-CH3的对称变形振动促使1 472 cm-1处峰的形成.此外,1 272、1 244和1 231 cm-1处的峰都包含有C-O的伸缩振动模式.图 3五种方法计算所得的红外光谱中,在低频部分5种方法一致度较高,而在高频部分B3LYP得到的结果出现了明显的红移现象,这种现象在文献[35]中也有报道.
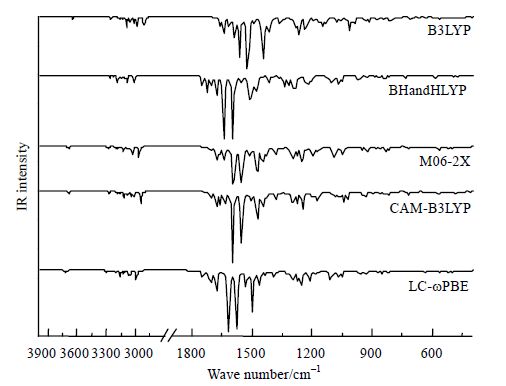
|
| 图 3 用不同理论方法在气相中计算的吉非替尼红外谱图 Fig. 3 Infared spectrum of Gefitinib calculated on different levels of methods in gas phase |
已知的红外实验数据及其相对应的理论计算值列于表 1,以表 1中的实验值[36]为纵坐标,与实验值对应的计算值为横坐标进行作图,得5条拟合直线对应方程列于图 4中.从拟合直线的相关系数中得到,用LC-ωPBE方法所得计算值与实验值的拟合度最好,R2为0.986 8,M06-2X和CAM-B3LYP方法次之.将计算所得红外光谱范围内(400~ 4 000 cm-1)的数值代入方程中,即得对应的理论实验值,二者统计结果如表 1所示.采用6-311++G**基组,考虑平均绝对偏差(MAD),发现B3LYP方法给出的计算结果最接近实验值,其平均绝对偏差在5种方法中最小,为17;CAM-B3LYP和M06-2X次之,分别为32和33.B3LYP的MAD在5种方法中最小, 然而计算值 与实验值的R2仅为0.980 6,为5种方法中拟合优度最低的方法.综合考虑拟合优度和MAD两个因素,最理想的用于预测吉非替尼红外振动频率的计算方法为CAM-B3LYP和M06-2X.Malik等人[37]研究1-苯基-1-硅代吗啡啉时得出CAM-B3LYP预测的频率值与实验值吻合度较好;Ricardo等人[38]用M06-2X/6-311+G(d,p)对二甲氧基查尔酮的红外光谱进行计算后与实验值进行比较,发现二者一致度较高.这些报道[37, 38]与本文所得结论一致.
| Vibrational assignments | Exp.a | B3LYP | BHandHLYP | M06-2X | CAM-B3LYP | LC-ωPBE |
| 6-311++G** | ||||||
| g =CH | 822 | 814 | 862 | 834 | 837 | 852 |
| g =CH | 860 | 842 | 883 | 860 | 860 | 872 |
| n C-O | 1 071 | 1 067 | 1 114 | 1 087 | 1 087 | 1 100 |
| n C-O | 1 161 | 1 133 | 1 195 | 1 161 | 1 164 | 1 166 |
| n C-O | 1 225 | 1 214 | 1 266 | 1 228 | 1 231 | 1 240 |
| n C-O | 1 276 | 1 264 | 1 302 | 1 273 | 1 272 | 1 273 |
| ds,CH3 | 1 366 | 1 445 | 1 512 | 1 473 | 1 472 | 1 476 |
| das,CH3 | 1 439 | 1 486 | 1 546 | 1 498 | 1 498 | 1 497 |
| n C=C | 1 498 | 1 524 | 1 592 | 1 548 | 1 548 | 1 569 |
| n C=C | 1 574 | 1 527 | 1 597 | 1 556 | 1 554 | 1 578 |
| n C=N | 1 632 | 1 587 | 1 678 | 1 639 | 1 637 | 1 680 |
| Mean Absolute Deviationb | 30 | 57 | 25 | 26 | 35 | |
| Mean Absolute Deviationc | 17 | 69 | 33 | 32 | 47 | |
| a: Cited from Ref. [36]; b: 已知红外实验数据与相对应理论数据的MAD; c: 红外光谱范围内计算值与理论实验值的MAD | ||||||
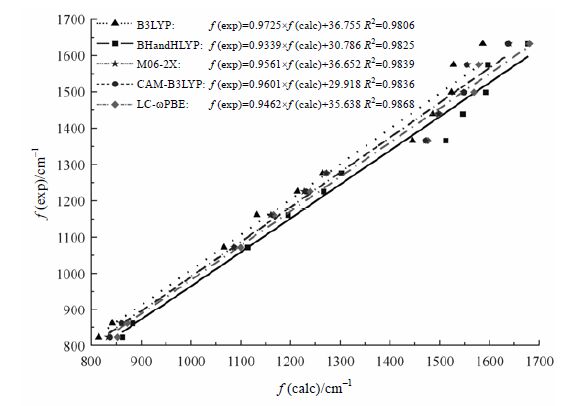
|
| 图 4 5种DFT方法与实验值的拟合曲线 Fig. 4 Linear fitting of five DFT methods with the experimental values for Gefitinib |
用B3LYP等5种DFT方法计算吉非替尼在气相中的紫外可见光谱.图 5是吉非替尼分子在5种DFT理论计算方法和6-311++G**基组水平下得到UV-Vis光谱图,由图可知,在紫外可见光谱范围内,吉非替尼分子有两个特征吸收峰.以CAM-B3LYP方法为例进行讨论,在150~250 nm范围内有一个强吸收峰;250~290 nm范围存在一个中等强度的吸收峰;说明分子中存在苯基.300 nm以上处存在吸收峰,说明吉非替尼中具有较大的共轭体系[39],该化合物中为喹唑啉环.B3LYP计算所得UV-Vis图在249.18 nm处存在一个肩峰,为苯基的特征 峰 .与其他方法相比,B3LYP的计算结果有红移现象,文献[40]中用B3LYP计算紫外可见光谱值时出现了相同现象.

|
| 图 5 用不同理论方法在气相中计算的吉非替尼紫外可见谱图 Fig. 5 UV-Vis spectra calculated for Gefitinib on different levels of methods in gas phase |
表 2给出5种方法下计算得到的紫外可见光谱参数.每种方法选取振子强度(f)最大的3组参数,其对应的电子跃迁轨道、激发能及吸收波长列于表 2中.从表中可以看出,B3LYP,BHandHLYP,M06-2X,CAM-B3LYP和LC-ωPBE得到的最大吸收波长分别为331.1,280.2,290.2,295.5,271.1 nm,它们几乎全部是由HOMO向LUMO跃迁所至.图 6列出CAM-B3LYP方法下的从HOMO-2~LUMO+2的前线轨道,其它方法下的前线轨道类似.由图 6可知,吉非替尼的最高占据轨道(HOMO)和最低未占轨道(LUMO)的轨道分布主要集中在3个存在共轭体系的环上,HOMO向LUMO跃迁主要是苯环上的电子向喹唑啉环上跃迁,它的最大吸收峰和最强吸收峰主要是π®π*电子跃迁.在前线轨道中,分子中的共轭体系贡献很大.LUMO+2轨道分布在3个具有共轭体系的环上,以集中在苯环上为主;LUMO+1,HOMO,HOMO-2轨道上的电子云均匀分布在3个环上;HOMO-1轨道上的电子云则集中在侧链的杂环上.HOMO-LUMO能隙差为4.197 4 eV,说明吉非替尼分子是非常稳定的.
| Method | Excited state | Electronic Transition | Excited energy/ev | λ/nm | f |
| B3LYP | S1 | 117®118(100%) | 3.7 | 331.1 | 0.4 |
| S2 | 117®121 (70% ) | 5.0 | 249.2 | 0.3 | |
| 117®122 (22% ) | |||||
| S3 | 115®120 (26.2%) | 5.6 | 221.8 | 0.4 | |
| 117®124 (26.1%) | |||||
| BHandHLYP | S1 | 115®118 (3.7%) | 4.4 | 280.2 | 0.5 |
| 117®118 (96.3%) | |||||
| S2 | 113®118 (17.2%) | 5.9 | 211.2 | 0.2 | |
| 117®125 (22.1%) | |||||
| S3 | 114®118 (24.4%) | 6.2 | 200.6 | 0.5 | |
| 114®120 (14.3%) | |||||
| M06-2X | S1 | 115®118 (3%) | 4.3 | 290.2 | 0.5 |
| 117®118 (97%) | |||||
| S2 | 114®118 (30.5%) | 6.0 | 205.6 | 0.4 | |
| 114®120 (13.6%) | |||||
| S3 | 113®118 (29%) | 6.2 | 201.3 | 0.4 | |
| 114®120 (17.7%) | |||||
| CAM-B3LYP | S1 | 115®118 (4.1%) | 4.2 | 295.5 | 0.5 |
| 117®118 (93.4%) | |||||
| S2 | 114®118 (21.3%) | 6.0 | 206.8 | 0.6 | |
| 114®119 (19%) | |||||
| S3 | 113®118 (46.5%) | 6.1 | 203.3 | 0.3 | |
| 114®119 (12.4%) | |||||
| LC-ωPBE | S1 | 115®118 (9.5%) | 4.6 | 271.1 | 0.5 |
| 117®118 (85.4%) | |||||
| S2 | 114®118 (41.9%) | 6.0 | 208.2 | 0.6 | |
| 117®121 (13.6%) | |||||
| S3 | 113®118 (18.2%) | 6.6 | 189.0 | 0.8 | |
| 115®118 (23.6%) |
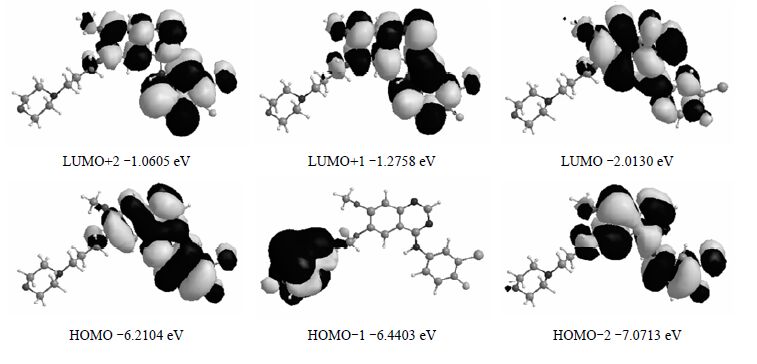
|
| 图 6 CAM-B3LYP方法得到的吉非替尼的前线分子轨道图 Fig. 6 The frontier molecular orbitals calculated for Gefitinib on CAM-B3LYP method |
采用B3LYP等5种计算理论和6-311++G**基组,分别运用CSGT和GIAO两种经典的方法计算吉非替尼分子1H NMR和13C NMR的化学位移.并采用极化连续介质模型(PCM)考虑CHCl3、(CH3)2SO、CH3OH三种溶剂的影响.为了便于和实验值进行比较,在化学位移的计算中,分别以四甲基硅烷(TMS)的氢和碳的化学位移来定标,并考虑其相应的溶剂效应.表 3与表 4中分别列出了用GIAO和CSGT方法在6-311++G**基组和不同的计算理论方法下计算的吉非替尼分子在(CH3)2SO中1H NMR和13C NMR化学位移值.原子编号如图 1所示.通过比较化学位移的计算值与实验值,得出不同理论计算方法的误差范围列于表 3和表 4中,从而确定最佳计算化学位移值的方法组合.
| Method | 1H NMR | ||||||||||||||
| dH32 | dH33 | dH34 | dH35 | dH36,37 | dH38,39 | dH40,41 | dH42~44 | ||||||||
| B3LYP | GIAO | 7.78 | 7.83 | 8.93 | 7.45 | 4.13 | 2.22 | 2.26 | 4.20 | ||||||
| CSGT | 6.73 | 6.61 | 7.56 | 6.18 | 3.52 | 1.71 | 1.71 | 3.49 | |||||||
| BHandHLYP | GIAO | 7.96 | 8.04 | 9.00 | 7.39 | 3.91 | 2.17 | 2.15 | 4.06 | ||||||
| CSGT | 7.13 | 7.01 | 7.87 | 6.42 | 3.61 | 1.90 | 1.88 | 3.66 | |||||||
| M06-2X | GIAO | 8.37 | 8.46 | 9.36 | 7.67 | 4.10 | 2.31 | 2.21 | 4.31 | ||||||
| CSGT | 7.38 | 7.22 | 8.09 | 6.60 | 3.48 | 1.74 | 1.72 | 3.48 | |||||||
| CAM-B3LYP | GIAO | 7.90 | 7.97 | 8.99 | 7.46 | 4.08 | 2.22 | 2.25 | 4.21 | ||||||
| CSGT | 6.91 | 6.79 | 7.67 | 6.20 | 3.54 | 1.76 | 1.76 | 3.56 | |||||||
| LC-ωPBE | GIAO | 8.05 | 8.14 | 9.02 | 7.45 | 3.94 | 2.15 | 2.17 | 4.11 | ||||||
| CSGT | 7.02 | 6.93 | 7.73 | 6.18 | 3.46 | 1.71 | 1.72 | 3.49 | |||||||
| Exp.a | 7.20 | 7.82 | 8.50 | 9.57 | 4.19 | 1.99 | 2.51 | 3.94 | |||||||
| Method | 1H NMR | Mean Absolute Deviation | |||||||||||||
| dH45 | dH46 | dH47 | dH48,49 | dH50,53 | dH54,55 | ||||||||||
| B3LYP | GIAO | 9.94 | 7.42 | 7.25 | 2.44 | 3.84 | 2.44 | 0.350 0 | |||||||
| CSGT | 8.29 | 6.18 | 5.96 | 1.95 | 3.21 | 1.95 | 0.730 0 | ||||||||
| BHandHLYP | GIAO | 10.10 | 7.64 | 7.45 | 2.34 | 3.69 | 2.34 | 0.370 0 | |||||||
| CSGT | 8.54 | 6.60 | 6.38 | 2.13 | 3.32 | 2.13 | 0.540 0 | ||||||||
| M06-2X | GIAO | 8.72 | 8.23 | 7.84 | 2.25 | 3.71 | 2.25 | 0.400 0 | |||||||
| CSGT | 8.71 | 6.77 | 6.57 | 1.94 | 3.13 | 1.94 | 0.640 0 | ||||||||
| CAM-B3LYP | GIAO | 10.20 | 7.58 | 7.39 | 2.41 | 3.82 | 2.41 | 0.370 0 | |||||||
| CSGT | 8.42 | 6.37 | 6.16 | 2.00 | 3.25 | 2.00 | 0.660 0 | ||||||||
| LC-ωPBE | GIAO | 10.32 | 7.74 | 7.54 | 2.35 | 3.71 | 2.35 | 0.430 0 | |||||||
| CSGT | 8.51 | 6.51 | 6.31 | 1.92 | 3.15 | 1.92 | 0.690 0 | ||||||||
| Exp.a | 8.13 | 7.45 | 7.79 | 2.41 | 3.59 | 2.41 | |||||||||
| a: Cited from Ref. [41] | |||||||||||||||
| Method | 13C NMR | |||||||||||
| dC1 | dC2 | dC3 | dC4 | dC5 | dC6 | dC8 | dC10 | dC12 | dC14 | dC15 | ||
| B3LYP | GIAO | 162.4 | 168.9 | 130.1 | 157.2 | 119.5 | 122.2 | 161.7 | 162.4 | 145.7 | 79.67 | 34.17 |
| CSGT | 157.5 | 164.4 | 125.4 | 151.6 | 113.2 | 117.1 | 156.4 | 157.3 | 140.9 | 77.55 | 33.78 | |
| BHandHLYP | GIAO | 160.2 | 168.4 | 130.2 | 158.7 | 118.4 | 123.8 | 163.2 | 165.5 | 146.5 | 75.62 | 32.92 |
| CSGT | 158.3 | 166.8 | 127.3 | 156.5 | 114.9 | 121.4 | 161.8 | 164.2 | 144.9 | 76.33 | 33.83 | |
| M06-2X | GIAO | 174.5 | 182.0 | 143.8 | 171.9 | 130.5 | 135.4 | 175.7 | 175.3 | 156.0 | 79.29 | 34.00 |
| CSGT | 170.7 | 178.9 | 140.3 | 167.0 | 125.7 | 131.6 | 170.9 | 172.1 | 154.6 | 78.03 | 33.22 | |
| CAM-B3LYP | GIAO | 163.0 | 170.0 | 132.0 | 159.2 | 120.1 | 124.5 | 164.6 | 166.2 | 147.5 | 77.87 | 33.73 |
| CSGT | 159.8 | 167.6 | 128.4 | 155.5 | 115.4 | 121.0 | 160.9 | 162.9 | 144.3 | 77.23 | 33.73 | |
| LC-ωPBE | GIAO | 162.7 | 170.7 | 133.7 | 159.5 | 119.8 | 126.9 | 166.6 | 167.9 | 147.0 | 75.54 | 31.52 |
| CSGT | 160.0 | 168.7 | 130.3 | 156.8 | 115.9 | 124.1 | 163.6 | 165.6 | 144.9 | 74.86 | 31.66 | |
| Exp.a | 148.3 | 154.4 | 102.4 | 146.9 | 108.8 | 107.2 | 152.5 | 155.9 | 136.9 | 67.07 | 25.85 | |
| Method | 13C NMR | Mean Absolute Deviation | ||||||||||
| dC16 | dC19 | dC20 | dC21 | dC22 | dC23 | dC24 | dC26,30 | dC27,29 | ||||
| B3LYP | GIAO | 58.75 | 65.36 | 126.5 | 135.8 | 163.6 | 123.8 | 124.9 | 58.39 | 72.82 | 9.780 | |
| CSGT | 57.38 | 62.94 | 121.5 | 130.6 | 157.8 | 118.6 | 120.5 | 56.91 | 70.92 | 6.240 | ||
| BHandHLYP | GIAO | 55.52 | 62.6 | 127.9 | 134.3 | 161.4 | 125.5 | 126.7 | 55.09 | 68.41 | 8.840 | |
| CSGT | 56.23 | 62.95 | 124.8 | 132.7 | 159.1 | 122.7 | 124.2 | 56.03 | 69.64 | 7.750 | ||
| M06-2X | GIAO | 59.31 | 64.49 | 146.2 | 146.8 | 173.8 | 137.6 | 141.3 | 59.17 | 71.16 | 18.12 | |
| CSGT | 57.31 | 63.9 | 135.5 | 142.8 | 168.7 | 133.5 | 135.5 | 56.83 | 70.49 | 14.78 | ||
| CAM-B3LYP | GIAO | 57.50 | 64.19 | 129.1 | 135.5 | 164.8 | 126.4 | 127.7 | 57.44 | 71.52 | 10.55 | |
| CSGT | 56.67 | 63.16 | 124.8 | 132.3 | 160.5 | 122.4 | 124.1 | 56.51 | 70.77 | 7.970 | ||
| LC-ωPBE | GIAO | 54.74 | 63.25 | 130.8 | 133.6 | 165.3 | 128.5 | 129.9 | 54.65 | 69.04 | 10.27 | |
| CSGT | 53.96 | 62.37 | 126.8 | 131.8 | 161.2 | 125.1 | 126.5 | 53.62 | 68.62 | 8.210 | ||
| Exp.a | 54.99 | 55.77 | 123.4 | 118.8 | 153.1 | 116.4 | 122.1 | 53.42 | 66.15 | |||
| a: Cited from Ref. [41] | ||||||||||||
以表 3中1H NMR的实验值为纵坐标,与实验值对应的计算值为横坐标进行作图,如图 7中的(a)和(b)所示,得10个线性拟合方程列于图 7.由10个1H NMR线性拟合方程的相关系数可知,无论是CSGT还是GIAO,5种方法中M06-2X的拟合度要优于其他方法;B3LYP、M06-2X和CAM-B3LYP这3种方法在GIAO水平的拟合度均比CSGT高,BHandHLYP在CSGT和GIAO两种水平下得到拟合曲线的相关系数相等,而LC-ωPBE得到的拟合曲线则是CSGT水平的拟合度较优.综上所述,就相关系数而言,M06-2X//GIAO方法下得到的拟合方程最优,M06-2X//CSGT与B3LYP//GIAO方法次之.

|
| 图 7 用5种DFT方法及两种NMR方法(GIAO,CSGT)在二甲亚砜中的计算值与实验值的拟合曲线 Fig. 7 Linear fitting of five DFT methods and two NMR calculated methods(GIAO,CSGT) with the experimental values in DMSO solvent for Gefitinib |
从表 3中看出,GIAO方法下产生的计算结果与实验数据相比,均比CSGT方法接近.用GIAO方法在B3LYP,BHandHLYP,M06-2X,CAM-B3LYP和LC-ωPBE水平下的计算结果与实验数据的平均绝对偏差分别是0.35,0.37,0.40,0.37,0.43.显然,B3LYP//GIAO获得吉非替尼在DMSO中1H NMR时能给出最好的结果.
以表 4中13C NMR的实验值为纵坐标,与实验值对应的计算值为横坐标进行作图,得到10个线性拟合方程,如图 7中的(c)和(d)所示.从相关系数可知,在5种方法中M06-2X方法的拟合度要优于其他方法;BHandHLYP和LC-ωPBE方法在CSGT水平的拟合度比GIAO高,B3LYP在CSGT和GIAO两种水平下得到拟合曲线的相关系数相等,而M06-2X与CAM-B3LYP得到的拟合曲线则是GIAO水平的拟合度较优.就相关系数而言,BHandHLYP//CSGT方法下得到的拟合方程最优,CAM-B3LYP//GIAO与CAM-B3LYP//CSGT方法次之,它们的拟合度大小相差不大.
从表 4中看出,CSGT方法下获得的13C NMR计算结果与实验数据相比,均比 GIAO方法更接近,这与1H NMR的结果相反.用CSGT方法在B3LYP,BHandHLYP,M06-2X,CAM-B3LYP和LC-ωPBE水平下的计算结果与实验数据的平均绝对偏差分别是6.24,7.75,14.78,7.97,8.21.均比GIAO方法的MAD值小,而其中B3LYP方法计算值与实验接近度最高.
B3LYP//GIAO方法获得13C NMR的计算结果与实验值最接近,BHandHLYP与CAM-B3LYP次之.由于各拟合方程的拟合度相差不大,本文以平均绝对偏差作为衡量方法优劣的因素.因而,B3LYP//CSGT获得吉非替尼在DMSD中13C NMR时能给出最好的结果.Günay等人[42]研究苯甲醛衍生物时得到了相同结论.
从表 3中各方法的MAD值中得出如下结论,不同的DFT方法计算吉非替尼1H NMR在DMSO溶剂中化学位移时,GIAO方法比CSGT方法更为准确;B3LYP//GIAO得到的氢谱化学位移值与实验值最吻合,其MAD为0.35,是5种方法中计算结果与实验值最为接近的.由表 4的统计分析结果表明,在计算13C NMR的化学位移时,CSGT方法更为准确,B3LYP//CSGT得到的碳谱化学位移值最接近实验值,其MAD为6.24.
在13C NMR化学位移值的比较中发现,无论是GIAO还是CSGT计算方法,均高于实验值.综上所述,计算吉非替尼在DMSO溶剂中化学位移,最合理的方法是1H NMR用B3LYP//GIAO计算,而13CNMR则用B3LYP//CSGT计算.基于以上比较所得出的
结果,运用最优计算方法对吉非替尼在CHCl3及CH3OH中的1H NMR和13C NMR的化学位移值进行了预测,结果见图 8.从上至下依次为吉非替尼在CHCl3和CH3OH中预测的NMR数据,其中正体为1H NMR,斜体为13C NMR.可见溶剂极性对化学位移产生稍许影响,极性增加,氢谱数值仅有细微增加,而碳谱数值则普遍稍有增加.

|
| 图 8 分别运用B3LYP//GIAO和B3LYP//CSGT预测吉非替尼的1H NMR和13C NMR (CHCl3和CH3OH环境中) Fig. 8 1H and 13C NMR chemical shifts calculated with B3LYP//GIAO and B3LYP//CSGT methods for Gefitinib,respectively,in CHCl3 and CH3OH environments |
用B3LYP,BHandHLYP,M06-2X,CAM-B3LYP和LC-ωPBE五种密度泛函理论方法在6-311++G**基组下对吉非替尼分子的红外光谱、紫外可见光谱及其核磁共振谱进行了计算.通过理论计算值与实验值进行对比分析,CAM-B3LYP方法是用于描述吉非替尼分子红外光谱的最好方法.吉非替尼的紫外可见光谱有两个特征吸收峰并且用不同方法描述时第一激发态均是由HOMO向LUMO电子跃迁所致.用于描述吉非替尼在DMSO中1H NMR和13C NMR化学位移值较为准确的理论方法分别是B3LYP//GIAO和B3LYP//CSGT.
| [1] | Wang Xia(王霞). Reversing drug resistance of lung cancer, change the treatment of lung cancer predicament—interviewed Professor Zhou, director of respiration department, southwest hospital, third military medical university, Chongqing(逆转肺癌耐药, 改变肺癌治疗窘境—访重庆第三军医大学西南医院呼吸科主任周向东教授)[J]. Chinese Mod Med(中国当代医药), 2014, 21(17): 1-3. |
| [2] | Jin Hui(金慧). Research progress of EGFR-TKI in treatment of non-small cell lung cancer drug resistance mechanism(EGFR-TKI治疗非小细胞肺癌耐药机制的研究进展)[J]. J Sun Yatsen Univ: Med Sci[中山大学学报(医学科学版)], 2009, 30(3S): 216-219. |
| [3] | Morgillo F, Kim W Y, Kim E S, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with Gefitinib[J]. Clin Cancer Res, 2007, 13(9): 2 795-2 803. |
| [4] | Ward R A, Anderton M J, Ashton S, et al. Structure- and reactivity-based development of covalent inhibitors of the activating and gatekeeper mutant forms of the epidermal growth factor receptor(EGFR)[J]. J Med Chem, 2013, 56(17): 7 025-7 048. |
| [5] | Wu Jin-zhi(吴金枝), Li Ning(李宁). Small molecule tyrosine kinase inhibitors in the treatment of 27 cases with advanced non squamous cell lung cancer(小分子酪氨酸激酶抑制剂治疗27例晚期非鳞状细胞肺癌临床观察)[J]. Guizhou Med J(贵州医药), 2013, 37(10): 904-906. |
| [6] | Rasmussen H S, MeCann P P. Metalloproteinase inhibitors as a novel anticancer: A review with special focus on batimastat and marimastat[J]. Pharmaeol Ther, 2005, 75(1): 69-75. |
| [7] | Cheng Gang(程刚). Iressa research progress in non-small cell lung cancer(易瑞沙在非小细胞肺癌的研究进展)[J]. Chinese J Clin Oncol(中国肿瘤临床), 2005, 32(23): 1 375-1 378. |
| [8] | Kim Y, Li Z, Apetri M, et al. Temporal resolution of autophosphorylation for normal and oncogenic forms of EGFR and differential effects of gefitinib[J]. Biochem, 2012, 51(25): 5 212-5 222. |
| [9] | Han Yu (韩宇), Xu Jian-ming(徐建明), Duan Hai-qing(段海清), et al. The relationship between EGFR gene mutation and curative effect and prognosis of Gefitinib in treatment of advanced non-small cell lung cancer(EGFR基因突变与吉非替尼治疗晚期非小细胞肺癌的疗效和预后的关系)[J]. Chinese J Oncol(中华肿瘤杂志), 2007, 29(4): 278-283. |
| [10] | Kim K S, Jeong J Y, Kim Y C, et al. Predictors of the response to Gefitinib in refractory non-small cell lung cancer[J]. Clin Cancer Res, 2005, 11(6): 2 244-2 251. |
| [11] | Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers[J]. Clin Cancer Res, 2005, 11(3): 1 167-1 173. |
| [12] | Chang C Y, Shen C C, Su H L, et al. Gefitinib induces apoptosis in human glioma cells by targeting bad phosphorylation[J]. J Neuro-Oncol, 2011, 105(3): 507-522. |
| [13] | Zhu Jing(朱静). Clinical analysis of 45 cases of Gefitinib in treatment of advanced non-small cell lung cancer(吉非替尼治疗45例晚期非小细胞肺癌的临床分析)[J]. J Med Theory & Prac(医学理论与实践), 2011, 24(11): 1 248-1 250. |
| [14] | Lv Zhen(吕真), Ni Guo-hua(倪国华). The clinical observation of Gefitinb third-line treatment of advanced non-small cell lung cancer(吉非替尼三线治疗晚期非小细胞肺癌临床观察)[J]. The Prac J Cancer(实用癌症杂志), 2010, 25(5): 528-529. |
| [15] | Ochs J, Grous J J, Warner K L, et al. Final survival and safety results for 21064 non-small cell lung cancer (NSCLC) patients who received compassionate use Gefitinib in a U.S expanded access program (EAP)[J]. Proc Am Soc Clin Oncol, 2004, 23: 628. |
| [16] | West H, Franklin W A. Gumerlock P H, et al. Gefitinib therapy for advanced bronchioalverlar lung cancer(BAC): Southwest oncology group (SWOG) study S0126[J]. Proc Am Soc Clin Oncol, 2004, 23: 618. |
| [17] | Carlson R W, O'Neill A, Vidaurre T, et al. A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer[J]. Brest Cancer Res Treat, 2012, 133(3): 1 049-1 056. |
| [18] | Casado J, Ortiz R P, Delgado M C R, et al. Multidisciplinary physicochemical analysis of oligothiophenes end-capped by nitriles: electrochemistry, UV-Vis-Near-IR, IR, and Raman spectroscopies and quantum chemistry[J]. J Phys Chem B, 2005, 109: 10 115-10 125. |
| [19] | Kronja O, Kohli T P, Mayr H, et al. The structure of the nonamethylcyclopentyl cation[J]. J Am Chem Soc, 2000, 122: 8 067-8 070. |
| [20] | Wu H S, Xu X H, Jiao H J. Structure and stability of C48 fullerenes[J]. J Phys Chem A, 2004, 108: 3 813-3 816. |
| [21] | Simoes R G, Bernardes C E, Diogo H P, et al. Energetics and structure of simvastatin[J]. Mol Pharmaceut, 2013, 10(7): 2 713-2 722. |
| [22] | Wang D J, Zhao X J, Vargek M, et al. Metal-Bound histidine modes in UV resonance Raman spectra of Cu, Zn superoxide dismutase[J]. J Am Chem Soc, 2000, 122: 2 193-2 199. |
| [23] | Marlies K, Van B, Smets J, et al. Matrix-isolation ftir studies and theoretical calculations of hydrogen-bonded complexes of imidazole. A comparison between experimental results and different calculation methods[J]. J Phys Chem A, 1997, 101: 2 397-2 413. |
| [24] | Wang Ling-yun(王凌云), Yang Ming-hui(杨明晖). Quantum chemical calculation on 1H NMR shifts of a AT1 receptor antagonist valsartan(AT1受体拮抗剂缬沙坦化学位移的量子化学计算研究)[J]. Chinese J Magn Reson(波谱学杂志), 2012, 29(4): 530-536. |
| [25] | Gao H W, Wei X J, Liu X T, et al. Comparison of different theory models and basis sets in the calculations of structures and 13C NMR Spectra of [Pt(en)(CBDCA-O, O')], an analogue of the antitumor drug carboplatin[J]. J Phys Chem B, 2010, 114(11): 4 056-4 062. |
| [26] | Anderson J A, Tschumper G S. Characterizing the potential energy surface of the water dimer with DFT: Failures of some popular functionals for hydrogen bonding[J]. J Phys Chem A, 2006, 110: 7 268-7 271. |
| [27] | Torrent S M, Anglada J M, Luis J M. Evaluation of the nonlinear optical properties for annulenes with Hückel and Möbius topologies[J]. J Chem Theory Comput, 2011, 7(12): 3 935-3 943. |
| [28] | Aragoó J, Sancho-García J C, Ortí E, et al. Ab initio modeling of donor–acceptor interactions and charge-transfer excitations in molecular complexes: The case of terthiophene–tetracyanoquinodimethane[J]. J Chem Theory Comput, 2011, 7(7): 2 068-2 077. |
| [29] | Stendardo E, Avila F F, Santoro F, et al. Vibrationally resolved absorption and emission spectra of dithiophene in the gas phase and in solution by first-principle quantum mechanical calculations[J]. J Chem Theory Comput, 2012, 8(11): 4 483-4 493. |
| [30] | Zhang Rui-qin(张瑞勤), Bu Yu-xiang(步宇翔), Li Shu-tang(李述汤). A available method of ab initio basis functions(一种选择从头算基函数的有效方法)[J]. Science China[中国科学(B辑)], 2000, 30(5): 419-427. |
| [31] | Wang X L, Liu J, Qiao J P, et al. Face hallucination based on csgt and pca[J]. Lect Notes Comput Sc, 2008, 5 264: 410-418. |
| [32] | Siehl H U. The interplay between experiment and theory: Computational NMR spectroscopy of carbocations[J]. Adv Phys Org Chem, 2007, 42: 125-165. |
| [33] | Harding M E, Gauss J, Schleyer P. Why benchmark-quality computations are needed to reproduce 1-adamantyl cation NMR chemical shifts accurately[J]. J Phys Chem A, 2011, 115(11): 2 340-2 344. |
| [34] | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, Revision A.01. [CP/DK]. Wallingford, CT: Gaussian Inc, 2009. |
| [35] | Roy T K, Carrington T, Gerber R B. Approximate first-principles anharmonic calculations of polyatomic spectra using MP2 and B3LYP potentials: Comparisons with experiment[J]. J Phys Chem A, 2014, 118(33): 6 730-6 739. |
| [36] | Yuan Li(袁立). Study on Synthesis of Iressa and Derivatives(Iressa及其衍生物的合成研究)[D]. Shenyang Pharmaceutical University(沈阳药科大学), 2005. |
| [37] | Malik M, Wysokiński R, Zierkiewicz W, et al. Raman and infrared spectroscopy, DFT calculations, and vibrational assignment of the anticancer agent picoplatin: Performance of long-range corrected/hybrid functionals for a platinum(II) complex[J]. J Phys Chem A, 2014, 118(34): 6 922-6 934. |
| [38] | Ricardo R, Ternavisk Ademir J, Camargo Francisco B C, et al. Synthesis, characterization, and computational study of a new dimethoxy-chalcone[J]. J Mol Model, 2014, 20(12): 2 526-2 536. |
| [39] | Meng Ling-zhi(孟令芝), Gong Shu-ling(龚淑玲), He Yong-bing(何永炳). Organic Spectral Analysis(有机波谱分析)[M]. Third Edition(第三版). Wuhan(武汉): Wuhan University Press(武汉大学出版社), 2010. |
| [40] | Chin K T, Wei Y H, Pao L Y, et al. Photo-physical properties of N-methyl-3, 4-fulleropyrrolidine and its derivatives: A DFT and TD-DFT investigation[J]. J Chin Chem Soc, 2013, 60(3): 251-260. |
| [41] | Chen Mu-zi(陈木子), Wang Lu(王璐), Zhang Hai-lu(张海禄), et al. NMR study of targeted drug Gefitinib(靶向新药吉非替尼的核磁共振波谱研究)[J]. Chinese J Magn Reson(波谱学杂志), 2011, 28(3): 413-418. |
| [42] | Günay N, Tarcan E, Avcinodot D, et al. Calculated on 1H and 13C NMR chemical shifts of 2,4-difluorobenzaldehyde isonicotinoylhydrazone and 2,3-dichlorobenzaldehyde isonicotinoylhydrazone with GIAO, IGIAM, and CSGT models[J]. Concept Magn Reson Part A, 2009, 34A(5): 297-304. |
 2015, Vol. 32
2015, Vol. 32




