文章信息
- 贺玉贵,冯继文,张志,王超,倪胜,胡少斌,王东,陈方,刘买利,刘朝阳
- HE Yu-gui, FENG Ji-wen, ZHANG Zhi, WANG Chao, NI Sheng, HU Shao-bin, WANG Dong, CHEN Fang, LIU Mai-li, LIU Chao-yang
- 脉冲动态核极化增强的NMR和MRI系统研究
- Development of Pulsed Dynamic Nuclear Polarization for Enhancing NMR and MRI
- 波谱学杂志, 2015, 32(2): 393-398
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 393-398
- http://dx.doi.org/10.11938/cjmr20150221
-
文章历史
- 收稿日期:2014-03-10
- 收修改稿日期:2015-05-08
2. 武汉光电国家实验室(筹), 华中科技大学 光学与电子信息学院, 武汉430074;
3. 中国科学院大学, 北京 100049
2. Wuhan National Laboratory for Optoelectronics, School of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan 430074;
3. University of Chinese Academy of Sciences, Beijing 100049]
Magnetic resonance imaging (MRI),as a non-radioactive and non-invasive mean,has become an important tool in clinical diagnostics and biomedical research,because it can provide information about both spatial structure and function of the sample. However,due to its low sensitivity,the conventional MRI usually uses high-concentration water proton signal to image[1, 2]. Thus,it is difficult to obtain other nuclear or molecular information (such as 13C,31P) which is important for biological metabolism and medical significance. Therefore,the challenge in MRI is to improve the sensitivity of nuclear magnetic resonance (NMR).
Dynamic nuclear polarization (DNP) is an electron-nuclear double resonance technique,where the polarization of high-γ electrons is transferred to the surrounding nuclei using microwave (MW) irradiation to enhance the NMR sensitivity[3]. It is an important method to enhance the MRI sensitivity of small biological molecules for several orders of magnitude relative to thermal equilibrium,thus promising direct image of low-concentration metabolites in vivo[4, 5, 6, 7]. DNP-enhanced molecular and metabolic imaging technologies can provide the unique molecular information for disease indication,metabolic profiling,and treatment monitoring[4]. For example,one can obtain the spatial distribution and the changes over time of several disease-related metabolic molecules by direct imaging[5]. This important molecular information is usually difficult to be obtained by other microscopic molecular imaging methods[8].
In general,the DNP enhancements in the solid and liquid are different. In solid,there are three main DNP enhancement processes: Solid Effect (SE)[9],Thermal Mixing (TM)[10] and Cross Effect (CE)[11]. These mechanisms are all in the continuous wave (CW) irradiation mode. With the developing of electronic technique,pulsed method was introduced into DNP and some new DNP mechanisms including rotating frame DNP[12],the integrated SE[13],NOVEL[14],the dressed SE[15] and PONSEE (polarization of nuclear spins enhanced by ENDOR)[16] were discovered. Unlike the solid paramagnetic centers in solid,the paramagnetic centers (radicals) in liquid is in motion,and the DNP in liquid is dominated by the Overhauser effect,originating from relaxation processes involving electron and nuclear spins coupled through the hyperfine interaction. However,only the solid-DNP based spectrometer for MRS is commercially available (Bruker,Inc.) up to now,commercial liquid-DNP based MRS and MRI spectrometers at any field are still lacking for biological applications. A scalable console for DNP-based MRS and MRI is highly expected to adapt to a variety of Overhauser DNP applications and innovative experimental technology.
Here we propose a distributed pulse spectrometer for DNP-MRS and DNP-MRI. It bases PCIe bus architecture and uses an external high-speed DDR to store PSB elements and FID data,which can greatly increase the transmission speed,improve execution speed pulse sequence and reduce the interval of TR (time of repetition). The designed system includes a host computer,an acquisition computer,a PCIe HUB,a console,four amplifiers (one RF power amplifier,one microwave power amplifier,one RF preamplifier,and one gradient amplifier),a magnet and a probe (Fig. 1). The console consists of transmitter,receiver,master and gradient and system clock module. The system clock module provides global clock to other modules to ensure the whole system synchronization. The transmitter,receiver,gradient module are all implanted with their own pulse programmers (PPG) which are connected to PCIe HUB,respectively. The spectrometer uses an acquisition computer as a whole system master controller,which translates the pulse sequence,and then sends the Pulse Sequence Binary (PSB) to Double Data Rate (DDR) memory in the corresponding pulse programmer (Pulse Programmer 1,2,3,…,n). A PCIe hub,with data transfer rate up to 5.0 Gbps,is used to complete a master-slave interconnection between the acquisition computer and PPGs. Moreover,using an external memory DDR to store NMR imaging pulse sequence effectively extends the data storage capacity to 4 GB or more. This approach dramatically increases the execution speed of pulse sequence and reduce the interval of TR,so we can guarantee that there has been a scan data at least in DDR after the experiment began until finished,because the transmit and receive channels in our design are completely independent.
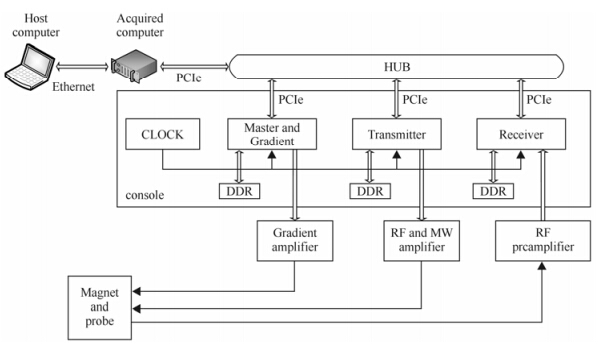
|
| Fig. 1 DNP spectrometer system structure |
As we know,a nanosecond pulse generator is a crucial technology of pulse DNP for controlling MW pulse and pulse sequences. With traditional avalanche transistor or lock-in pulse generator,one couldn’t yield less than 2 ns timing resolution due to its relative long (~ns) pulse rising time and complex circuit. Here we propose a new high-resolution pulse generator that utilizes the phase-shift capability of the phase locked loop (PLL) in field programmable gate array (FPGA). The newly designed pulse generator can produce pulse accurately with 1ns resolution via the different clock along in a single-chip FPGA only.
We show here two primary DNP experimental results,e.g. DNP enhanced spectrum and DNP enhanced image at 0.35 T,to demonstrate the performance of our spectrometer. A homebuilt data processing software was used to control the DNP spectrometer. The samples contain 10 mmol/L nitroxide free radical 4-Hydroxy-2,2,6,6-tetrame- thylpiperidin-1-oxyl (TEMPOL) dissolved in de-ionized water solution. For DNP-MRS and DNP-MRI experiments,approximate 5 μL of solution was sealed in glass capillary with 1 mm outer diameter. DNP experiments were preformed by on-resonant irradiating the sample at 9.58 GHz [one of the Electron Paramagnetic Resonance (EPR) lines of the 14N labelled TEMPOL free radicals] using our custom built microwave driver. Fig. 2 shows the proton spectra of water sample with DNP enhancement (red) and without enhancement (blue). The microwave irradiation time is 1 s,with the power of 50 W (resonator input terminal). We observe very large water 1H signal enhancement up to -170.

|
| Fig. 2 1H Spectra of 10 mmol/L TEMPOL in water with MW irradiation (red) and without (blue). A thermally polarized spectrum is multiplied by 128 times. The enhancement factor is about 170 |
In general,it is necessary to saturate EPR transitions using high microwave (MW) power in a relatively long time period to achieve high nuclear polarization. However,microwave-induced heating is a fatal weakness in DNP-based magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI),especially for heat-sensitive biological samples. Pulsed methodologies are expected to minimize heating effect using a train of short saturation pulses with a very short duty cycle. In order to reduce the influence of microwave heating of the sample,we use a train of microwave saturation pulses for MRI. Fig. 3 shows the images of water 1H with (right) and without (left) microwave irradiation. Obviously,the image contrast of the water sample in a capillary with 1 mm outer diameter is clear in the case of DNP enhancement,but invisible without DNP enhancement.
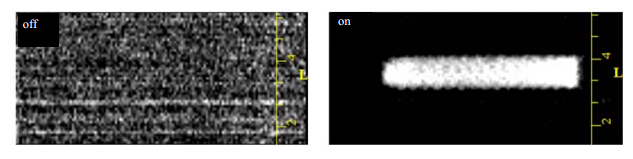
|
| Fig. 3 Image of 1H of water with (right) and without (left) microwave irradiation. TE = 25 ms,TR = 1.5 s, FOV = 10 mm*4 mm,phase encode = 128,MW power = 1.26 W,pulse width = 1 ms,duty cycle = 50% |
It is worth pointing out that in our DNP design we propose a PCIe-based distributed pulse programmer,to make up for deficiencies of the conventional pulse sequence generator in communication speed and storage capacity. The PCIe-based distributed pulse programmer is extensively reconfigurable to construct a variety of medical/scientific instruments for NMR/EPR/MRI. The DNP system presented here can also be used in conjunction with other biomedical instrumentations to broaden its application. The further development of our PCIe-based scalable console should exploit its advantages and apply to the high performance NMR spectrometers or human whole-body MRI scanners with multi-channel transceivers at high field. In addition,due to the limit of probe and MW amplifier,we can not achieve a shorter microwave pulse (several nanoseconds) at this time,and we will develop own multi-resonant cavity based LGR structure,which supports 1H and 13C imaging.
| [2] | Li H D, Zhang Z Y, Han Y Q, et al. Lung MRI Using Hyperpolarized Gases[J]. Chinese J Magn Reson, 2014, 31(3):307-320 |
| [3] | Overhauser A W. Polarization of nuclei in metals[J]. Phys Rev, 1953, 92:411-415. |
| [4] | Gallagher Ferdia A, Kettunen Mikko I, Day Sam E, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C labelled bicarbonate[J]. Nature, 2008, 453:940-944. |
| [5] | Golman K, Int Zandt R, Thaning M. Real-time metabolic imaging[J]. Proc Natl Acad Sci, 2006, 103(30):11 270-11 275. |
| [6] | Kohler S J, Yen Y, Wolber J. In vivo 13C arbon metabolic imaging at 3 T with hyperpolarized 13C-1-pyruvate[J]. Magn Reson Med, 2007, 58:65-69. |
| [7] | Su Y C, Andreas L, Griffin R G. Magic angle spinning NMR of proteins high frequency dynamic nuclear polarization and 1H detection[J]. Annu Rev Biochem, 2015, 84:1-35.33. |
| [8] | Wang Y Q, Li C G, Pielal Gary J. In-cell protein magnetic resonance spectroscopy[J]. Chinese J Magn Reson, 2012, 29(4):475-488. |
| [9] | Abragam A. Overhauser effect in nonmetals[J]. Phys Rev, 1955, 98:1 729-1 735. |
| [10] | Borghini M. Spin-temperature model of nuclear dynamic polarization using free radicals[J]. Phys Rev Lett, 1968, 20:419-421. |
| [11] | Hwang C F, Hill D A. New effect in dynamic polarization[J]. Phys Rev Lett, 1967, 18:110-112. |
| [12] | Farrar C T, Hall D A, Gerfen G J, et al. High-frequency dynamic nuclear polarization in the nuclear rotating frame[J]. J Magn Reson, 2000, 144:134-141. |
| [13] | Henstra A, Dirksen P, Wenckebach W T. Enhanced dynamic nuclear polarization by the integrated solid effect[J]. Phys Rev A, 1988, 134:134-136. |
| [14] | Henstra A, Wenckebach W T. The theory of nuclear orientation via electron spin locking (NOVEL)[J]. Mol Phys, 2008, 106:859-871. |
| [15] | Weis V, Bennati M, Rosay M, et al. Solid effect in the electron spin dressed state-A new approach for dynamic nuclear polarization[J]. J Chem Phys, 2000, 113:6 795-6 802. |
| [16] | Morley G W, van Tol Johan, Ardavan A, et al. Efficient dynamic nuclear polarization at high magnetic fields[J]. Phys Rev Lett, 2007, 98:220501. |
 2015, Vol. 32
2015, Vol. 32




