文章信息
- 王粉粉,陈铁红,孙平川
- WANG Fen-fen, CHEN Tie-hong, SUN Ping-chuan
- 先进固体核磁共振揭示苯硼酸-壳聚糖纳米粒子非均匀结构和相容性
- Heterogeneous Structure and Miscibility of Phenylboronic Acid-Rich Chitosan Nanoparticles as Revealed by Advanced Solid-State NMR
- 波谱学杂志, 2015, 32(2): 354-362
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 354-362
- http://dx.doi.org/10.11938/cjmr20150217
-
文章历史
- 收稿日期:2015-02-11
- 收修改稿日期:2015-05-08
2. 药物化学生物学国家重点实验室,南开大学,天津 300071;
3. 天津化学化工协同创新中心(天津),天津 300071
2. State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin 300071;
3. Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300071
Biopolymer-based nanoparticles (NPs) have attracted booming interests in drug and gene delivery[1], device-based therapy, molecular imaging[2], electronics and photonics[3] as well as pharmaceutical applications due to their excellent biocompatibility and biodegradability. In particular, cationic polysaccharide chitosan (CS)-based NPs have shown particularly promising results in biological applications. For instance, chitosan NPs have been used as carriers for targeted delivery of anticancer drugs and poly-siRNA[4], cell culture, and biosensor[5]. Boronic acid can react with diols and saccharides to form the cyclic boronate esters, which have been widely used in boron-rich biopolymer-based NPs[6]. Recently, novel boronic acid-rich chitosan NPs were successfully prepared and exploited for doxorubicin delivery[7]. Although advantaged by good cellular uptake and improved stability, in practice, the properties of such phenylboronic acid-rich chitosan NPs were found to depend on the micro-phase structure and miscibility of different components in the NPs. Moreover, the particle size, nanostructure, and blend morphology of NPs are of great importance for practical applications. For example, in respect of organic solar cell applications, the dimension of phase separation must be in the range of the exciton diffusion length, commonly a few tens of nanometers. Thomas et al [8]. utilized miniemulsion process to blend polymers in each individual nanoparticle so that the dimension of phase separation may be controlled in a range similar to the particle size. However, relevant study on detailed information of the structure and inter-polymer miscibility of these chitosan-containing NPs was scarce. Thus, understanding the nanostructure and miscibility between polymer and biopolymer of the NPs is crucial for practical applications of these materials.
A variety of experimental techniques including dynamic light scattering (DLS)[9], transmission electron microscopy (TEM), scanning electron microscope (SEM)[10], and nuclear magnetic resonance (NMR) have been utilized for investigating the structure and miscibility of NPs. Nonetheless, due to the poor electron density contrast between chitosan and other polymers, detailed morphology (homogenous or core-shell separated) of NPs usually cannot be well determined by TEM technique. Moreover, DLS and SEM methods, which are capable of providing information on particle size and surface structure of NPs, they are handicapped for determining the inner morphology. In this context, solid-state NMR (SSNMR) spectroscopy has been proven to be a nondestructive and powerful multiscale technique for studying the micro-domain structure of polymers in a length scale from atomic level to several tens of nanometers[11]. In a previous study, 2D 1H-1H spin-exchange NMR spectra were used to elucidate the 1H-1H proximity in organic solids such as α-glycine[12], for probing the miscibility of polymer blends[13] as well as for studying the water-polymer interaction of biomacromolecules. 2D 13C-1H wide-line separation spectroscopy (WISE) and chemical shift filter technique were reported to determine the componential dynamics and morphology of the NPs[14, 15]. In addition, heteronuclear correlation (HETCOR) NMR experiments have been used to identify the structure of solid proteins at natural abundance, providing key structure information in these functional residues, which was invisible in the spectra obtained from the CRAMPS (Combined Rotation and Multiple Pulse Sequences) technique[16].
In this work, a variety of advanced SSNMR techniques were used to characterize the structure and inter-polymer miscibility of CS-PAPBA NPs aiming to address the following issues: (1) heterogeneous structure of NPs, (2) miscibility between polymer (PAPBA) and biopolymer (chitosan), and (3) length scale of possible phase separation. To achieve these goals, 13C CPMAS technique was utilized to detect the conformational change of chitosan when incorporated with phenyl boronic acid. 2D 13C-1H HETCOR experiment was used to determine the interfacial mixing of chitosan and PAPBA. Moreover, spectra obtained from 2D 1H-1H spin-exchange with spin-diffusion were analyzed to determine the length scale of possible phase separation and the domain size of the NPs. 1 Materials and experimental procedure
Materials. Chitosan (CTS) (Mn = 5 k) was purchased from Yuhuan Biomedical Company. The degree of deacetylation of chitosan was determined as 83% by NMR spectroscopy. 3-acrylamidophenyl-boronic acid (APBA) was purchased from Frontier Scientific (FSI). K2S2O8 was purchased fromJ&K Chemical. Other analytical reagents were used as received.
Preparation of the chitosan-poly(N-3-acrylamidophenyl-boronic acid) nanoparti- cles (CS-PAPBA NPs). PAPBA was first synthesized following a known recipe[17]. Subsequently, CS-PAPBA NPs were prepared through the radical polymerization of APBA in the presence of chitosan in an aqueous medium. The feeding molar ratio glucosamine unit/APBA of NPs was 1∶1. In brief, 120 mg of APBA was dissolved in 10 mL distilled water, followed by adding 100 mg chitosan to the system after the solution became clear at 70 ℃. Upon complete dissolution of chitosan, the system was allowed to cool to room temperature (25 ℃), then, the desired amount of K2S2O8 as initiator was added to the solution. Upon adjusting to pH 5.0 with sodium hydroxide solution, the system was degasified and allowed to polymerize at 90 ℃ under N2 atmosphere for 2 h. The resultant solution was dialyzed against 1 L distilled water for 24 h using a dialysis bag (MWCO 100 k). The dialyzed solution was then lyophiled at -50 ℃.
Characterization methods. SEM experiments were measured on a Shimadzu SS-550 scanning electron microscopy. Prior to the measurements, samples were dispersed in ethanol, drop-cast onto glass slips, and dried. SSNMR experiments were performed on a Varian Infinitplus-400 wide-bore (89 mm) NMR spectrometer at room temperature, corresponding to Larmor frequencies of 399.72 and 100.52 MHz for 1H and 13C, respectively. For 1H and 13C CPMAS NMR experiments, a T3 probe was used. Sample was placed in a 4 mm zirconia PENCIL rotor having a volume of ca. 52 μL. The MAS frequency was automatically controlled for all 1D and 2D experiments. The 1H and 13C chemical shifts were referenced to external tetramethylsilane (TMS, δ 0) and hexamethylbenzene (HMB, δ 17.3 for CH3 resonance), respectively. The pulse sequences of all SSNMR experiments are illustrated in Fig. 1.

|
| Fig. 1 SSNMR pulse sequences used in this study: (a) 13C CPMAS with SPINAL-64 dipolar decoupling, (b) 2D 13C-1H HETCOR, and (c)2D 1H-1H spin-exchange CRAMPS with eDUMBO-1 homonuclear decoupling during t1. The 90° pulse length of 1H and 13C were 2.5 ms for all experiments, respectively. The DUMBO-1 and eDUMBO-1 decoupling cycle time was set to 32 ms divided in 64 discrete phase steps of 500 ns duration each, and 1H decoupling RF field strength of DUMBO-1 and eDUMBO-1 were set to 100 kHz in both dimensions |
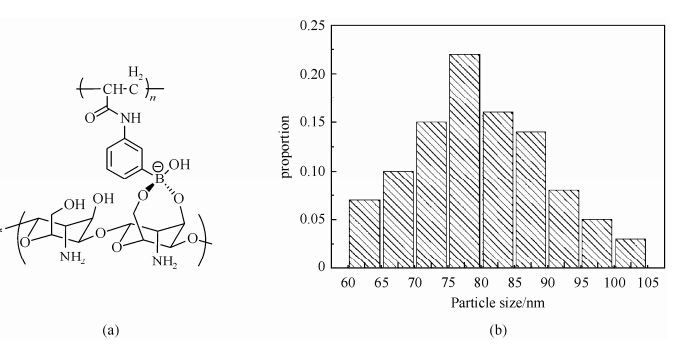
External morphological structure of CS-PAPBA NPs. As shown in Fig. 2(a), the PAPBA segments grafted from chitosan through APBA tends toform cyclic ester with double hydroxyl of chitosan (CS) in specific pH environment. Grafted chain of PAPBA together with some free PAPBA molecules self-assembled into the hydrophobic interior of the CS-PAPBA NPs, while the CS chains constitute the hydrophilic exterior. The morphological structure of CS-PAPBA NPs was determined by SEM and the particle size distribution is shown in Fig. 2(b). All NPs exhibited a spherical shape with a narrow particle size distribution of ca. 80 nm, in agreement with our previous report (S1 in Supporting Information)[15].
|
| Fig. 2 (a) Schematic structure and (b) particle size distribution statistics of the CS-PAPBA NPs |
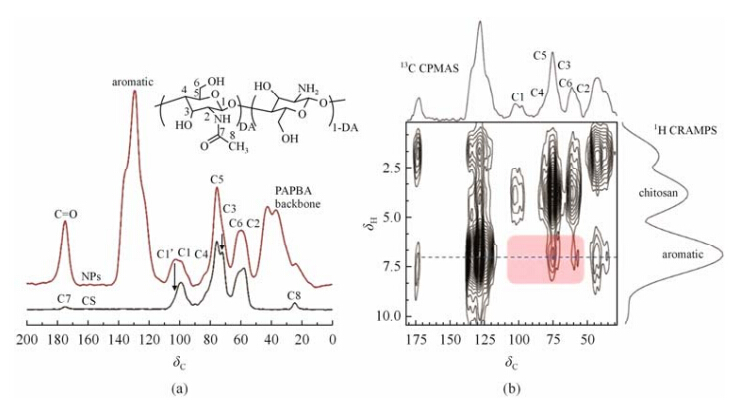
Composition and interfacial mixing of CS-PAPBA NPs determined by 13C CPMAS and 2D 13C-1H HETCOR experiments. Intermolecular coordination interactions between CS and PAPBA are considered to be the driving force for their miscibility in NPs. Therefore, it has a crucial effect on the structure and properties of the material, especially the final conformation of chitosan. However, few work was reported regarding to the effect of coordination interactions on the structural change of chitosan in the NPs. High-resolution 13C CPMAS experiment, which is highly sensitive for probing local chemical environments, is a powerful method to investigate the intermolecular coordination interactions and the change of crystal structure[18, 19]. Especially the C1 and C5 of chitosan are very sensitive to the formation of coordination bonds and can be directly detected by 13C CPMAS techniques. The 13C CPMAS NMR spectra of CS-PAPBA NPs and pristine CS are shown in Fig. 3(a). Compared with pure chitosan, a notable change in 13C CPMAS spectrum of bound chitosan in the NPs was observed. It is noteworthy that two C1 peaks at ca. δC 102 and 98 in NPs were observed, while that of pure CS shows only a narrow C1 peak at ca. δC 98. The low field peak at δC 102 is attributed to C1 in the proximity of B[4] (tetrahedral boronate form) in the interphase region. The presence of C1′ peak is a clear and direct evidence of the intermolecular coordination interactions between CS and PAPBA.
|
| Fig. 3 (a) 13C CPMAS spectra of CS-PAPBA NPs (top) and CS (bottom); (b) 2D 13C-1H HETCOR spectrum of the CS-PAPBA NPs recorded with a mixing time of 200 ms |
The coordination interactions and interfacial mixing between CS and PAPBA are further confirmed by 2D 13C-1H HETCOR experiment, which has been proven to be a powerful technique for providing chemical shift and dipolar coupling information relevant to structure, hydrogen bonding, and differential dynamics that may be detected at 13C natural abundance. The technique has been applied for the analyses of 1H-13C and other heteronuclear correlations in a wide variety of crystalline and amorphous materials of practical interest[20]. The advantage of this experiment is that simultaneous 13C and 1H chemical shift information may be obtained along with distance-dependent dipolar coupling. As such, proximity of different groups in macromolecules can be identified. In general, at very short mixing time (< 200 ms), only signals originated from carbons associated with proximate hydrogens (< 0.35 nm)are observed, since polarization is transferred from each type of hydrogen to nearby carbons in the absence of 1H-1H spin diffusion. Whereas at long mixing time, carbon signals transferred from more distant hydrogens can be detected.
Fig. 3(b) shows the contour plot obtained from the13C-1H HETCOR experiments of NPs at a mixing time of 200 ms. The projections along the first (1H CRAMPS; F1) and second (13C CPMAS; F2) dimensions are also displayed. Take the advantage of highly efficient dipolar decoupling facilitated by the continue wave modulation (eDUMBO) technique, the resolution in F1 dimension is high enough to resolve three peaks, corresponding to the CS at δH 3.4 and PAPBA at δH 7.1 (aromatic) and δH 2.0 (back bone), respectively. Most of the signals observed in the contour plot arose from carbons directly bonded with hydrogens in PAPBA, e.g., the PAPBA back bone and aromatic groups at δC 45, 128, and 175, respectively. Although expected, such peaks are informative, as the conformational dependence of both the 13C and 1H. Of much more interest are the correlations arising from the CS carbon at δC 95-102 and signals originated from aromatic groups of PAPBA. Two CS-correlated 13C signals are detected for aromatic protons of PAPBA:one with 13C chemical shift of ca. δC 60 (C6+C2) by which polarization is transferred from aromatic protons to carbonsand the other two carbons with chemical shift of ca. δC 75 (C3+C5). The above results clearly demonstrate that a portion of CS should be closely bound with PAPBA through boronic coordination interactions in the interphase region.
Heterogeneous structure and domain size of CS-PAPBA NPs determined by 2D 1H-1H spin-exchange NMR. Proton spin-diffusion experiments allow for characterization of heterogeneity within a length scale from about one monomer unit up to 150 nm[21]. Recently, 2D high-resolution 1H-1H spin-exchange experiments have been successfully used to elucidate the proximity among protons from different groups in organic solids and the water-polymer interaction in polymers[11(b), 22]. In principle, with the increase in mixing time, the proximity of different groups can be detected by their through-space 1H-1H spin-exchange. Therefore, by using this technique, the interfacial mixing and proximity of CS and PAPBA in the interphase region at different length scale can be elucidated. Since a given value of mixing time corresponds to a certain distance related to spin-exchange, therefore a specific 1H-1H distance (r) in typical blends may be estimated accordingly. The dynamics of the 1H magnetization can be approximated as a diffusive process characterized by the mean square displacement, given by the equation:
where Deff is the effective spin-diffusion coefficient, and tm denotes the characteristic mixing time of spin-diffusion. It is worth noting that the proton spin-diffusion coefficient in rigid organic solids is similar for various samples, typically Deff ~ 0.6-0.8 nm2/ms.
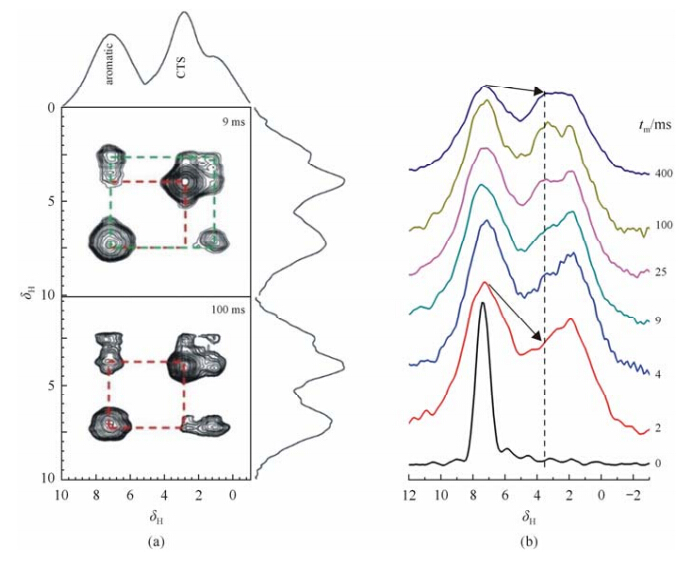
Fig. 4(a) shows the typical 2D 1H-1H spin-exchange spectra of CS-PAPBA NPs observed at a mixing time of 9 and 100 ms plotted based onthe normalized peak intensities of aromatic signals of PAPBA. The cross-peaks associated with PAPBA aromatic and CS groups can be clearly observed at a very short mixing time of 0.2 ms, indicating that the interfacial proximity of these two components mainly arose from the 4-coordinated boron B[4] bridge. Detailed information of the 1H spin-diffusion from the PAPBA aromatic to CS can be extracted from the F1 slice of the 2D spin-exchange spectrum in the signal region of PAPBA aromatic groups (δ7.1), as shown in Fig. 4(b). Upon increasing the mixing time of spin diffusion, the signal intensity of the CS protons at ca. δ3.5 gradually increases, the spin-diffusion reaches the equilibrium state at a considerable long mixing time of ca. 400 ms, indicating the presence of micro-phase separation in the NPs. As the effective spin-diffusion coefficient Deff under fast MAS frequency of 25 kHz is only 20% of that at static conditions, a quantitative analysis by Eq. (1) using the values of Deff = 0.6*20% nm2/ms and tm = 400 ms yields an average distance (r) that represents the length scale of micro-phase separation in the NPs of ca. 15-20 nm.
|
| Fig. 4 (a)2D 1H-1H spin-exchange MAS NMR spectra of CS-PAPBA NPs at different mixing times; (b) A series of 1H-1H spin-exchange spectra sliced along F1 dimension of the 2D NMR spectra corresponding to the aromatic groups of PAPBA (δ7.1) in NPs atvarious 1H spin-diffusion times (tm) from 0 to 400 ms |
Advanced solid-state NMR techniques have been exploited to investigate the heterogeneous structure and the miscibility of the CS-PAPBA NPs. Results obtained from 13C CPMAS NMR experiments indicate that hydrogen bonding interactions among the chitosansegments and resulting crystalline structure are inhibited by the incorporation of phenylboronic acid side chains. Spectrum obtained from 2D 13C-1H HETCOR further demonstrated that a portion of chitosan is closely bound with PAPBA in the interphase through boronic coordination interactions. Moreover, results from 2D 1H-1H spin-exchange with spin-diffusion studies verify the presence of interfacial mixing of CS and PAPBA in the NPs with an estimated micro-phase separation of ca. 15-20 nm. These NMR results provide important information for the precise design and efficient biological applications of chitosan-based biomaterials.
Acknowledgment: This work was supported by the National Science Fund for Distinguished Young Scholars (20825416), and the National Natural Science Foundation of China (21374051 and 21174072), “973 program” (2012CB821503), PCSIRT (IRT1257) and CERS-1-61.| [1] | (a) Guo P, Martin C R, Zhao Y, et al. General method for producing organic nanoparticles using nanoporous membranes[J]. Nano Lett, 2010, 10 (6):2 202-2 206;(b) Liu Z, Jiao Y, Wang Y, et al. Polysaccharides-based nanoparticles as drug delivery systems[J]. Adv Drug Deliver Rev, 2008, 60(15):1 650-1 662. |
| [2] | Schirhagl R. Bioapplications for molecularly imprinted polymers[J]. Anal Chem, 2014, 86 (1), 250-261. |
| [3] | Huang G, Yin Y, Pan Z, et al. Fabrication of 3D photonic crystals from chitosan that are responsive to organic solvents[J]. Biomacromolecules, 2014, 15(12):4 396-4 402. |
| [4] | Lee S J, Huh M S, Lee S Y, et al. Tumor-homing poly-siRNA/Glycol chitosan self-cross-linked nanoparticles for systemic siRNA delivery in cancer treatment[J]. Angew Chem Int Edit, 2012, 51 (29), 7 203-7 207. |
| [5] | Anitha A, Chennazhi K P, Nair S V, et al. 5-Flourouracil loaded N,O-carboxymethyl chitosan nanoparticles as an anticancer nanomedicine for breast cancer[J]. J Biomed Nanotechnol, 2012, 8(1):29-42. |
| [6] | Wang B, Ma R, Liu G, et al. Effect of Coordination on the Glucose-Responsiveness of PEG-b-(PAA-co-PAAPBA) Micelles[J]. Macromol Rapid Commun, 2010, 31(18):1 628-1 634. |
| [7] | Wang X, Zhen X, Wang J, et al. Doxorubicin delivery to 3D multicellular spheroids and tumors based on boronic acid-rich chitosan nanoparticles[J]. Biomaterials, 2013, 34(19):4 667-4 679. |
| [8] | Kietzke T, Neher D, Landfester K, et al. Novel approaches to polymer blends based on polymer nanoparticles[J]. Nature Mater, 2003, 2(6):408-412. |
| [9] | Liu X, Dai Q, Austin L, et al. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering[J]. J Am Chem Soc, 2008, 130:2 780-2 782. |
| [10] | Khoa N T, Kim S W, Yoo D H, et al. Fabrication of Au/graphene-wrapped ZnO-nanoparticle-assembled hollow spheres with effective photoinduced charge transfer for photocatalysis[J]. ACS Appl Mater Inter, 2015. |
| [11] | (a) Fu W G, Sun P C. Solid-state NMR study of hydrogen bonding, miscibility, and dynamics in multiphase polymer systems[J]. Front Chem China, 2011, 6(3):173-189;(b) He X, Liu Y, Zhang R, et al. Unique interphase and cross-linked network controlled by different miscible blocks in nanostructured epoxy/block copolymer blends characterized by solid-state NMR[J]. J Phys Chem C, 2014, 118(24):13 285-13 299. |
| [12] | Brus J, Petříčková H, Dybal J. Influence of local molecular motions on the determination of 1H-1H internuclear distances measured by 2D 1H spin-exchange experiments[J]. Solid State Nucl Magn Reson, 2003, 23(4):183-197. |
| [13] | Caravatti P, Neuenschwander P, Ernst R R. Characterization of heterogeneous polymer blends by two-dimensional proton spin diffusion spectroscopy[J]. Macromolecules, 1985, 18:119-122. |
| [14] | Zhang R C, Sun P C. Aplications of advanced solid-state NMR techniques in studying the structure and dynamics of polymers[J]. Chinese J Magn Reson, 2012, 29(3):157-174. |
| [15] | Wang F, Zhang R, Wu Q, et al. Probing the nanostructure, interfacial interaction, and dynamics of chitosan-based nanoparticles by multiscale solid-state NMR[J]. ACS Appl Mater Inter, 2014, 6(23):21 397-21 407. |
| [16] | Jeffery L W, Mirau P A. Heteronuclear correlation in solid polymers:idelntification of hydrogen bond donors and acceptors in miscible polymer blends[J]. Macromolecules, 1994, 27:1 648-1 650. |
| [17] | Zhang L Z, Lin Y, Wang J J, et al. A Facile strategy for constructing boron-rich polymer nanoparticles via a boronic acid-related reaction[J]. Macromol Rapid Commun, 2011, 32(6):534-539. |
| [18] | (a) Dang Q Q, Lu S D, Yu S, et al. Novel silk fibroin/montmorillonite nanocomposites:effect of pH on the conformational transition and clay dispersion[J]. Biomacromolecules, 2010, 11(7):1 796-1 801;(b) Yamamoto H, Hori F. CP/MAS 13C NMR analysis of the crystal transformation induced for vdonia cellulose by annealing at high temperatures[J]. Macromolecules, 1993, 26:1 313-1 317. |
| [19] | Zhang Z F, Yang J. Solid-state NMR stuieson amyloid fibris:recent progresses[J]. Chinese J Magn Reson, 2013, 30(2):157-174. |
| [20] | White J L, Mirau P A. Heteronuclear correlation in solid polymers:identification of hydrogen bond donors and acceptors in miscible polymer blends[J]. Macromolecules, 1994, 27:1 648-1 650. |
| [21] | Schmidt-Rohr S H. Multiple Dimensional NMR and Polymers. Multiple Dimensional NMR and Polymers[M]. San Diego:Academic Press, 1994. |
| [22] | Li B, Xu L, Wu Q, et al. Various types of hydrogen bonds, their temperature dependence and water-polymer interaction in hydrated poly(acrylic acid) as revealed by 1H solid-state NMR spectroscopy[J]. Macromolecules, 2007, 40:5 776-5 786. |
 2015, Vol. 32
2015, Vol. 32





