文章信息
- 卢秀秀, 顾嘉琦, 蓝文贤, 王春喜, 麻锦彪, 曹春阳
- LU Xiu-xiu, GU Jia-qi, LAN Wen-xian, WANG Chun-xi, MA Jin-biao, CAO Chun-yang
- Lin28特异性结合let-7 RNA结构基础
- Structural Basis for Lin28 Specific Interaction with let-7 RNA
- 波谱学杂志, 2015, 32(2): 318-328
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 318-328
- http://dx.doi.org/10.11938/cjmr20150214
-
文章历史
- 收稿日期:2015-03-06
- 收修改稿日期:2015-05-11
2. 复旦大学 生命科学学院, 遗传工程国家重点实验室, 上海 200433
2. State Key Laboratory of Genetic Engineering, School of Life Sciences, Fudan University, Shanghai 200433
MicroRNAs (miRNAs) are a class of small noncoding RNAs. Post-transcriptional regulation by miRNAs is found in nearly every biological process including differentiation,proliferation,survival,apoptosis,metabolism,and stress response. The mammalian let-7 family,which was identified as the first mammalian miRNAs,comprises twelve members expressed from eight distinct loci (let-7a-1,-2,-3; let-7b; let-7c; let-7d; let-7e; let-7f-1,-2; let-7g; let-7i and miR-98)[1, 2]. Let-7 controls cell differentiation and proliferation by regulating oncogenes,such as KRAS and MYC,and cell cycle related genes,cyclin D1/2[3]. Mass spectrometric analyses revealed that the two predominant interacting proteins were Lin28a and Lin28b[4]. Lin28 (also known as Lin28a) is one of the reprogramming factor used in concert with KLF4,SOX2 and NANOG,to induce pluripotency in adult human fibroblast cells[5]. Lin28 is activated in many human tumors (15%) and appears to be associated with less differentiated cancers[6]. Studies with patient samples show correlation between overexpression or mutation of Lin28 with ovarian cancer[7, 8] and colon cancer[9].
Lin28 binds conserved terminal loop of pre-let-7 elements and induces terminal uridylation (addition of uridine nucleotides) of pre-let-7 via recruitment of Zcchc11,a terminal uridylyl transferase (TUTase),and TUT4. Dicer is unable to cleave uridylated pre-let-7 transcript,thus blocking the processing of mature let-7 miRNAs[10, 11]. It has been recently shown that Lin28b inhibits let-7 via different mechanisms by directly binding pre-let-7 transcript in the nucleus and impeding its processing by the microprocessor complex of Drosha and DGCR8[12]. The detailed distinct mechanisms by which Lin28 and Lin28b control let-7 miRNA biogenesis have been unraveled and covered by three excellent reviews[11, 13, 14].
Lin28 contains two RNA-binding domains (RBDs): an N-terminal cold-shock domain (CSD) and a C-terminal Zn-knuckle domain (ZKD) composed of two tandem arranged retroviral-type CCHC Zn knuckles. Several biochemical and structural studies showed that the specificity of this interaction is mainly mediated by the ZKD with a conserved GGAG or GGAG-like motif [11, 15, 16]. To address how Lin28 interacts with RNA containing GGAG or GGAG-like motif,the structural studies were performed by NMR and X-ray technologies separately,where the information about RNA are a little contradict,especially for the conformation of the RNA backbone. Thus,to double confirm which conformation is more possible in the biological system,we here determined the solution structure of Lin28 ZKD in complex with let-7 RNA.
1 Materials and methods 1.1 Protein and RNA preparationA construct encoding amino acids 134-179 of human Lin28 (NM_024674; Q9H9Z2) was subcloned into pET28a vector with a His6-tag using NheI and HindIII restriction sites. Site-directed mutagenesis was performed using a QuikChange site-directed mutagenesis kit (Stratagene Inc.). The constructs were verified by DNA sequencing,and the plasmids were transformed into Escherichia coli BL21(DE3) competent cells. The transformed cells were grown at 37 ℃ in a Luria Broth (LB) medium containing 50 g/mL kanamycin and were induced (24 h,18 ℃) by the addition of 0.1 mmol/L isopropyl-d-thio-β-D-galactopyranoside (IPTG) when the OD600 value was measured as 0.5-0.6. The cells were harvested and resuspended in lysis buffer (50 mmol/L phosphate,pH 7.5,500 mmol/L NaCl),lysed with 10 μg/mL PMSF by sonication on ice. The lysates were clarified by centrifugation (30 min,16 000 rpm),and the soluble proteins were purified by nickel-affinity chromatography (GE Healthcare) throngh a linear gradient of 0-500 mmol/L imidazole in the lysis buffer. Protein fractions were collected and dialyzed twice at 4 ℃ against the lysis buffer. To remove the His6-tag,the fusion protein was digested with thrombin protease overnight at 4 ℃,followed by running a second nickel column. The accurate concentrations of Lin28 and its variants were determined using their A280 absorption constants.
For isotope labeling,M9 minimal medium was supplemented with 15NH4Cl or/and 2 g/liter [U-13C]-glucose (Cambridge Isotope Laboratories,USA). Two single-stranded (ss) RNAs with the sequences 5′-A-2A-1G1G2A3G4-3′ and 5′-A-2A-1G1G2A3G4-3′ were commercially synthesized at the HPLC grade from Shanghai Sangon Biological Engineering Technology,China.
1.2 Isothermal Titration Calorimetry (ITC) binding assayTo obtain direct binding affinities between Lin28 (134-179) (and its variants) and ssRNA,the isothermal titration calorimetry (ITC) binding assay was performed. An ITC-200 microcalorimeter (GE Healthcare) was used with buffer A containing 20 mmol/L Tris,150 mmol/L NaCl,pH 7.0 2 mmol/L MgCl2,0.2 mmol/L TCEP and 5% glycerol. Before running ITC assay,the protein solution and the RNA solution were first centrifuged to remove any particulates,and degassed at 25 ℃. Protein and RNA were then exchanged with buffer A. A solution of 0.50 mmol/L Lin28 was titrated with ssRNA at the concentration of 0.05 mmol/L. The reference titration of small molecules in the buffer was subtracted from the experimental data,and the data were fitted using the Origin 7.0 (OriginLab Corporation) software.
1.3 NMR titration for binding assayTo preliminarily determine ssRNA (5′-A-2A-1G1G2A3G4-3′) binding sites on Lin28,NMR chemical shifts perturbation assay was performed at the buffer condition similar to that for structural determination. For correctly examining NMR chemical shifts changes of Lin28 in the presence of ssRNA,the assignments of cross peaks in two dimensional (2D) 1H-15N HSQC spectra were confirmed by performing NMR stepwise titration experiments through increasing the stoichiometric ratio of Lin28: ssRNA as follows 1∶0,1∶0.5,1∶1,1∶1.2,and 1:5. The 1H-15N HSQC spectra were collected after each addition.
1.4 NMR measurements for structure determinationAll NMR experiments were performed on 1 mmol/L hLin28 mixed with 1.2 mmol/L RNA at 20 ℃ on a Varian Unity Inova 600 NMR spectrometer (with cryo-probe) equipped with triple resonances and pulsed-field gradients. The standard suite of experiments for assigning the 1H,13C and 15N backbone,determining the side-chain chemical shifts of Lin28 in complex with the RNA 5′-A-2A-1G1G2A3G4-3′ and collecting the NOE-based distance restraints were measured[17, 18]. These experiments included 2D 13C-edited HSQC and 15N-edited HSQC; 3D HNCA,HNCO,HN(CO)CA,HNCACB,CBCA(CO)NH,15N-resolved HSQC-TOCSY and HCCH-TOCSY in both aliphatic and aromatic regions; 15N-resolved HSQC-NOESY; 13C-resolved HSQC-NOESY for both aliphatic and aromatic resonances; and 2D hbcbcgcdceheA and hbcbcgcdhdA spectra for the correlation of Cβ and Hδ or Hε in the aromatic ring which are used for aromatic proton assignment. The proton NMR signals of the bound ssRNA were assigned by analyzing the 2D 13C-filtered,15N-filtered and J-resolved NOESY and TOCSY spectra recorded for the 13C- and 15N-labeled protein with the unlabeled ssRNA and the 2D 1H-1H COSY,NOESY and TOCSY spectra recorded for the unlabeled free ssRNA in the NMR buffer mentioned above,respectively. The intermolecular NOEs between the labeled protein and the unlabeled ssRNA were obtained by analyzing the 3D 13C-F1 edited and 13C/15N-F3 filtered NOESY spectra. The spectra were processed with the NMRPipe program[19] and analyzed using Sparky 3 (http://www.cgl. ucsf.edu/home/sparky/).
1.5 NMR structure determinationThe NMR structure calculations were performed using a standard simulated annealing protocol implemented in the XPLOR-2.19 program (NIH version)[20]. The inter-proton distance restraints derived from the NOE intensities were grouped into three distance ranges,namely 1.8-2.9 (10-1 nm),1.8-3.5 (10-1 nm) and 1.8-6.0 (10-1 nm),which corresponds to strong,medium and weak NOEs,respectively. The dihedral angles phi and psi were derived from the backbone chemical shifts (HN,HA,CO and CA) using the program TALOS[21]. Slow-exchanging amide protons identified in the 2D 15N-1H HSQC spectra recorded after the H2O buffer was exchanged for a D2O buffer were used in the structure calculated with the NOE distance restraints to generate hydrogen bonds for the final structure calculation as previously described in the literature. Constraints between the protein and the zinc ion were added using a previously reported procedure[22, 23]. A total of ten iterations were performed (50 structures in the initial eight iterations). In total,100 structures were computed during the last two iterations,and the 20 conformers with the lowest energy were used to represent the 3D structures. The conformers of the bundles (Lin28 in complex with the ssRNA) do not violate the following constraints: NOE > 0.03 nm and dihedral angle >3°. The entire structure statistics were evaluated with PROCHECK and PROCHECK-NMR and are summarized in Table 1. All of the structure figures were generated using the PyMOL (http://pymol.org/) and MOLMOL programs.
2 Results and discussion 2.1 Lin28 specially binds to RNA let-7 5′-A-2A-1G1G2A3G4-3′It was reported that the Lin28 Zinc-Knuckle domain specifically recognizes GGAG or GGAG-Like motifs[11]. The core bases G1 and G4 in the RNA sequence were sequence-specifically recognized by the Znfs in mouse Lin28 (mLin28)[11]. Although RNA sequences both containing the conserved nucleotides are not the same,as shown in Fig. 1(a) and 1(b),the dissociation constants (KD) measured by ITC assay are 3.4 μmol/L for human Lin28 (hLin28) interacting with ssRNA 5′-A-2A-1G1G2A3G4-3′,and 9.0 μmol/L with ssRNA 5′-A-2A-1G1A2G3G4-3′,respectively,in line with the data reported[15]. This dissociation constant reveals that Lin28 binds to RNA 5′-A-2A-1G1G2A3G4-3′ about 3-fold more strongly than that to RNA 5′-A-2A-1G1A2G3G4-3′. Thus,we focused our NMR structural studies on how Lin28 interacts with RNA 5′-A-2A-1G1G2A3G4-3′.
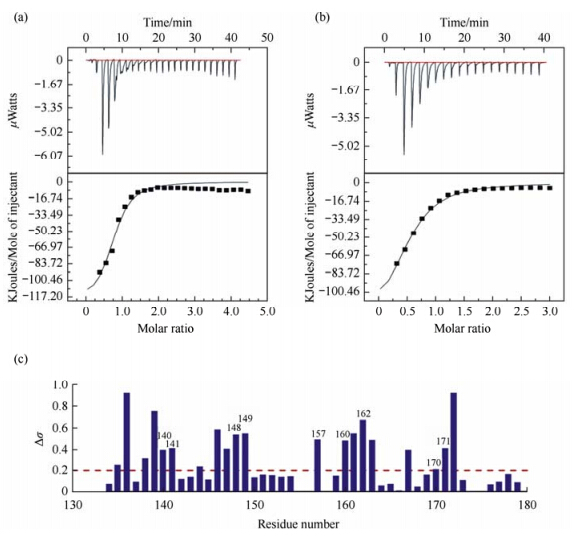
|
| Fig. 1 Lin28(134-179) interacts with let-7 RNA. Binding affinities of Lin28 to RNA (a) 5′-A-2A-1G1G2A3G4-3′ (Kd = 3.4 μmol/L) and (b) 5′-A-2A-1G1G2A3G4-3′ (Kd= 9.0 μmol/L) measured by ITC assay. (c) NMR chemical shift changes of Lin28(134-179) backbone atoms amide 1H and 15N upon RNA 5′-A-2A-1G1G2A3G4-3′ binding by the equation $\Delta {\delta _{{\rm{av}}}} = {\{ 0.5 \times [\Delta \delta {({\rm{NH}})^2} + 0.2 \times \Delta \delta {{(^{15}}{\rm{N}})^2}]\} ^{1/2}}$ |
Before doing NMR titration assay,we completed the assignments of more than 98% of the main-chain and 96% of the side-chain atoms of the residues for both free and bound Lin28. Large chemical shift changes were then found when 5′-A-2A-1G1G2A3G4-3′ was added into Lin28,as shown in Fig. 1(c). Residues with chemical shifts perturbation more than δ0.2 are mainly around the Zn knuckles [shown in Fig. 2(b)],implying that Zn knuckles in Lin28 play important roles in the interaction between Lin28 and RNA.
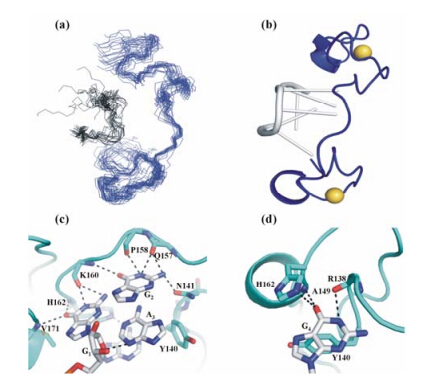
|
| Fig. 2 Structure features of Lin28 (134-179) in complex with 5′-A-2A-1G1G2A3G4-3′. (a) Backbone view of the ensemble of the 20 lowest-energy complex structures; (b) 3D representative structure in ribbon representation. Lin28 is shown in blue,while RNA is displayed in black or in grey. The ball represented zinc ion. (c)-(d) Interaction between Lin28 and RNA. Residues in Lin28 (cyan) are highlighted in orange,bases in RNA are displayed in blue and grey stick mode,highlighted in purple,respectively. The dotted lines indicate hydrogen bonds formation |
The solution structure of Lin28 bound to the ssRNA 5′-A-2A-1G1G2A3G4-3′ was calculated with 689 NOESY-derived NOE distance con¬straints (including 30 intermolecular NOE constraints),24 hydrogen bonds and 39 dihedral angle constraints,resulting in a well-defined ensemble of 20 structures [shown in Fig. 2(a)],in which the RMSD was 0.63 ± 0.14 (10-1 nm) for the heavy atoms in the secondary structural regions (Table 1).
| Complex(RNA) | |
| Distance restraints from NOEs | |
| Total | 689(30) |
| Intra-residue | 213(22) |
| Sequential (|i-j| = 1) | 196(6) |
| Medium range (1 < | i-j | ≤ 5) | 117(2) |
| Long range (| i-j | > 5) | 53(0) |
| Inter hydrogen bonds | 24 |
| Dihedral angels | 39(13) |
| Inter NOE | 33 |
| r.m.s.d versus the mean structure (0.1 nm) | |
| All backbone atoms | 2.25± 0.36 |
| All heavy atoms | 2.99 ± 1.03 |
| Backbone atoms (2nd structural region) | 0.52 ± 0.15 |
| Heavy atoms (2nd structural region) | 0.92 ± 0.20 |
| Structural statistics | |
| Rms Deviations versus the mean structure | |
| NOE distances (0.1 nm) | 0.049 ± 0.013 |
| Dihedral angles (deg.) | 0.986 ± 0.198 |
| Rms Deviations from idealized geometry | |
| Bonds (0.1 nm) | 0.0036 ± 0.00016 |
| Angles (deg.) | 0.912 ± 0.035 |
| Impropers (deg.) | 0.449 ± 0.018 |
| Structure Analysis | |
| residues in most favored regions | 74% |
| residues in additionally allowed regions | 23.7% |
| residues in generously allowed regions | 2.3% |
| residues in disallowed regions | 0.0% |
The structure (shown in Fig. 3) shows that the bound Lin28 only contains one α-helix (residues 149-152) and one 310-helix (residues 171-173). The Zn2+ ions coordinate with the residues C139,C142,H147,C152 in the first CCHC Zn knuckle (the first zinc finger,i.e.,ZnF1),and with the residues C161,C164,H169,C174 in the second CCHC Zn knuckle (the second zinc finger,i.e.,ZnF2). Different from our current complex structure,the conformation of Lin28 bound to 5′-A-1G1G2A3G4A5U6-3′ in previously reported NMR structure (PDB code 2LI8) contains only one helix located in ZnF1[15],while the other helix is absent,which however appears in the crystal structure of Lin28 in complex with RNA (PDB code 1A1T) (shown in Fig. 3)[16]. From this point,the conformation of Lin28 bound to RNA 5′-A-2A-1G1G2A3G4-3′ in current NMR structure looks to be more similar to that in the crystal structure,which might be caused by two more nucleotides after the conserved base G4 in 2LI8[16].
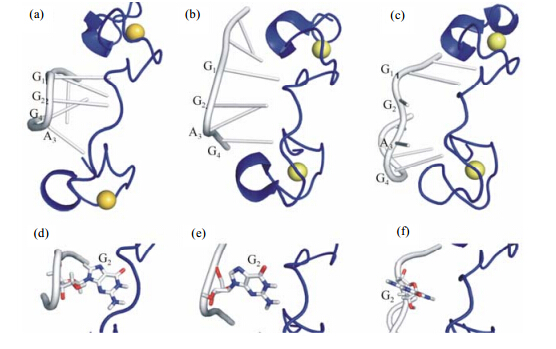
|
| Fig. 3 Structural differences among (a) the current NMR structure,(b) the crystal structure of mLin28 with let-7 RNA,and (c) NMR structure of hLin28 in complex with let-7 RNA. All structures are shown in ribbon mode. The ball represented zinc ion. In both (a) and (b),the backbones of the region G1G2A3G4 of RNA are bent due to Lin28 binding,while in (c),RNA backbone is not bent. (d)-(e) Anti-conformation of the base G2 in RNA in structures of both (a) and (b). (f) Syn-conformation of the base G2 in RNA in structure of (c). In all figures,Lin28 is shown in blue,while RNA is displayed in grey,and the base G2 is shown in stick mode,respectively |
Upon binding with Lin28,all the bases in the RNA sequence 5′-A-2A-1G1G2A3G4-3′ adopt anti-conformation in the complex,which is favorable in energy and consistent with the observations in the complex structure of mouse Lin28a (mLin28) with G1G2A3G4 (PDB code 1A1T). A distinctive hydrogen bond forms between ribose O and N3 of A3,resulting in a “kink-turn” like bending in the RNA backbone. This strong bending of the RNA backbone was not observed in the previous NMR structure[15],which,however,was also found in the crystal structure[16].
Except the first base A-2,A-1 and the core bases G1G2A3G4 have massive interactions with the Znfs of hLin28,where ZnF1 contacts A3 and G4,ZnF2 contacts A-1,G1 and G2,respectively. G1 is recognized by three hydrogen bonds with the main chain atoms of residues V171,H162 and K160 in hLin28. Although the base A-1 is not an essential base when RNA is recognized by Lin28[15],it forms both aromatic interaction and hydrogen bonds with the imidazole ring of H162. To confirm the hydrophobic interaction,H162 was replaced by W162 and A162,the binding affinities of the variants to RNA 5′-A-2A-1G1G2A3G4-3′ were measured by ITC assay as 1.7 μmol/L for Lin28 H162W variant (increased by 2-fold,compared to wild type (WT) protein),and 15 μmol/L for Lin28 H162A variant (decreased by 5-fold,compared to wild type protein),respectively[15]. The C-6 carbonyl of the base G4 forms hydrogen bonds with the back¬bone amides of the residues Y140 and A149 in human Lin28,while the backbone carbonyl of R138 forms hydrogen bond with the N-1 amine and the imino group of this guanine. These observations are consistent with the results from NMR titration assay,where all these key residues have large chemical shift changes during the NMR titration.
The rigid conformation of G2 was fixed by a hydrogen bond network formed by the residues in Lin28 and the base adjacent to G2 in RNA,i.e.,between the nitrogen atom of the side chain of residue N141,the backbone carbonyl group of residues Q157,P158,K160 and the N8 atom of the base A3. To confirm these interactions,the side-chain of N141 was intentionally removed by mutating N141 into A141,the binding affinity was then measured as 13 mol/L,decreased by about 4-fold,compared to the WT protein. When N141 was replaced by D141,the binding affinity became to be 97 μmol/L,decreased by 30-fold,further indicating the importance of this hydrogen bond[15]. To confirm that the side-chain of Q157 was not involved in the interaction,Q157 was replaced by A157 and R157,the binding affinities were measured as 7.1 μmol/L and 4.7 μmol/L,respectively,slightly weaker than the WT protein. These interactions were similar to those observed in the previously reported crystal complex structure of Lin28 with RNA 5′-G-8G-7A-6C-5U-4A-3G-2C-1G1G2A3G4 G5C6U7A8G9 U10C11C12-3′ (PDB code 1A1T)[16],but distinct from those observed in previously published NMR structure of Lin28 in complex with RNA (5′-A-1G1G2A3G4A5U6-3),where G2 is distorted in a syn conformation and has no interactions with Lin28 at all[15]. The binding of the base A3 to Lin28 is mainly mediated by the π-π stacking interaction with Y140,which also packs against the base G1,as well as the base G2[16]. This was confirmed by the following mutation studies. When Y140 was replaced by A140,the binding affinity was switched into about 34 μmol/L,decreased by about 10-fold,compared with the WT protein. When Y140 was mutated into F140,the binding affinity was 2 μmol/L,almost similar to that of the WT protein (3.4 μmol/L),indicating that the -OH group in the side-chain of Y140 is not involved in the interactions between the protein and RNA.
In conclusion,we here determined NMR solution structure of human Lin28 in complex with pre-let-7 RNA,which further confirms the structural basis for how Lin28 specially recognizes this RNA. We also demonstrated the RNA backbone bending in the structure,which was suggested to be important to directly hinder Dicer[16].
Acknowledgments: This work was supported by the grants from the Ministry of Science and Technology of China (2011CB966300) and the National Natural Science Foundation of China (21272261,21472229 and 21275154),the Science and Technology Commission of Shanghai Municipality (15ZR1449300).| [1] | Pasquinelli A E, Reinhart B J, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA[J]. Nature, 2000, 408(6 808):86-89. |
| [2] | Johnson S M, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family[J]. Cell, 2005, 120(5):635-647. |
| [3] | Charles D J, Aurora E K, Giovanni S, et al. The let-7 microRNA represses cell proliferation pathways in human cells[J]. Cancer Res, 2007, 67(17):7 713-7 722. |
| [4] | James E T, Richard I G. How does Lin28 let-7 control development and disease[J]? Trends Cell Biol, 2012, 22(9):474-482. |
| [5] | Yu J, Vodyanik M A, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells[J]. Science, 2007, 318(5 858):1 917-1 920. |
| [6] | Viswanathan S R, Powers J T, Einhorn W, et al. Lin28 promotes transformation and is associated with advanced human malignancies[J]. Nat Genet, 2009, 41(7):843-848. |
| [7] | Peng S, Maihle N J, Huang Y, et al. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer[J]. Oncogene, 2010, 29(14):2 153-2 159. |
| [8] | Permuth-Wey J, Kim D, Tsai Y Y, et al. Lin28B polymorphisms influence susceptibility to epithelial ovarian cancer[J]. Cancer Res, 2011, 71(11):3 896-3 903. |
| [9] | King C E, Cuatrecasas M, Castells A, et al. Lin28B promotes colon cancer progression and metastasis[J]. Cancer Res, 2011, 71(12):4 260-4 268. |
| [10] | Heo I, Joo C, Cho J, et al. Lin28 mediates the terminal uridylation of let-7 precursor microRNA[J]. Mol Cell, 2008, 32(2):276-284. |
| [11] | Heo I, Joo C, Kim Y K, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation[J]. Cell, 2009, 138(4):696-708. |
| [12] | Polesskaya A, Cuvellier S, Naguibneva I, et al. Lin28 binds IGF2 mRNA and participates in skeletal myogenesis by increasing translation efficiency[J]. Genes Dev, 2007, 21(9):1 125-1 138. |
| [13] | Thornton J E, Gregory R I. How does Lin28 let-7 control development and disease[J]? Trends Cell Biol, 2012, 22(9):474-482. |
| [14] | Viswanathan S R, Daley G Q. Lin28:A microRNA regulator with a macro role[J]. Cell, 2010, 140(4):445-449. |
| [15] | Loughlin F E, Gebert L F, Towbin, H, et al. Structural basis of pre-let-7 miRNA recognition by the zinc knuckles of pluripotency factor Lin28[J]. Nat Struct Mol Biol, 2012, 19(1):84-89. |
| [16] | Nam Y, Chen, C, Gregory R I, et al. Molecular basis for interaction of let-7 microRNAs with Lin28[J]. Cell, 2011, 147(5):1 080-1 091. |
| [17] | Bax A, Grzesiek S. Methodological advances in protein NMR[J]. Acc Chem Res, 1993, 26(4):131-138. |
| [18] | Clore G M, Gronenborn A. Determining the structures of large proteins and protein complexes by NMR[J]. Trends Biotechnol, 1998, 16(1):22-34. |
| [19] | Delaglio F, Grzesiek S, Vuister G W, et al. NMRPipe:a multidimensional spectral processing system based on UNIX pipes[J]. J Biomol NMR, 1995, 6(3):277-293. |
| [20] | Kuszewski J, Clore G M. Sources of and solutions to problems in the refinement of protein NMR structures against torsion angle potentials of mean force[J]. J Magn Reson, 2000, 146(2):249-254. |
| [21] | Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology[J]. J Biomol NMR, 1999, 13(3):289-302. |
| [22] | Neuhaus D, Nakaseko Y, Schwabe J W, et al. Solution structures of two zinc-finger domains from SWI5 obtained using two-dimensional 1H nuclear magnetic resonance spectroscopy. A zinc-finger structure with a third strand of beta-sheet[J]. J Mol Biol, 1992, 228(2):637-651. |
| [23] | Cao C Y, Kwon K, Jiang Y L, et al. Solution structure and base perturbation studies reveal a novel mode of alkylated base recognition by 3-methyladenine DNA glycosylase I[J]. J Biol Chem, 2003, 278(48):48 012-48 020. |
 2015, Vol. 32
2015, Vol. 32




