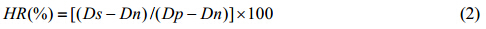文章信息
- 肖研,湛游洋,薛蓉,李晓晶,裴奉奎,冯江华,占博涵
- XIAO Yan, ZHAN You-yang, XUE Rong, LI Xiao-jing, PEI Feng-kui, FENG Jiang-hua, ZHAN Bo-han
- 一种肝脏类生物相容性氨基酸共聚物磁共振成像造影剂
- A Biocompatible Gadolinium (III)-Poly (Aspartic Acid-Co-Phenylalanine) for Liver Magnetic Resonance Imaging Contrast Agent
- 波谱学杂志, 2015, 32(2): 273-282
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 273-282
- http://dx.doi.org/10.11938/cjmr20150210
-
文章历史
- 收稿日期:2015-01-30
- 收修改稿日期:2015-05-19
2. 厦门大学 电子科学系, 厦门 361005
2. Department of Electronic Science, Xiamen University, Xiamen 361005
In the past few decades, magnetic resonance imaging (MRI) has become one of the most important diagnostic techniques in the clinic[1, 2, 3]. To enhance the contrast of imaging, nearly 50% of MRI examinations employ the use of MRI contrast agents[4, 5]. Currently, the commercial MRI contrast agents are gadolinium (III)-based complexes with the molecular weights of ~650, such as Gd-DTPA (Magnetvist), Gd-DOTA (Dotarem) and Gd-HPDO3A (Prohance)[6]. However, these small gadolinium (III)-chelates present low T1-relaxivities, nonspecific distribution in the body, resulting in heavy administration in the patients to obtain the desired imaging[6, 7, 8]. Furthermore, the clinical gadolinium (III)-based coordination complexes are extracellular fluid space (EFS) agents and their blood circulation time is too short to detect lesions[9, 10, 11]. The ideal MRI contrast agent would have high relaxivity, specificity and safety as well as suitable blood circulation time.
On the basis of the Solomon-Bloembergen-Morgan theory, loading small molecular gadolinium (III)-agents into the macromolecular compounds can increase the rotational correlation time (tR), resulting in improved relaxivity[12]. Compared with the small contrast agents, the macromolecular agents can present higher relaxation rates, and the properties of these macromolecules could cause better tissue or organ-specificity and low toxicity[13]. Currently, many gadolinium (III)-macromolecular MRI contrast agents have been prepared and reported and they are based on the covalent or non-covalent binding between Gd-DOTA or Gd-DTPA and macromolecules. Various natural polymers such as serum albumin[14], dextran[15], and synthetic biocompatible polymers such as polyamidoamine[16] have been chosen as the carriers for small MRI agents. The gadolinium (III)-macromolecules, however, have some shortcomings[17, 18] such as: non-biodegradability and low organ-specificity. The ideal gadolinium (III)-based macromolecular agents should be biocompatible, biodegradable and low-immunogenic macromolecules[18].
Poly (aspartic acid) is a water-soluble polymer with biodegradability, non-immunogenicity and biocompatibility[19], which satisfies the aforementioned requirements. Poly (aspartic acid) and its derivates have been extensively used as the pharmaceutical carriers[20, 21, 22]. In this respect, poly (aspartic acid) might also be used as the small gadolinium (III)-chelates carriers. Liver-selective imaging is related to the lipophilicity of MRI contrast agent[23]. Inserting phenyl groups and lipid derivatives into the backbone or side-chains of MRI contrast agents can enhance the liver-selectivity[23, 24]. L-phenylalanine, an essential amino acid, possesses the physiological activity and phenyl structure[25]. Previous works have shown that adding L-phenylalanine into the macromolecular polymer enhanced the lipophilicity[26, 27]. Therefore, we chose the poly (aspartic acid-co-phenylalanine) as the carrier for Gd-DOTA.
Here, we report a new biocompatible gadolinium (III)-based macromolecular conjugate (AP-EDA-DOTA-Gd) developed for a potential liver MRI contrast agent. AP-EDA-DOTA-Gd has been synthesized and characterized. The biodegradability, and hemocompatibility of this gadolinium (III)-macromolecule has been investigated. The evaluation of AP-EDA-DOTA-Gd for liver contrast agent was conducted by T1-relaxivity measurement and in vivo studies of MRI in rats. 1 Methods 1.1 Materials and methods
All reagents in this research were A.R. (analytical reagent) and used without further purification. L-aspartic acid, L-phenylalanine, phosphoric acid, N, N-dimethylformamide (DMF), ethylenediamine, ethyl ether (Et2O), ethyl alcohol (EtOH), and dimethylsulfoxide (DMSO) were purchased from Sinopharm Chemical Reagent. N-hydroxysulfosuccinimide sodium salt (sulfo-NHS), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl), 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid (DOTA), gadolinium (III) chloride (GdCl3), ethylene diamine tetraacetic acid (EDTA). The red blood cells (RBC) were purchased from College of Basic Medical Sciences, Jilin University. 1.2 Preparation of the MRI contrast agent AP-EDA-DOTA-Gd 1.2.1 Synthesis of ploy (aspartic acid -co- phenylalanine) (AP)
L-aspartic acid (6.53 g, 49 mmol) and L-phenylalanine (3.47 g, 21 mmol) were mixed with 5 mL 85% phosphoric acid, and the mixture was poured into a round-bottom flask equipped with a rotary evaporator. The reaction mixture was stirred under reduced pressure (24 mmHg) for 5 h at 200 ℃. 50 mL DMF was added into the round-bottom flask to dissolve the mixture. The resulting solution was precipitated with 250 mL deionized water and filtered. The crude product was washed several times with deionized water. The final product (AP, 6.9 g) was obtained by vacuum drying. 13C NMR (DMSO-d6): 173.8 (-CH2CON-, carbonyl carbon of succinimide unit), 172.7 (-CHCON-, carbonyl carbon of succinimide unit), 169.6 (-CONH-, carbonyl carbon of phenylalanine unit), 137.7-126.9 (C6H5, phenyl carbons of phenylalanine unit), 55.8-54.0 (-CHCH2C6H5, methyne carbon of phenylalanine unit), 47.7 (-CHCO-, methyne carbon of succinimide unit), 33.8-32.9 (-CH2CO-, methylene carbon of succinimide unit; -CHCH2C5H6, methylene carbon of phenylalanine unit). 1.2.2 Synthesis of aminated ploy (aspartic acid -co- phenylalanine) (AP-EDA)
Both AP (2.0 g) and ethylenediamine (24 mL) were dissolved in 24 mL DMF, respectively. The ethylenediamine solution was drop-wise added into the AP solution and stirred for 4 h at 25 ℃. The resulting solution was precipitated with 240 mL Et2O-EtOH (2∶1) and filtered. The crude product was added into 100 mL NaOH aqueous solution (0.1 mol×L-1) and stirred for 1 h at 25 ℃. The residue solution was obtained via dextran Sephadex G-25 column. After condensation and lyophilization, the final product (AP-EDA, 1.2 g) was obtained. 13C NMR (D2O): 173.1-172.7 (-CH2CONH-, CHCONH-, carbonyl carbon of aspartic acid unit), 172.2-171.7 (-CH2COONa, -CHCOONa, -CH2CONHCH2 -, -CHCONHCH2 -, carbonyl carbon of aspartic acid unit), 170.7 (-CONH-, carbonyl carbon of phenylalanine unit), 137.7-126.6 (C6H5, phenyl carbons of phenylalanine unit), 55.8-54.0 (-CHCH2C6H5, methyne carbon of phenylalanine unit), 47.7 (-CHCO-, methyne carbon of aspartic acid unit), 38.9 (-CONHCH2CH2NH2, methylene carbon of aspartic acid unit), 36.8 (-CONHCH2CH2NH2, methylene carbon of aspartic acid unit), 35.6-33.8 (-CH2CO-, methylene carbon of aspartic acid unit; -CHCH2C5H6, methylene carbon of phenylalanine unit). 1.2.3 Conjugation of DOTA to aminated ploy (aspartic acid -co- phenylalanine) (AP-EDA-DOTA)
DOTA (0.8 g, 1.98 mmol) was activated with sulfo-NHS (0.4 g, 1.82 mmol) and EDC×HCl (0.45 g, 2.35 mmol) in 20 mL deionized water for 4 h at 4 ℃. AP-EDA (25 mg×mL-1, 12 mL) was added to the reacting solution, and the solution was stirred for 12 h at 4 ℃. The resulting solution was purified via dextran Sephadex G-25 column. After condensation and lyophilization, the final product (AP-EDA-DOTA, 0.83 g) was obtained. 13C NMR (D2O): 177.7-177.3 (C-7, C-7′, C-7″/DOTA carboxyl), 173.1-172.7 (-CH2CONH-, CHCONH-, carbonyl carbon of aspartic acid unit), 172.2-171.7 (-CH2COOH, -CHCOOH, -CH2CONHCH2-, -CHCONHCH2-, carbonyl carbon of aspartic acid unit), 170.7 (-CONH-, carbonyl carbon of phenylalanine unit), 160.3-158.8 (C-1/DOTA CONH carbon), 137.7-126.6 (C6H5, phenyl carbons of phenylalanine unit), 64.8-63.7 (C-2/DOTA CH2 carbon), 56.5 (C-5, C-5′, C-5″/DOTA carbons), 55.1-54.0 (-CHCH2C6H5, methyne carbon of phen ylalanine unit; C-6, C-6′, C-6″, C-6′′′/DOTA CH2 carbons), 52.9 (C-3, C-3′/DOTA CH2 carbons), 50.7 (C-4, C-4′/DOTA CH2 carbons), 48.2 (-CHCO-, methyne carbon of aspartic acid unit), 39.5 (-CONHCH2CH2NH-, methylene carbon of aspartic acid unit), 36.3-34.8 (-CH2CO-, methylene carbon of aspartic acid unit; -CHCH2C5H6, methylene carbon of phenylalanine unit). 1.2.4 Preparation of gadolinium conjugate (III) (AP-EDA-DOTA-Gd)
2 mL of an aqueous solution of GdCl3 (0.25 mol×L-1) was added to AP-EDA-DOTA solution (0.5 g, 20 mL), and the mixture was stirred for 2 h at 25 ℃. Xylenol Orange (the indicator for free gadolinium (III)) was added into the resulting solution. A suitable solution of EDTA (the chelating agent of free gadolinium (III)) was added until the color of the solution changed from pink to bright yellow. The resulting solution was further purified via a dextran Sephadex G-50 column. After condensation and lyophilization, the final product (AP-EDA-DOTA-Gd) was obtained. 1.3 Characterization methods
The 13C NMR spectra were obtained on a Bruker Avance 400 NMR spectrometer. The molecular weights of AP-EDA-DOTA-Gd were determined by a Waters gel permeation chromatography (GPC) instrument, respectively. The content of DOTA in AP-EDA-DOTA was determined by a reverse complexometric titration[28]. The contents of gadolinium (III) in AP-EDA-DOTA-Gd and bio-samples were obtained on a POMES TJA inductively couple plasma mass spectroscopy/spectrophotometer (ICP-OES). 1.4 In vitro relaxation time
The longitudinal relaxation time (T1) of AP-EDA-DOTA-Gd was measured on a 60 NMR spectrometer, using the standard inversion-recovery method. The T1 values of AP-EDA-DOTA-Gd and Gd-DOTA were measured in D2O-H2O (m/m=1/3) at the concentrations of gadolinium (III) (0, 0.5, 1.0, 2.0, 3.5 and 5.0 mmol×L-1), respectively. The longitudinal relaxivity (r1) was calculated on the basis of the following Eq. (1):
where, (1/T1)obs and (1/T1)inh are the observed water relaxation rates in the presence and absence of gadolinium (III), respectively. [Gd] is gadolinium (III) concentration. 1.5 Biodegradation of AP-EDA-DOTA-Gd
AP-EDA-DOTA-Gd was dissolved in phosphate buffered solution (pH=5.5) to get a concentration of 6.5 mg×mL-1. Based on the previous work[29], cathepsin B was chosen as an activator, and it was added to the mixture to get a final concentration of 20 unit×mL-1. The mixture was incubated in a constant temperature incubator at 37 ℃. At the scheduled time intervals, solutions were collected, and the molecular weights of the gadolinium (III)-complex were measured by GPC. 1.6 Hemolytic toxicity
The RBC (2 mL) was added into 100 mL normal saline to obtain a 2% RBC suspension. The RBC suspension was mixed with normal saline (Negative Control) and deionized water (Positive Control), respectively. AP-EDA-DOTA-Gd was added to RBC suspension to get the concentrations of gadolinium (III) to be 2-972 mmol×L-1. The mixed solutions were incubated in a constant temperature incubator for 1 h at 37 ℃. Subsequently, the solutions were centrifuged (4 000 rpm/min, 10 min) to separate the complete RBC. The absorbance of the supernatant solutions were obtained by a UV-VIS spectrophotometer at 545 nm. The hemolytic rates (HR) were calculated by the following Eq. (2):
where, Ds, Dp and Dn are the absorbance of the Positive Control and Negative Control sample solution, respectively. 1.7 In vivo MRI experiment
In vivo MRI experiments were performed on a 4.7 T MR imaging system at 25 ℃. The male Wistar rats (weight 100-120 g) were purchased from College of Basic Medical Sciences, Jilin University (Jilin, China). All the animal studies were carried out in accordance with current China legislation. The Wistar rats were anesthetized by 20% ethyl carbamate solution before injecting AP-EDA-DOTA-Gd and Gd-DOTA (0.1 mmol/kg, gadolinium (III)/body weight) via the vein, respectively. A series of ventral MRI images were acquired at the scheduled time intervals. T1-weight images were obtained by using a multi-slice and multi-echo (MSME) sequence. The experimental parameters were echo time (TE)/repetition time (TR) = 13.6 ms/300 ms, field of view (FOV) = 5.5×5.5 cm2, slice thickness = 1.5 mm, and a 128×128 matrix. The enhancement (ME) was described by the following Eq. (3):
where, SI(t) and SI(0) are the signal intensity obtained at time point t and the signal intensity obtained at time of pre-injection, respectively. 2 Results and discussion 2.1 Preparation and properties of AP-EDA-DOTA-Gd
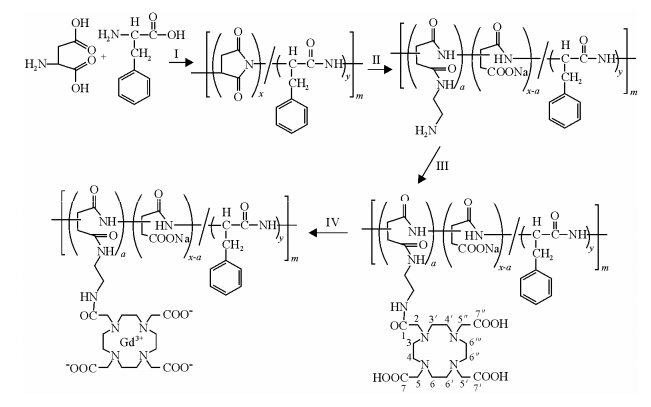
The successful synthesis route of AP-EDA-DOTA-Gd is shown in Scheme 1. Poly (aspartic acid-co-phenylalanine) was synthesized, modified via ethylenediamine, conjugated with 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid (DOTA) and finally chelated gadolinium (III), yielding gadolinium (III)-based macromolecule (AP-EDA-DOTA-Gd). The average molecular weight of AP-EDA-DOTA-Gd was 58.4 k. The average number of conjugated Gd-DOTA per AP-EDA-DOTA-Gd was 32-33, which was measured by the reverse complexometric method as well as ICP-OES. The relaxivity (15.93 mmol-1×L×s-1) of AP-EDA-DOTA-Gd was 2.9 times than that (5.59 mmol-1×L×s-1) of the clinical contrast agent (Gd-DOTA) at 1.5 T and 25 ℃. The high relaxivity of AP-EDA-DOTA-Gd could be mainly attributed to an increase in rotational correlation time due to the macromolecular size of this gadolinium (III)-copolymer[35, 36].
|
| Scheme 1 The synthetic route of AP-EDA-DOTA-Gd (I: phosphoric acid, II: ethylenediamine, DMF, III: DOTA, sulfo-NHS, EDC.HCl, IV: GdCl3) |
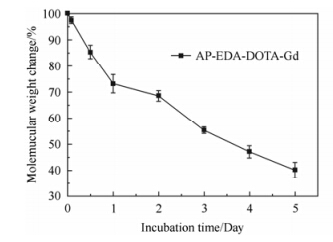
The enzymatic degradation of AP-EDA-DOTA-Gd was carried out in the cathepsin B solution, using GPC to analyze the changes of the gadolinium (III)-polymeric molecular weights at the scheduled time intervals. The molecular weight of AP-EDA-DOTA-Gd decreased as early as 2 h after incubating with cathepisn B in PBS, and finally 59.2% during the 5 days (Fig. 1). It was speculated that AP-EDA-DOTA-Gd was degraded through two ways: (1) the cleavage of gadolinium (III)-polymeric backbone (2) the hydrolysis of the amide linkages in the polymeric side-chains[33, 34].
|
| Fig. 1 In vitro degradation of AP-EDA-DOTA-Gd |
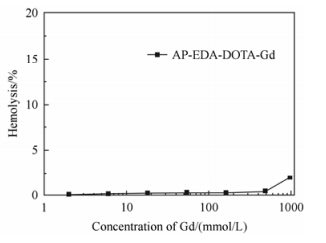
Hemocompatibility is an important factor to assess the in vivo application of parenteral biomaterials[37, 38]. The hemolytic effect of AP-EDA-DOTA-Gd was quantified by measuring the release of hemoglobin and calculated by Eq. (2). As shown in Fig. 2, AP-EDA-DOTA-Gd exhibited almost no hemolysis (<5%), even at a high concentration of gadolinium (III). AP-EDA-DOTA-Gd is hemocompatible and appropriate for the intravenous administration.
|
| Fig. 2 Hemolysis of AP-EDA-DOTA-Gd (n = 3, mean ±SD, SD: standard deviation) |
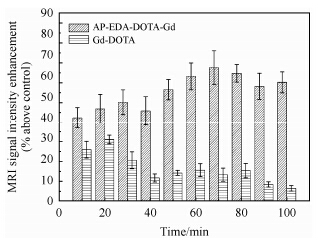
In vivo MRI experiment was performed with AP-EDA-DOTA-Gd (0.095±0.003 mmol/kg, gadolinium (III)/body weight) and Gd-DOTA (0.098 ±0.005 mmol/kg, gadolinium (III)/body weight). Fig. 3 and Fig. 4 illustrate the liver MR signal intensities of AP-EDA-DOTA-Gd and Gd-DOTA, respectively. Compared with Gd-DOTA, AP-EDA-DOTA-Gd showed stronger signal, with obviously different pattern. After the administration of this gadolinium (III)-macromolecular coordination complex, a significant enhancement was achieved in liver within 10 min and persisted throughout the entire MRI experiment. The liver enhancement of AP-EDA-DOTA-Gd was 63.5±6.1% during the maximum enhancement time (50-80 min). In contrast, significantly lower enhancement (24.2±2.9%, 10-30 min) of Gd-DOTA was achieved. Therefore, AP-EDA-DOTA-Gd led to a remarkable increase of the image signal in the liver, most probably due to its molecular size and structure. Owing to the large molecular size of AP-EDA-DOTA-Gd, the ability of gadolinium (III)-polymer crossing the capillaries was limited, resulting in the prolonged blood circulation time. The liver is a well blood-perfused organ, and the T1-effect of AP-EDA-DOTA-Gd in the blood could enhance the liver signal intensity[32, 40]. Furthermore, the degradation of this gadolinium (III)-based macromolecule in the blood could cause the Gd-DOTA-fragments to diffuse into the hepatic interstitium, resulting in a T1-effect in the liver. Numerous aromatic rings in this gadolinium (III)-copolymer enhanced the lipophilicity which could make the contrast agent accumulated in the liver[5]. Therefore, AP-EDA-DOTA-Gd provided remarkable liver imaging and a longer MRI time-window than Gd-DOTA, which enables satisfactory liver MR imaging. These results indicate that AP-EDA-DOTA-Gd could be applied in the MRI diagnosis of liver.

|
| Fig. 3 Axial T1- images of rat liver pre-injection (a); 10 min (b), 40 min (c), and 80 min (d) post-injection of AP-EDA-DOTA-Gd |
|
| Fig. 4 The mean percentage enhancement of liver at the scheduled time intervals following the intravenous administration of AP-EDA-DOTA-Gd and Gd-DOTA. (n = 3, mean ±SD, SD: standard deviation) |
In summary, a new biocompatible gadolinium (III)-macromolecular MRI contrast agent (AP-EDA-DOTA-Gd) with high T1-relaxivity has been successfully prepared. This gadolinium (III)-macromolecule shows almost no hemolysis. In vivo, AP-EDA-DOTA-Gd could provide remarkable contrast enhancement in the liver and a prolonged MRI time-window. Therefore, AP-EDA-DOTA-Gd might be applied in the MRI diagnosis of liver.
| [1] | Werner E J, Datta A, Jocher C J, et al. High-relaxivity MRI contrast agents:where coordination chemistry meets medical imaging[J]. Angew Chem, Int Ed, 2008, 47(46):8 568-8 680. |
| [2] | Yu K C, Lv Z Y, Yao Y, et al. Recent progress in development of bio-active MRI contrast agents[J]. Chinese J Magn Reson, 2010, 27(3):355-368. |
| [3] | Yu K C, Wang G P, Ding S W, et al. Recent progresses in the development of contrast agents used in magnetic resonance imaging[J]. Chinese J Magn Reson, 2004, 21(4):505-525. |
| [4] | Major J L, Meade T J. Bioresponsive cell-penetrating and multimeric MR contrast agents[J]. Acc Chem Res, 2009,42(7):893-903. |
| [5] | Yan G P, Robinson L, Hogg P. Magnetic resonance imaging contrast agents:overview and perspectives[J]. Radiography, 2007, 13:e5-e19. |
| [6] | Chan K W Y, Wong W T. Small molecular gadolinium (III) complexes as MRI contrast agents for diagnostic imaging[J]. Coordin Chem Rev, 2007, 251(17):2 428-2 451. |
| [7] | Weinmann H J, Ebert W, Misselwitz B, et al. Tissue-specific MR contrast agents[J]. Eur J Radiol, 2003, 46(1):33-44. |
| [8] | Tsitovich P B, Burns P J, McKay A M, et al. Redox-activated MRI contrast agents based on lanthanide and transition metal ions[J]. J Inorg Biochem, 2014, 133:143-154. |
| [9] | Jao J C, Lu H C, Lu H Y, et al. The imaging behavior of an MRI contrast agent [Gd (TTDA-BOM)] 2-in a mice liver tumor model at 3 Tesla[J]. J Med Biol Eng, 2010, 30(3):139-144. |
| [10] | Caravan P, Ellison J J, McMurry T J, et al. Gadolinium (III) chelates as MRI contrast agents:structure, dynamics, and applications[J]. Chem Rev, 1999, 99(9):2 293-2 352. |
| [11] | Tang J, Sheng Y, Hu H, et al. Macromolecular MRI contrast agents:Structures, properties and applications[J]. Prog Polym Sci, 2013, 38(3):462-502. |
| [12] | Li Y, Beija M, Laurent S, et al. Macromolecular ligands for gadolinium MRI contrast agents[J]. Macromolecules, 2012, 45(10):4 196-4 204. |
| [13] | Nwe K, Milenic D, Bryant L H, et al. Preparation, characterization and in vivo assessment of Gd-albumin and Gd-dendrimer conjugates as intravascular contrast-enhancing agents for MRI[J]. J Inorg Biochem, 2011, 105(5):722-727. |
| [14] | Fasano M, Curry S, Terreno E, et al. The extraordinary ligand binding properties of human serum albumin[J]. IUBMB Life, 2005, 57(12):787-796. |
| [15] | Wang S C, Wikström M G, White D L, et al. Evaluation of Gd-DTPA-labeled dextran as an intravascular MR contrast agent:imaging characteristics in normal rat tissues[J]. Radiology, 1990, 175(2):483-488. |
| [16] | Rudovský J, Botta M, Hermann P, et al. PAMAM dendrimeric conjugates with a Gd-DOTA phosphinate derivative and their adducts with polyaminoacids:the interplay of global motion, internal rotation, and fast water exchange[J]. Bioconjugate Chem, 2006, 17(4):975-987. |
| [17] | Pouponneau P, Bringout G, Martel S. Therapeutic magnetic microcarriers guided by magnetic resonance navigation for enhanced Liver chemoembilization:a design review[J]. Ann Biomed Eng, 2014, 42(5):929-939. |
| [18] | Bryson J M, Reineke J W, Reineke T M. Macromolecular imaging agents containing lanthanides:can conceptual promise lead to clinical potential?[J]. Macromolecules, 2012, 45(22):8 939-8 952. |
| [19] | Obst M, Steinbüchel A. Microbial degradation of poly(amino acid)s[J]. Biomacromolecules, 2004, 5(4):1 166-1 176. |
| [20] | Yan G P, Liu M L, Li L Y. Polyaspartamide gadolinium complexes containing sulfadiazine groups as potential macromolecular MRI contrast agents[J]. Bioconjugate Chem, 2005, 16(4):967-971. |
| [21] | Giammona G, Cavallaro G, Maniscalco L, et al. Synthesis and characterisation of novel chemical conjugates based on α,β-polyaspartylhydrazide and β-cyclodextrins[J]. Eur Poly J, 2006, 42(10):2 715-2 729. |
| [22] | Oh N M, Oh K T, Youn Y S, et al. Poly (l-aspartic acid) derivative soluble in a volatile organic solvent for biomedical application[J]. Colloid Surface B, 2012, 97:190-195. |
| [23] | Ferroud C, Borderies H, Lasri E, et al. Synthesis of a novel amphiphilic GdPCTA-[12] derivative as a potential micellar MRI contrast agent[J]. Tetrahedron Lett, 2008, 49(41):5 972-5 975. |
| [24] | Wojciechowski F, Suchy M, Li A X, et al. A robust and convergent synthesis of dipeptide-DOTAM conjugates as chelators for lanthanide ions:new PARACEST MRI agents[J]. Bioconjugate Chem, 2007, 18(5):1 625-1 636. |
| [25] | Sprenger G A. From scratch to value:engineering Escherichia coli wild type cells to the production of L-phenylalanine and other fine chemicals derived from chorismate[J]. Appl Microbiol Biotechnol, 2007, 75(4):739-749. |
| [26] | Akagi T, Higashi M, Kaneko T, et al. Hydrolytic and enzymatic degradation of nanoparticles based on amphiphilic poly (γ-glutamic acid)-graft-l-phenylalanine copolymers[J]. Biomacromolecules, 2006, 7(1):297-303. |
| [27] | Sage H J, Fasman G D. Conformational studies on poly-L-glutamic acid and copolymers of L-glutamic acid and L-phenylalanine[J]. Biochemistry, 1966, 5(1):286-296. |
| [28] | Corot C, Schaefer M, Beaute S, et al. Physical, chemical and biological evaluations of CMD-A2-Gd-DOTA. A new paramagnetic dextran polymer[J]. Acta Radiol, 1996, 412:91-99. |
| [29] | Kishore B K, Lambricht P, Laurent G, et al. Mechanism of protection afforded by polyaspartic acid against gentamicin-induced phospholipidosis. II. Comparative in vitro and in vivo studies with poly-L-aspartic, poly-L-glutamic and poly-D-glutamic acids[J]. J Pharm Exper Ther, 1990, 255(2):875-885. |
| [30] | Lebdušková P, Kotek J, Hermann P, et al. A gadolinium (III) complex of a carboxylic-phosphorus acid derivative of diethylenetriamine covalently bound to inulin, a potential macromolecular MRI contrast agent[J]. Bioconjugate Chem, 2004, 15(4):881-889. |
| [31] | Chong H S, Garmestani K, Bryant L H, et al. Synthesis and evaluation of novel macrocyclic and acyclic ligands as contrast enhancement agents for magnetic resonance imaging[J]. J Med Chem, 2006, 49(6):2 055-2 062. |
| [32] | Sun G, Feng J, Jing F, et al. Synthesis and evaluation of novel polysaccharide-Gd-DTPA compounds as contrast agent for MRI[J]. J Magn Magn Mater, 2003, 265(2):123-129. |
| [33] | Zhang G, Zhang R, Melancon M P, et al. The degradation and clearance of Poly (N-hydroxypropyl-L-glutamine)-DTPA-Gd as a blood pool MRI contrast agent[J]. Biomaterials, 2012, 33(21):5 376-5 383. |
| [34] | Ke T, Feng Y, Guo J, et al. Biodegradable cystamine spacer facilitates the clearance of Gd (III) chelates in poly (glutamic acid) Gd-DO3A conjugates for contrast-enhanced MR imaging[J]. Magn Reson Imaging, 2006, 24(7):931-940. |
| [35] | Armitage F E, Richardson D E, Li K C P. Polymeric contrast agents for magnetic resonance imaging:synthesis and characterization of gadolinium diethylenetriaminepentaacetic acid conjugated to polysaccharides[J]. Bioconjugate Chem, 1990, 1(6):365-374. |
| [36] | Secchi F, Di Leo G, Papini G D E, et al. Optimizing dose and administration regimen of a high-relaxivity contrast agent for myocardial MRI late gadolinium enhancement[J]. Eur J Radiol, 2011, 80(1):96-102. |
| [37] | Yalkowsky S H, Krzyzaniak J F, Ward G H. Formulation-related problems associated with intravenous drug delivery[J]. J Pharm Sci, 1998, 87(7):787-796. |
| [38] | Dekie L, Toncheva V, Dubruel P, et al. Poly-L-glutamic acid derivatives as vectors for gene therapy[J]. J Control Release, 2000, 65(1):187-202. |
 2015, Vol. 32
2015, Vol. 32