文章信息
- 郁桂云, 彭路明
- YU Gui-yun, PENG Lu-ming
- 固体核磁共振谱学研究层状双氢氧化物
- Solid-State NMR Studies of Layered Double Hydroxides:A Review
- 波谱学杂志, 2015, 32(2): 228-247
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 228-247
- http://dx.doi.org/10.11938/cjmr20150207
-
文章历史
- 收稿日期:2015-02-11
- 收修改稿日期:2015-05-11
2. 南京大学 化学化工学院,介观化学教育部重点实验室,南京210093
2. Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093
Layered double hydroxides (LDHs) have been known for over 150 years since the discovery of the mineral hydrotalcite [Mg6Al2CO3(OH)16·4(H2O)][1, 2]. LDHs are a class of ionic lamellar compounds made up of positively charged brucite-like layers with an interlayer region containing charge compensating anions and water. The cations occupy the centers of edge sharing octahedra,whose vertexes contain hydroxide ions that connect to form 2D sheets. The general formula of common LDHs may be written as: [M2+1−xM3+x(OH)2][An−]x/n·zH2O (0.17 ≤ x ≤ 0.33)[3],where M2+,M3+ and An− are divalent cations (e.g.,Mg2+,Zn2+ and Ni2+),trivalent cations (e.g.,Al3+,Ga3+,Fe3+ and Mn3+),and charge compensating inorganic or organic anions,respectively. M+ and M4+ cations can also be present in LDHs but these are limited to specific examples such as Li+ and Ti4+. LDHs are very important inorganic supramolecular materials,in which the compositions can be controlled for a variety of applications including environmental applications[4, 5],catalysis[6],photochemistry[7, 8, 9],electrochemistry[10, 11],and biomedical sciences[12].
It is important to understand the cation ordering (distribution of M2+ and M3+) which controls the interactions of anions and hydroxides sheets,as well as the binding mode of anions in the interlayer space at molecular scale,to design and prepare LDHs with improved properties. The conventional diffraction methods are not well suited for most extensively explored MgAl-LDHs (LDHs containing Mg and Al),due to the very similar scattering power of Mg and Al,and the presence of significant turbostratic disorder and stacking faults[13]. Solid-state NMR spectroscopy represents a powerful tool with which to extract the local structure and dynamics information for inorganic materials[14, 15, 16, 17],thus should be perfect to study LDHs. In the past 40 years,details of the LDH structure in the atomic/molecular level have been obtained with solid-state NMR methods,and in particular,key advances have been made only very recently. In this presentation,the progress in the characterization of the structure of LDHs by solid-state NMR spectroscopy is reviewed. 1 1H NMR
With nearly 100 % natural abundance and high gyromagnetic ratio,the spin-1/2 1H is considered to be an NMR friendly nucleus. 1H NMR spectroscopy has been extensively used to study the structure of organic and inorganic substances. The first 1H NMR characterization of LDH was reported by Marcellin and co-workers in 1989[18]. Their results revealed the dynamics and ordering of intercalated water and the interaction of water molecules with hydroxyl species in the hydroxide layer. The water molecules are found to be hydrogen-bonded to the hydroxyl groups,leading to a configuration that water molecules lie perpendicular to the plane of the layers,while retain their translational mobility. This ordering and the interactions between the mobile water and the structural OH group were further investigated by other researchers[19, 20]. These results indicate that interlayer water possesses rotational freedom around the C2 axis which is also parallel to the crystallographic c axis of LDHs. Reinholdt et al. also studied the motional dynamics of surface and interlayer water in LDHs by analyzing the 1H spin lattice NMR relaxation (T1) and the 1H static NMR over a range of relative humidities (RHs)[21].
Depègeand coworkers studied the polymerization of silicates in LDHs by using 1H magic angle spinning (MAS) NMR at a spinning rate of 6 000 Hz[22]. The 1H NMR spectra of Zn3-Al-SiO4 show that there are two well-resolved peaks at δ1.4 and 4.2,due to hydroxyl groups bound to octahedral metal ions and interlayer water. However,in most cases,the high concentration of interlayer water and hydroxyl groups results in strong 1H homonuclear dipolar coupling and there is no sufficient resolution to identify different hydroxyl species despite applying a moderate MAS frequency (i.e. below 10 kHz)[22, 23, 24, 25]. Since the 1H line widths scale approximately linearly with the inverse of the spinning frequency[26],the first approach to overcome the broadening due to 1H dipolar coupling and retrieve chemical shift information is to simply use very fast MAS. Recently,Sideris and coworkers showed that ultrafast MAS 1H NMR spectroscopy could be applied to distinguish the signals arising from two different hydroxyl groups,i.e.,Mg3OH and Mg2AlOH,as well as interlayer water[13]. Fig. 1(a) compares one dimensional 1H spectra recorded from 10 kHz to 60 kHz MAS of MgAl-LDHs with 33% Al and 67% Mg. The drastic improvement in resolution can be observed with increasing MAS rate and two distinct 1H resonances are clearly resolved at 40 kHz or higher.
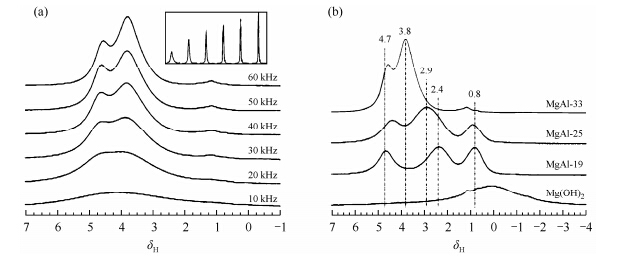
|
| Fig. 1 (a) The 1H MAS NMR spectra of MgAl-LDH (Al = 33%)at 14 T as a function of spinning speed: Only the isotropic resonances are shown. The inset shows the same spectra side by side to emphasize the gain in resolution. (b) The effect of Al content on the single-pulse 1H MAS NMR spectra collected at a spinning speed of 60 kHz. Hydrotalcite-like LDH containing 19%,25%,and 33% Al are shown. (Reproduced from Ref. [13]) |
By fitting the 1H NMR peaks arising from three different species,the cation ordering in the hydroxide layer is unveiled. Each OH group in the layer is coordinated to three metal ions,leading to four possible hydroxyl local environments: Mg3OH,Mg2AlOH,MgAl2OH,and Al3OH,if Al3+ cations are randomly distributed [Fig. 2(c)],while only the first two are possible in a non-random distribution model assuming Al-Al avoidance [Fig. 2(c)]. Quantitative 1H MAS NMR experiments revealed that LDH with Al = 33% has mostly Mg2AlOH hydroxyl groups (97%) and negligible Mg3OH (3%) hydroxyl groups,which is consistent with the non-random distribution model. In addition,the lack of signals from MgAl2OH and Al3OH species clearly indicates that there is no Al3+-Al3+ contacts in the hydroxide layers. For LDH with different Al molar percentages (Al = 19 and 25%),the experimental results of the hydroxyl groups are also in good agreement with the relative concentrations calculated based on non-random models that assumed Al-Al avoidance [Fig. 1(b)].
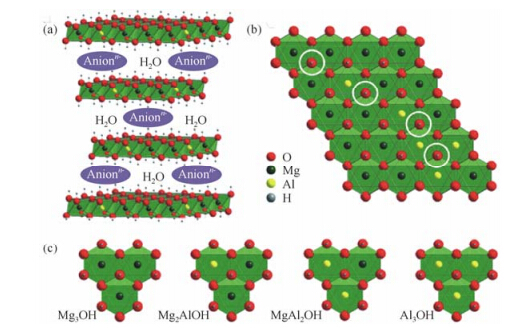
|
| Fig. 2 (a) The schematic representation of the structure of LDH materials with (b) random Al distribution and (c) the 4 different possible oxygen local environments in the cation layer.(Reproduced from Ref. [54]) |
However,Cadars et al. claimed that even within a largely ordered structure,a small concentration of defects (i.e.,MgAl2OH),can exist in Al rich LDH[27]. In their LDH sample (Al = 33%),a small 1H signal at 5.3 is observed that can be attributed to the MgAl2OH moieties. This resonance also appears in 1H-1H double- quantum (DQ) recoupling experiments (Fig. 3),correlating the peak at δ3.8 which arises from the dominant Mg2AlOH species. MgAl2OH is about 10 % of the total hydroxyl species in the hydroxide sheets.
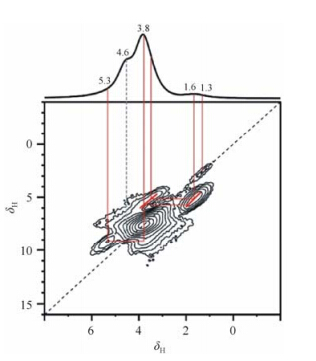
|
| Fig. 3 2D 1H-1H double quantumNMR spectrum ofthe LDH sample MgAl-LDH (Al = 33%),collected at 17.6 T at a MAS frequency of 64 kHz. (Reproduced from Ref. [26]) |
This matter was further investigated by Sideris et al[28].The diagonal peaks at 2.9-3.4 and 1.1-1.4 are very intense in the 2D DQ data,which implies a significant clustering of Mg2AlOH and Mg3OH hydroxyl groups,respectively. Strong off-diagonal peaks linking the two different hydroxyl clusters proves that the two environments observed in the proton spectra are in the same metal hydroxide layer. These results supported the conclusion that there is Al-Al avoidance in LDHs and suggested that the presence of MgAl2OH defects in LDH prepared by Cadars is more likely due to different sample preparation method.
1H NMR spectroscopy also proves to be helpful in illustrating the cation ordering of LDH with different M3+ cations other than Al. For example,the local environment and composition of MgGa-LDH was carefully investigated by using a high magnetic field (21.1 T,900 MHz) and fast spinning (35 kHz)[29]. Three hydroxyl groups (i.e.,Mg3OH,Mg2GaOH and interlayer water) were assigned on the basis of 1H-71Ga heteronuclear correlation (HETCOR) NMR results with different contact time. The isotropic chemical shifts are very similar with that of MgAl-LDH. The observations of only two different hydroxy groups in the layer indicate that the presence of similar cation ordering in MgGa-LDH (i.e.,Ga-Ga avoidance).
While ultrafast MAS spinning can provide high resolution for 1H to extract cation ordering information,it is difficult to reintroduce dipolar coupling at this condition in order to explore fundamental and key interactions between cations and anions with double resonance NMR techniques. Yu and coworkers used a different approach to narrow the spectral broadening due to 1H dipolar coupling by isotopic dilution of 1H with deuterium[30]. High resolution 1H solid-state NMR spectra of LDH can be obtained conveniently at medium to low spinning speeds on deuterated samples (Fig. 4). Cation ordering information in LDH can then be extracted and similar conclusions have been made as the ultrafast MAS NMR studies of non-deuterated sample. At relatively low spinning rate,double resonance NMR methods,such as Transfer of Populations in Double Resonance (TRAPDOR),can be applied to investigate the spatial correlation between 1H and its neighboring 27Al ions. The 1H NMR peak at 2.9 with a large reduction in intensity from TRAPDOR experiment can be assigned to Mg2AlOH species due to the short H-Al distance (Fig. 5). The shoulder peak at 1.1 assigned to Mg3OH is associated with a small but non-negligible TRAPDOR fraction,indicating the nearest Al ions around Mg3OH are not in the first but in the second/third coordination shell (Fig. 5). Combined with numerical simulations,TRAPDOR experiments with different Al irradiation time can be performed on deuterated LDH and test cation ordering models in superlattices. These results show that double resonance experiments [31] can be applied conveniently to investigate internuclear proximities,superstructure models on deuterated LDHs because of significantly less 1H-1H homonuclear dipolar coupling,and thus this approach can be extended to study the key supramolecular host - guest interactions in LDHs.
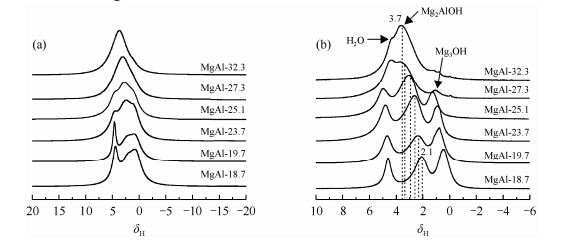
|
| Fig. 4 1H MAS NMR spectra of as prepared (a) and deuterated (b) LDH with different Al molar percentages.Spinning speed,20 kHz; recycle delay,2 s. (Reproduced from Ref. [29]) |
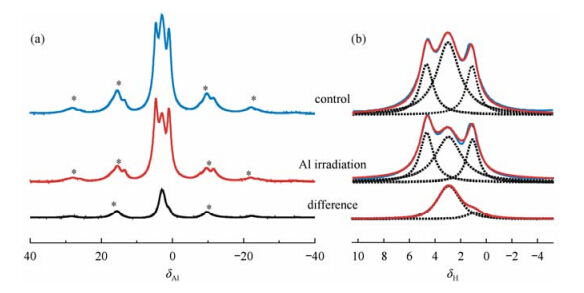
|
| Fig. 5 1H-27Al TRAPDOR NMR spectra (a) and fitting results (b) of deuterated MgAl-25.1 at a spinning rate of 5 kHz. Al irradiation time: 0.2 ms.(Reproduced from Ref. [29]) |
1H MAS NMR spectroscopy can also be used to study the interlayer anions,for example,distinguishing unambiguously the mobile and immobile pesticide[25] and differentiate aliphatic hydrocarbon chain protons[32]. 2 27Al NMR
Although 27Al is a spin-5/2 quadrupolar nucleus,due to a relatively high gyromagnetic ratio,a small quadrupole moment and 100 % in natural abundance,27Al NMR spectroscopy have been widely applied in materials science. It is often used to distinguish 6-and 4-coordinated Al ions and to track Al coordination changes on thermal treatment or redydration process of Al containing LDHs. Uncalcined Al containing LDHs exhibit a narrow,symmetric resonance,at about 9,which is attributed to Al 6-coordinated to OH groups[19, 28, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42]. The narrow,symmetric peak indicates that Al occupies highly ordered and symmetric octahedral sites. Slight variations of the frequency and linewidth are most likely due to the degree of distortion of the surrounding Mg atoms or changes in the nature and strength of hydrogen bonding between the Al(OH)6 octahedra and interlayer species. More disorder is seen for the lower Al content samples,along with a noticeable reduction in the intensities of the satellite transitions which is partially ascribed to mobility of the water within the layers[28].
However,Rocha et al. found that the uncalcined samples contains another type of Al sites at δ11 in addition to the peak at 9[43]. The high frequency peak displays a slightly larger distribution of isotropic chemical shifts,which can be ascribed to the presence of a range of slightly different local Al environments caused by the random insertion of small amounts of Al in the layers. This resonance is also associated with a larger quadrupole coupling constant,corresponding to more distorted local Al environments. The results were further confirmed by 27Al 3QMAS NMR by Benito[44] and Vyalikh[32, 45]. Benito and coworkers found that the 27Al MAS NMR spectra of MgAl-LDH and ZnAl-LDH intercalated with carboxylate also display a main peak at ca. 9.7 and a broad low-frequency shoulder,both of which can be assigned to octahedral Al species[44]. It is also found that the intensity of the broad peak monotonously decreases and essentially disappears after aging treatment for 180 min in microwave oven,indicating that microwave irradiation can restore the local symmetry of some Al environments and improve crystallinity of the samples. In combination of 27Al MAS NMR,27Al DQ-filtered NMR and DFT calculations,it was further proved that the broad peak in 27Al NMR are the results from defects in the cation ordering[27]. Signals due to more distorted environments are observed at ca. δ0,yielding a typical asymmetric line shape. The DQ-filtered spectrum [Fig. 6(c)] and the DFT calculations of 27Al NMR parameters [Fig. 6(e)-(g)] give additional evidences that the broad peak corresponds to the less-symmetric environment of Al(Mg)5(Al),Al(Mg)4(Al)2 and/or Al(Mg)3(Al)3 moieties.
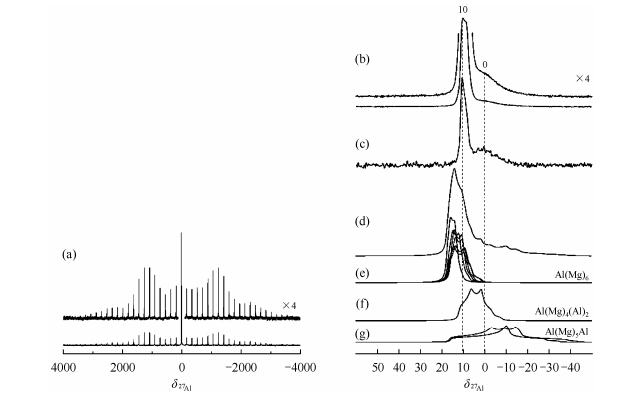
|
| Fig. 6 (a)-(c) 27Al NMR spectra of the LDH material (Al =33%),conducted at 7.0 T and a MAS frequency of 14 kHz. (a) Direct excitation 27Al MAS spectrum and (on top) magnification of the satellite transition region on both sides of the central transition. (b) Hahn echo 27Al NMR spectrum (and magnification on top),focusing on the central-transition region. (c) 27Al-27Al DQ-filtered spectrum revealing only pairs (or clusters) of Al atoms in close spatial proximity. (d) 27Al NMR central transition spectrum calculated for the disordered LDH model. (e) -(g) Details of the individual 27Al signals calculated for (e) Al(Mg)6 sites corresponding to ordered Al environments and for (f) Al(Mg)4(Al)2 and (g) Al(Mg)5(Al) environments generated by the permutation of one Al and one Mg site in the disordered LDH model. (Reproduced from Ref. [26]) |
Ishihara and coworkers studied the structures of paramagnetic NiAl-LDH by 27Al MAS NMR[46]. Unlike MgAl-LDH,27Al NMR spectra of NiAl-LDH are strongly shifted to negative chemical shift. The change of shifts are ascribed to the Fermi-contact shift (hyperfine shift) interaction which arises from the through-bond transfer of spin-density from the unpaired electrons of the Ni2+ ions (3d8,S = 1),via the intervening filled oxygen p orbitals to Al atoms.
After calcining LDHs at high temperature,the spectra show a broad resonance for Al in octahedral coordination (δ0-15) and a broader resonance in the range of δ 60-90 for Al in tetrahedral coordination (AlO4)[19, 33, 34, 35, 36, 37, 38, 39, 40, 41]. The AlO4 resonance exhibits a broad asymmetric lineshape characteristic of combinations of significant distributions of isotropic chemical shift and second-order quadrupolar broadening. Mackenzie and coworkers applied 27Al MAS NMR to study LDHs after being heated at different temperatures[42]. A small peak due to 4-coordinated Al at δ74 appears after the removal of the interlayer water at 200 ℃. The peak intensity continues to increase with increasing temperature. Eventually,the ratio of 4-coordinated Al : 6-coordinated Al is similar to that of g-Al2O3 (30-32%). The change of the Al coordination for LDHs calcined at different temperatures was further investigated by 27Al 1D MAS and 27Al 2D z-filtered 3QMAS solid-state NMR spectroscopy[47]. The 27Al 1D MAS show that the tetrahedral site gradually increases as the Al resonance at octahedral site became smaller and broader. According to the 27Al 2D z-filtered 3QMAS spectra,as the calcination temperature rises,the contour corresponding to the tetr ahedral Al ions becomes bigger,and the contour associated with the octahedral sites becomes broader. The 2D lineshapes were observed to smear near the octahedral site region which indicates the existence of amorphous Al2O3 phase,consistent with the 27Al 1D MAS NMR results. Hou et al. applied 27Al MAS NMR to study the thermal evolution of the LiAl2-Cl LDH[38]. For the LDH sample heated under 200 ℃,there is no new peak in the spectra,indicating that the hydroxide-layer structure is unchanged. After calcination at 300 ℃,a broad resonance at 71.5 due to AlO4 appears and an additional resonance due to AlO6 occurs as a shoulder at about 13 on the higher frequency side of the main peak at 7.8,implying the formation of an amorphous phase. At 850 ℃,the appearance of the peak at 17.3 can be attributed to a-LiAlO2 with distorted NaCl-like structure containing cross-linked sheets of alternating AlO6 and LiO6 octahedra. After calcination at 1 100 ℃,the resonance at δ 17 is no longer present and a small peak at δ79.3 and a very small shoulder at δ73 appeared which can be best simulated with the γ-LiAlO2 and LiAl5O8. It can be concluded that the change of overall AlO6/AlO4 ratio indicates the occurrence of Al redistribution during thermal treatment. Using a similar approach,the structural change of Pt-doped Ni/Mg(Al)O and Mg(Ga)O catalysts prepared from LDHs were monitored[39, 48].
One of the unusual features of LDH is the “memory effect” in which the mixed oxide derived from heating LDH can recover its original structure upon contact with water or aqueous solutions containing certain anions. This property makes it possible to intercalate specific anions for various applications[49, 50, 51, 52, 53]. Consequently,monitoring the structure change in the process is crucial for the rational design of better LDHs and 27Al NMR can provide key information[5, 32, 34, 41, 43, 45, 54]. For MgAl-LDH and ZnAl-LDH,the 27Al MAS NMR spectra of rehydrated LDH show a measurable decrease and a corresponding increase for 4- and 6- coordinated Al,respectively,compared to the parent mixed oxides,indicating that the tetrahedral Al ions are transformed back to the octahedral sites[34]. Detailed analysis of the 27Al NMR spectra by Rocha and coworkers also suggests that the process is not always entirely reversible[43]. All regenerated samples contain a small amount of 4-coordinated Al (around 5-14%). Rehydration of the LDHs calcined at 350,450 and 550 ℃generates samples which show a sharper and more symmetrical 6-coordinated peak in 27Al NMR,indicating less disordered Al local environment. The 27Al NMR spectrum of the rehydrated LDH obtained from the LDH heated above 650 ℃shows a peak for 4-coordinated Al and a broad peak for 6-coordinate Al. The 27Al MAS NMR spectrum of rehydrated sample derived from calcining LDH at 1 000 ℃displays mixed signals for MgAl-carbonate LDH,MgO (periclase) and MgAl2O4 (spinel). All these phenomena suggest that the reconstruction become more difficult after the LDHs are calcined at higher temperature. 27Al MAS NMR and 1H→27Al CP MAS NMR can also be used to explore regeneration mechanism of LDHs[41]. The spectrum of MgAl-CO3 LDH shows that,a small amount of tetrahedral aluminum is extracted from the LDH network after regeneration of the LDH using “memory effect” for three times. These 4-coordinated Al are concluded to locate at the surface of LDHs (as defects such as corners,edges,etc.),since the signal for 4-coordinated Al is more intense in the CP spectrum than in the one-pulse spectrum. 317O NMR
Oxygen is the other major component of all LDHs except H,thus 17O NMR can be a direct method to investigate the structure of all LDHs. The chemical shift of 17O is very sensitive to the structure change because of the large chemical shift range of more than δ 1 000. Since 17O is a spin-5/2 quadrupolar nucleus,the local environments of oxygen ions can also be monitored with quadrupolar interactions. Therefore 17O solid-state NMR spectroscopy should be an ideal method to study LDH and provide considerable information. However,no paper about the 17O NMR studies of LDH was published before 2013,due to the low natural abundance of 17O (0.037%) and the resulting difficulties in efficient and economical isotopic enrichment[55].
Recently,Zhao et al. used an efficient and general 17O enrichment method based on “memory effect” to obtain the 17O-enriched LDH[56]. The single pulse 17O MAS NMR spectra of four LDHs with different interlayer species and Al concentrations show (Fig. 7) that the major signal appears at around 10 to -180 which possesses a characteristic second order quadrupolar line shape with a non-zero asymmetry parameter. This peak can be assigned to hydroxyl group in the hydroxide layers (i.e.,Mg3OH and Mg2AlOH). The slim difference in the line shapes of different samples is due to relative amount of the Mg3OH and Mg2AlOH sites. The small peak at approximately 40 observed for all LDHs except the CO32--containing LDH can be assigned to OH- in the interlayer spaces. The signals in Mg3OH and Mg2AlOH environments can be resolved in the isotropic dimension in the 17O MQMAS NMR (Fig. 8). The F1 dimension (isotropic dimension) shows two resolved resonances with centers of gravity,δ1 of 53.0 and 64.8,respectively. The resonance at δ1 = 53.0 can be simulated with an asymmetry parameter h of 0,thus is assigned to oxygen atoms in Mg3OH environments,consistent with the fact that the oxygen ions at Mg3OH sites are in an axial environment with a C3 rotational axis. The other resonance at δ1 = 64.8 is assigned to Mg2AlOH sites,in agreement with the non-zero value for h (0.3).

|
| Fig. 7 17O MAS NMR spectra of 17OO enriched LDH with different Al concentrations and interlayer species at 9.4 T. “M” and “D” represent that the enrichment are via “memory effect” and “direct enrichment approach”,respectively; 22.5,26.1 and 30.4 stand for the Al mole percent in each sample; OH-Oand CO32-show the major interlayer species. Spinning speed: 20 kHz,recycle delays: 0.1 s. A total of 80 000 scans were used to collect each spectrum. (Reproduced from Ref. [54]) |
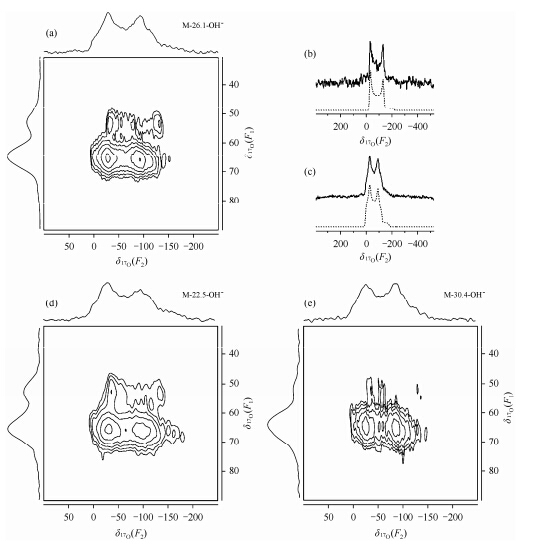
|
| Fig. 8 17 O 3Q MAS NMR spectra of M-26.5-OH- (a). M-22.5-OH- (d),and M-30.4-OH- (e) at 9.4 T under MAS frequency of 23 kHz. Projections of the anisotropic and isotropic dimensions are shown on the top and left side of the 2D spectrum,respectively. Cross sections (full lines) extracted parallel to anisotropic dimension of the 2D 3Q MAS spectrum for M-26.1-OH-at 53.0 (b) and 64.8 (c) in F1. (Reproduced from Ref. [54]) |
The isotropic dimension data of the 17O MQMAS spectra for a variety of LDH with different Al concentrations are shown in Fig. 9. Due to the fact that the values of the quadrupolar product PQ are very similar (a difference of <5%) for the Mg3OH and Mg2AlOH species,the intensities in isotropic dimension can be used to quantify the amounts of the two hydroxyl groups without applying any correction. In the three LDH with different Al-containings,the relative concentrations of Mg3OH and Mg2AlOH in the hydroxide layer which are generally in good agreement with the results obtained by Sideris et al. and Cadars et al. and with the predictions from the ordered structure in which no Al-O-Al linkage exist (Fig. 9). The results show that 17O solid-state NMR spectroscopy can be used to quantitatively analyze the cation distribution in LDH and again confirms the non-random distribution of cations in the layers. It is worth mentioning that the 17O MQMAS NMR data obtained at moderate spinning speed provide better resolution as compared to 1H ultrafast MAS NMR results for LDHs with OH- in the interlayer,suggesting the large advantage of using 17O NMR spectroscopy to separate the distinct local structures in investigations more complicated LDH materials.
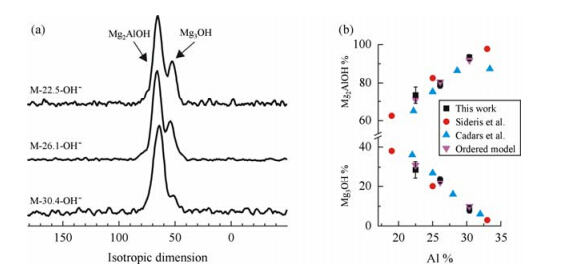
|
| Fig. 9 (a) Isotopic projections of 17O MQMAS NMR spectra of 17O M-LDH-OH- with different Al concentrations at 9.4 T. (b) The mole percentages of Mg3OH and Mg2AlOH in comparison with previous work by Sideris et al. and Cadars et al.,and predictions of ordered model (non-random Al distribution). (Reproduced from Ref. [54]) |
Sahoo used 17O as the structure probe to study the existence of the equilibrium involving carbonate anions and water in the interlayer of CO32--containing LDH[57]. The 17O MAS NMR spectra contain two resolved signals at around δ −80 and 100−200. The signal at around −80 is assigned to water molecules with reduced dynamics and symmetry,possibly because of the formation of a hydrogen bonded network between the interlayer water molecules and carbonate anions. The other NMR signal at around 100−200 can be ascribed to carbonate anions. The results reveal that there is an equilibrium involving interlayer carbonate anions and 17O-labeled interlayer water which leads to the transfer of 17O atom between water and carbonate anions. 425Mg NMR
As a spin-5/2 quadrupolar nucleus with around 10% natural abundance and low gyromagnetic ratio,25Mg is not very NMR friendly at first sight. However,the second-order quadrupole broadening of the central transition (1/2--1/2) of 25Mg is about 9 times greater than that for 27Al in a site with the same electric field gradient in the same applied magnetic field. Therefore,25Mg NMR spectroscopy is more sensitive to the structure and axially symmetry of the cation local environment.
25Mg NMR can be used to investigate the change of structure of LDH heated at various temperature[42]. Although the 25Mg MAS NMR spectra of unheated LDHs show a single Mg site,due to 6-coordinated Mg ions similar as in Mg(OH)2,the positons and line shapes are different because of the presence of Al,leading to larger EFG than brucite. For the heated sample,the change of the spectra is dramatic,thus it can be included that the NMR parameters of 25Mg (δ,CQ,h) is sensitive to the change of structure and axially symmetry of Mg local environment which is consistent with the change of the environment and coordination of Al in thermal treatment.
Sideriset al. combined 1H MAS and 25Mg 3QMAS NMR to elucidate that the Mg and Al cations are not randomly distributed and that they are globally ordered in a honeycomb arrangement for MgAl-LDH with 33 % Al[13]. By comparing the 25Mg MAS NMR spectra of brucite,MgAl-19 LDH,MgAl-25 LDH,and MgAl-33 LDH [Fig. 10(a)],it can be found that,although both the spectra of brucite and the MgAl-33-LDH contain only a single axially symmetric environment,the two local environments are completely different. For MgAl-19 LDH and MgAl-25 LDH,both the two components (as in brucite and MgAl-33-LDH) are present at the same time. 25Mg 3QMAS NMR spectra were used to separate the overlapping resonances of 25Mg nucleus. The 25Mg 3QMAS NMR spectrum clearly shows only one Mg local environment for MgAl-33 LDH [Fig. 10(b)]. By careful simulating the projection of the anisotropic dimension,the measured CQ (4.6 MHz) is noticeably different from that of brucite,proving that the 25Mg electric-field gradient is extremely sensitive to different local environments in LDHs. The asymmetry parameter (h= 0.00) indicate that the Mg ions are in an axial environment. All of the these prove that the Mg anions are located in an ordered C3 symmetry [Mg(OMg)3(OAl)3] environment,and which can form only one local hydroxyl environment (Mg2AlOH).
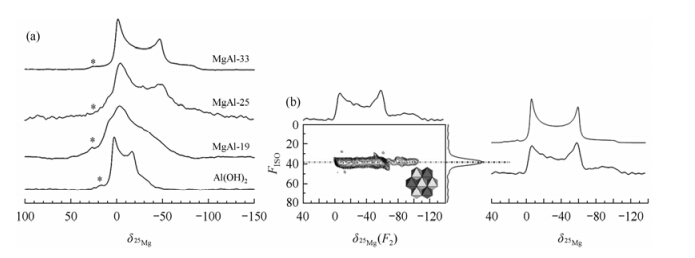
|
| Fig. 10 (a) The 25Mg MAS NMR spectra of Brucite [Mg(OH)2] and the three LDH collected at 21.1 T at Pacific Northwest National Laboratory. (Reproduced from Ref. [13]) |
Sideris and coworkers further investigated the different Mg local and long-range structure in NO3- and CO32--containing LDH with various Al contents[28]. For the MgAl-19-NO3-LDH sample,at least three different magnesium environments can be resolved in the 25Mg SPAM-3QMAS spectrum. The peak at 26.6 has a CQ (~3 MHz) very close to that of brucite and is assigned to the Mg(OMg)6 brucite-like environment. The site at 40.6 have a much larger quadrupole coupling constant (~4.5 MHz) which are similar to that of MgAl-33-NO3-LDH with configuration Mg(OAl)3(OMg)3. The third peak is associated with the largest CQ (~ 5 MHz) and an asymmetry parameter (h= 0.2),implying an increased distortion of the magnesium hydroxide octahedron. This Mg environment is assigned to the configuration Mg(OAl)2(OMg)4. The reason of the larger quadrupole coupling constant and the most asymmetric parameter for the third site is the nonaxial symmetrical cation arrangements [Mg(OAl)2(OMg)4] and possibly the interaction between Mg hydroxide octahedron and an impurity anion with higher charge density (e.g CO32-). Similarly,three sites were resolved in the 25Mg SPAM MQMAS spectrum of MgAl-25-NO3- LDH at similarlocation of F1 dimension (δ1= 28.4,42.5 and 46.0). The significant presence of both Mg(OMg)3(OAl)3 and Mg(OMg)6 sites indicates that the Mg(OMg)4(OAl)2 ordering schemes do not predominate in MgAl-25-NO3-. The amounts of the Mg(OMg)6 and Mg(OAl)3(OMg)3 resonances,predicted by the model of the honeycomb ordered lattice randomly replaced by Mg to obtain the desired samples (Mg-Al-19,Mg-Al-25,Mg-Al-33),are in good agreement with the experimentally observed intensities. 5 NMR of other nuclei in the hydroxide layer
It is also be practical to investigate LDHs containing other tri- and tetravalent cations (Ga,Sn) and univalent cation (Li) with solid-state NMR spectroscopy.
6,7Li NMR. Both the nuclei of Li used for NMR spectroscopy have advantages and disadvantages. 7Li has a higher natural abundance (92.5%),but its large quadrupole moment usually lead to relatively broad lines for Li ions in non-symmetrical sites. The Larmor frequency and natural abundance of 6Li are much lower,but its significantly lower quadrupole moment is associated with weaker quadrupolar interactions,giving narrower spectral lines. Hou et al. first reported the thermal decomposition and structural evolution of LiAl2(OH)6Cl.H2O along with increasing the temperature by 27Al,35Cl,and 6,7Li NMR[38]. The 6Li MAS NMR spectra of the samples have very high resolutions. The unheated samples and those heated below 200 ℃have similar 6Li spectra,with peak maxima between -0.04 and -0.06 which was attributed to the octahedral Li ions. The 300 ℃sample yields another narrow peak with a maximum at -1.10 which was assigned to the crystalline LiCl. The presence of this peak in samples calcined at all of the temperatures indicates that essentially all of the Li ions in the calcined samples retain the sixfold coordination. The 6Li NMR spectra also show that a-LiAlO2,LiAl5O8 and g-LiAlO2 appear gradually with an increase in the calcination temperature. Kozlova and coworkers studied the structure of LiAl2(OH)6Cl·xH2O by 7Li NMR[58]. It was found that the incorporation of crystalline water (x = 0.5-0.6) into the interlayer space do not cause any significant changes in the lineshape of the 7Li spectrum,except a slight change of the quadrupolar coupling constant and the broadening factor. These data prove that the local environment of the Li basically remains unchanged. Upon further hydration (x > 1),the 7Li NMR spectra are significantly narrowed. This can be attributed to the change in the 7Li electric field gradient because of the disordering of the chloride anions in the interlayer space.
71Ga NMR. Although both 69Ga and 71Ga are spin 3/2 quadrupolar nuclei with a reasonable NMR receptivity,the latter is generally preferred due to a relatively smaller quadrupole moment and higher gyromagnetic ratio. Similar to 27Al NMR,the isotropic chemical shift of Ga can be used to distinguish tetra-,penta- and octahedral Ga sites in the structure and the quadrupole coupling parameters are sensitive to the symmetry of the coordination site. Thus,71Ga NMR should be an effective method to explore the local environment in Ga containing LDH,e.g.,MgGa-LDH. However,few solid-state NMR studies of MgGa-LDHs have been reported to investigate their structure at molecular level. Aramendia[23, 34, 57]and coworkers compared the difference of the 71Ga MAS NMR spectra of as prepared and heated sample. Like Al3+,the non-calcined LDH exhibited a single typical signal at about 20 which revealed the presence of octahedral 71Ga,while the spectra of heated sample show another signal from 55 to 60 which can be ascribed to tetrahedrally coordinated Ga. The results that Ga containing LDH has a similar structure with Al containing LDH werefurther evidenced by Prihod[39]. Recently,Petersen and co-workers have applied 71Ga MAS and 3QMAS NMR spectra to prove the presence of a single 71Ga site in MgGa-LDH (Ga = 33%),and no sign of Ga-O-Ga connectivities[29].
119Sn NMR. Velu et al. reported the synthesis of a new Sn-incorported LDH as early as 1999[59]. A series of MgIIAlIIISnIV LDH were obtained by a simple coprecipitation method at room temperature. 119Sn NMR spectroscopy with/without proton decoupling can be used to provide valuable structure information for Sn LDH. The119Sn spectra of LDHs (Sn = 7 and 24%) without proton decoupling exhibit a broad signal with the peak maximum centered at around 600,indicating that Sn ions are present in an octahedral environment in both samples. The signal intensity reduces dramatically with proton decoupling in the LDH (24% Sn)demonstrates that the Sn nuclei have a strong magnetic interaction with neighboring H nuclei and MgSn(OH)6 is formed as a major phase. In contrast,there is only a small change in the intensity of the peak in LDH (7% Sn) with decoupling,implying that the broadening is due to the fact thatSn ions are in a distorted octahedral environment as a consequence of isomorphous substitution in the brucite-like lattice. 6 NMR of other nuclei in the interlayer regions
A variety of organic and inorganic anions have been intercalated into LDHs. Solid-state NMR spectroscopy can also be applied to study the anion dynamics and states in the interlayer regions.
11B NMR. The 11B NMR spectra of MgAl-borateLDH show that there are tetrahedral and trigonal boron species in the interlayer space. According to the relative intensities of two NMR signals,the presence of only tetraborate species in the interlayer space is concluded[37].In another study,Ay et al. acquired 11B MAS NMR and 2D 11B MQMAS spectra of borate-LDH samples treated at different pH values[60]. The ratio of triborate to tetraborate is varied between 1 and 2,corresponding to the intercalation of [B4O5(OH)4]2- or [B3O3(OH)4]- species or both.
13C NMR. Since different organic molecules can be intercalated to give LDH different functions,13C NMR can be used to detect the presence organic anions and/or their dynamics in the interlayer[25, 61, 62, 63, 64],as well as the interaction of the intercalated organic anions with the host hydroxide layers[44, 46, 65, 66]. In addition,static 13C NMR can be used to study the ordering of interlayer carbonate in MgAl-CO3 LDH. These data indicate that the molecular symmetry axes of carbonate (C3) are oriented parallel to the crystallographic c axis of hydrotalcite[20]. Ishihara[67]and Sahoo[57 ] investigaed the exchange situation between atmospheric CO2 and carbonate anion intercalated within LDH by 13C NMR spectroscopy and other characterization methods. According to their observations,MgAl-CO3 LDH can dynamically exchange on time scale of hours with atmospheric CO2 under atmospheric conditions,while the Mg/Al ratio of LDH has a significant influence on the carbonate anion exchange dynamics.
15N NMR. Hou et al. revealed that the nitrate ions in interlayer regions and adsorbed on the surface exhibit very different dynamic and structural behavior by using 15N NMR[68]. The former is associated with temperature independent uniaxial chemical shift anisotropy (CSA) powder pattern,while the dynamic behaviors of the latter depend substantially on temperature and relative humidity (RH).
29SiNMR.Depege[22] and del Arco[37] investigated the activity of silicate anions inside the interlamellar space. The change of 29Si chemical shift is mainly due to the occurrence of layered polymerization of SiO44- in which the silicon environment becomes either Si(OSi)3-y(OAl)y(OH) or Si(OSi)3-y(OAl)y. This leads to a more negative chemical shift as the aluminum content increases in the coordination polyhedral. Recently,29Si MAS NMR spectroscopy was also used to analyze whether or not the inorganic-organic hybrid layered compounds were successfully synthesized[61, 69, 70, 71, 72].
31PNMR.The 31P MAS NMR data can be used todifferentiate the phosphate speciation for LDHs obtained at different pH values[73]. The peak at about 3 indicates the presence of HPO42- which exists largely in the pH = 9 sample. In contrast,the peak at about 6 corresponds to PO43- which is mainly foundin the pH = 12 sample.
35Cl NMR. Although the 35Cl NMR spectrum for CaAl-Cl LDH shows poorly resolved signal,the data demonstrates the presence of dynamical order-disorder phase transitions at different temperatures and different RHs,as well as significant differences in the transition temperatures[74]. Careful analysis of 35Cl NMR data of LiAl2-Cl LDH shows that there are three types of Cl-,depending on the temperature and hydration level. One type of Cl-is located on the surface of LDH and is associated with a sharp solution-like peak at 0,while the other two types of Cl-are in the interlayer spaces,one with uniaxial CSA and the other with structureless NMR peak[75]. By using the same method,the structure and dynamical behavior of ClO4-intercalated into the interlayers of LiAl2-Cl LDH were studied. 35Cl NMR data show that ClO4-site is almost unchanged at low RHs and temperatures,but it undergoes isotropic reorientation at high RHs and in pastes[76].Hou et al. also studied the thermal decomposition and structural evolution of LiAl2-Cl LDH in the temperature range of 20-1 100 ℃by 35Cl NMR[38].It is found that 35Cl NMR spectra can provide important information about the Cl-sites present in the samples heated at different temperatures. For example,a temperature above 300 ℃ can lead to the exsolution of crystalline LiCl whose shift vary from 2.7 to 5.1 due to the change of quadrupolar effects associated with disorder or small particle size.
77Se NMR. 77Se NMR can be used to study the difference of swelling properties and dynamical behaviors of MgAl-SeO4and MgAl-SeO3LDH[77]. For MgAl-SeO4,the change of the 77Se CSA shows the structural transition with the increasing relative humidity (RH),and 77Se CSA is uniaxial due to the combined effects of hydrogen bonding to the hydroxyls of the layer and interlayer water molecules,and Coulombic interaction with the cations of the main layer.For MgAl-SeO3,the observations that uniaxial CSA powder pattern is independent on the RH indicates that SeO32- is rigidly held or perhaps rotates on its 3-fold axis,which is consistent with the observed lack of expansion at all hydration conditions. 7 Conclusions
Solid-state NMR spectroscopy has become a powerful tool to extract rich structure information of LDHs. With the development of improved NMR hardware,new NMR techniques,as well as new isotopic labeling methods,the solid-state NMR approach will provide more valuable information on the structure,dynamics and interactions of LDHs to help rational design and preparations of LDHs with desired properties.
| [1] | Martin K J, Pinnavaia T J. Layered double hydroxides as supported anionic reagents. Halide-ion reactivity in zinc chromium hexahydroxide halide hydrates [Zn2Cr(OH)6x·nH2O](x=Cl, i)[J]. J Am Chem Soc, 1986, 108(3):541-542. |
| [2] | Cavani F, Trifirò F, Vaccari A. Hydrotalcite-type anionic clays:Preparation, properties and applications[J]. Catal Today, 1991, 11(2):173-301. |
| [3] | De Roy A, Forano C, Besse J. Layered double hydroxides:Synthesis and post-synthesis modification[J]. Layered Double Hydroxides:Present and Future, 2001:1-39. |
| [4] | He S, Zhao Y F, Wei M, et al. Fabrication of hierarchical layered double hydroxide framework on aluminum foam as a structured adsorbent for water treatment[J]. Ind Eng Chem Res, 2012, 51(1):285-291. |
| [5] | Torres-Rodríguez D A, Lima E, Valente J S, et al. CO2 capture at low temperatures (30-80℃) and in the presence of water vapor over a thermally activated Mg-Al layered double hydroxide[J]. J Phys Chem A, 2011, 115(44):12 243-12 250. |
| [6] | Morandi S, Manzoli M, Prinetto F, et al. Supported Ni catalysts prepared by intercalation of layered double hydroxides:Investigation of acid-base properties and nature of Ni phases[J]. Micropor Mesopor Mat, 2012, 147(1):178-187. |
| [7] | Primo A, Marino T, Corma A, et al. Efficient visible-light photocatalytic water splitting by minute amounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method[J]. J Am Chem Soc, 2011, 133(18):6 930-6 933. |
| [8] | Zhang M C, Han D M, Lu C, et al. Organo-modified layered double hydroxides switch-on chemiluminescence[J]. J Phys Chem C, 2012, 116(10):6 371-6 375. |
| [9] | Shi W, Ji X, Zhang S, et al. Fluorescence chemosensory ultrathin films for Cd2+ based on the assembly of benzothiazole and layered double hydroxide[J]. J Phys Chem C, 2011, 115(42):20 433-20 441. |
| [10] | Shao M, Ning F, Zhao J, et al. Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins[J]. J Am Chem Soc, 2012, 134(2):1 071-1 077. |
| [11] | Faour A, Mousty C, Prevot V, et al. Correlation among structure, microstructure, and electrochemical properties of NiAl-CO3 layered double hydroxide thin films[J]. J Phys Chem C, 2012, 116(29):15 646-15 659. |
| [12] | Darder M, López-Blanco M, Aranda P, et al. Bio-nanocomposites based on layered double hydroxides[J]. Chem Mater, 2005, 17(8):1 969-1 977. |
| [13] | Sideris P J, Nielsen U G, Gan Z, et al. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy[J]. Science, 2008, 321(5 885):113-117. |
| [14] | MacKenzie K J, Smith M E. Multinuclear Solid-State Nuclear Magnetic Resonance of Inorganic Materials[M]. Elsevier, 2002. |
| [15] | Lesage A. Recent advances in solid-state NMR spectroscopy of spin I=1/2 nuclei[J]. Phys Chem Chem Phys, 2009, 11(32):6 876-6 891. |
| [16] | Ashbrook S E. Recent advances in solid-state NMR spectroscopy of quadrupolar nuclei[J]. Phys Chem Chem Phys, 2009, 11(32):6 892-6 905. |
| [17] | Sutrisno A, Huang Y N. Multinuclear solid-state NMR and quantum chemical investigations of layered transition metal disulfides [J]. Chinese J Magn Reson, 2013, (4):461-487. |
| [18] | Marcelin G, Stockhausen N, Post J, et al. Dynamics and ordering of intercalated water in layered metal hydroxides[J]. J Phys Chem, 1989, 93(11):4 646-4 650. |
| [19] | Rey F, Fornés V, Rojo J M. Thermal decomposition of hydrotalcites. An infrared and nuclear magnetic resonance spectroscopic study[J]. J Chem Soc Faraday T, 1992, 88(15):2 233-2 238. |
| [20] | Van der Pol A, Mojet B, Van de Ven E, et al. Ordering of intercalated water and carbonate anions in hydrotalcite. An NMR study[J]. J Phys Chem, 1994, 98(15):4 050-4 054. |
| [21] | Reinholdt M X, Babu P K, Kirkpatrick R J. Proton dynamics in layered double hydroxides:A 1H T1 relaxation and line width investigation[J]. J Phys Chem C, 2009, 113(24):10 623-10 631. |
| [22] | Depège C, El Metoui F Z, Forano C, et al. Polymerization of silicates in layered double hydroxides[J]. Chem Mater, 1996, 8(4):952-960. |
| [23] | Aramendia M A, Borau V, Jimenez C, et al. Synthesis, characterization, and 1H and 71Ca MAS NMR spectroscopy of a novel Mg/Ga double layered hydroxide[J]. J Solid State Chem, 1997, 131(1):78-83. |
| [24] | Aramendía M a A, Borau V, Jiménez C, et al. Xrd and 1H MAS NMR spectroscopic study of mixed oxides obtained by calcination of layered-double hydroxides[J]. Mater Lett, 2000, 46(6):309-314. |
| [25] | Combourieu B, Inacio J, Delort A M, et al. Differentiation of mobile and immobile pesticides on anionic clays by 1H HR MAS NMR spectroscopy[J]. Chem Commun, 2001, (21):2 214-2 215. |
| [26] | Yesinowski J P, Eckert H. Hydrogen environments in calcium phosphates:Proton MAS NMR at high spinning speeds[J]. J Am Chem Soc, 1987, 109(21):6 274-6 282. |
| [27] | Cadars S, Layrac G, Gerardin C, et al. Identification and quantification of defects in the cation ordering in Mg/Al layered double hydroxides[J]. Chem Mater, 2011, 23(11):2 821-2 831. |
| [28] | Sideris P J, Blanc F, Gan Z, et al. Identification of cation clustering in Mg-Al layered double hydroxides using multinuclear solid state nuclear magnetic resonance spectroscopy[J]. Chem Mater, 2012, 24:2 449-2 461. |
| [29] | Petersen L B, Lipton A S, Zorin V, et al. Local environment and composition of magnesium gallium layered double hydroxides determined from solid-state 1H and 71Ga NMR spectroscopy[J]. J Solid State Chem, 2014, 219:242-246. |
| [30] | Yu G, Shen M, Wang M, et al. Probing local structure of layered double hydroxides with1h solid-state NMR spectroscopy on deuterated samples[J]. J Phys Chem Lett, 2014, 5(2):363-369. |
| [31] | He Y Y, Wang X M, Li F, et al. Determination of 1H-27Al dipolar coupling constant by 1H/27Al S-RESIDOR experiment under fast MAS[J]. Chinese J Magn Reson, 2013, 30(1):93-102. |
| [32] | Vyalikh A, Costa F R, Wagenknecht U, et al. From layered double hydroxides to layered double hydroxide-based nanocomposites- a solid-state NMR study[J]. J Phys Chem C, 2009, 113(51):21 308-21 313. |
| [33] | Reichle W, Kang S, Everhardt D. The nature of the thermal decomposition of a catalytically active anionic clay mineral[J]. J Catal, 1986, 101(2):352-359. |
| [34] | Beres A, Palinko I, Bertrand J C, et al. Dehydration-rehydration behaviour of layered double hydroxides:A study by X-ray diffractometry and MAS NMR spectroscopy[J]. J Mol Struct, 1997, 410:13-16. |
| [35] | Weir M R, Kydd R A. Synthesis of heteropolyoxometalate-pillared Mg/Al, Mg/Ga, and Zn/Al layered double hydroxides via ldh-hydroxide precursors[J]. Inorg Chem, 1998, 37(21):5 619-5 624. |
| [36] | Aramendía M A, Avilés Y, Borau V, et al. Thermal decomposition of Mg/Al and Mg/Ga layered-double hydroxides:A spectroscopic study[J]. J Mater Chem, 1999, 9(7):1 603-1 607. |
| [37] | del Arco M, Gutierrez S, Martin C, et al. Effect of the Mg:Al ratio on borate (or silicate)/nitrate exchange in hydrotalcite[J]. J Solid State Chem, 2000, 151(2):272-280. |
| [38] | Hou X, Kirkpatrick R J. Thermal evolution of the Cl-LiAl2 layered double hydroxide:A multinuclear MAS NMR and xrd perspective[J]. Inorg Chem, 2001, 40(25):6 397-6 404. |
| [39] | Prihod'ko R, Sychev M, Kolomitsyn I, et al. Layered double hydroxides as catalysts for aromatic nitrile hydrolysis[J]. Micropor Mesopor Mat, 2002, 56(3):241-255. |
| [40] | Hsueh H B, Chen C Y. Preparation and properties of LDHs/epoxy nanocomposites[J]. Polymer, 2003, 44(18):5 275-5 283. |
| [41] | Martínez-Ortiz M d J s, Lima E, Lara V, et al. Structural and textural evolution during folding of layers of layered double hydroxides[J]. Langmuir, 2008, 24(16):8 904-8 911. |
| [42] | Mackenzie K J D, Meinhold R H, Sherriff B L, et al. 27Al and 25Mg solid-state magic-angle-spinning nuclear-magnetic- resonance study of hydrotalcite and its thermal-decomposition sequence[J]. J Mater Chem, 1993, 3(12):1 263-1 269. |
| [43] | Rocha J, del Arco M, Rives V, et al. Reconstruction of layered double hydroxides from calcined precursors:A powder xrd and 27Al MAS NMR study[J]. J Mater Chem, 1999, 9(10):2 499-2 503. |
| [44] | Benito P, Labajos F M, Mafra L, et al. Carboxylate-intercalated layered double hydroxides aged under microwave-hydrothermal treatment[J]. J Solid State Chem, 2009, 182(1):18-26. |
| [45] | Vyalikh A, Massiot D, Scheler U. Structural characterisation of aluminium layered double hydroxides by 27Al solid-state NMR[J]. Solid State Nucl Magn Reson, 2009, 36(1):19-23. |
| [46] | Ishihara S, Deguchi K, Sato H, et al. Multinuclear solid-state NMR spectroscopy of a paramagnetic layered double hydroxide[J]. RSC Advances, 2013, 3(43):19 857-19 860. |
| [47] | Park T J, Choi S S, Kim Y. 27Al solid-state NMR structural studies of hydrotalcite compounds calcined at different temperatures[J]. Bull Korean Chem Soc, 2009, 30(1):149. |
| [48] | Zhan Y, Li D, Nishida K, et al. Preparation of ""intelligent"" pt/Ni/Mg (Al)O catalysts starting from commercial Mg-Al LDHs for daily start-up and shut-down steam reforming of methane[J]. Appl Clay Sci, 2009, 45(3):147-154. |
| [49] | Ueno S, Yoshida K, Ebitani K, et al. Hydrotalcite catalysis:Heterogeneous epoxidation of olefins using hydrogen peroxide in the presence of nitriles[J]. Chem Commun, 1998, (3):295-296. |
| [50] | Corma A, Navarro M, Pariente J P. Synthesis of an ultralarge pore titanium silicate isomorphous to MCM-41 and its application as a catalyst for selective oxidation of hydrocarbons[J]. J Chem Soc Chem Commun, 1994, (2):147-148. |
| [51] | Di Cosimo J, Apestegui&a C R, Gines M J L, et al. Structural requirements and reaction pathways in condensation reactions of alcohols on Mgy AlOx catalysts[J]. J Catal, 2000, 190(2):261-275. |
| [52] | Rao K K, Gravelle M, Valente J S, et al. Activation of Mg-Al hydrotalcite catalysts for aldol condensation reactions[J]. J Catal, 1998, 173(1):115-121. |
| [53] | Abelló S, Medina F, Tichit D, et al. Aldol condensations over reconstructed Mg-Al hydrotalcites:Structure-activity relationships related to the rehydration method[J]. Chem Eur J, 2005, 11(2):728-739. |
| [54] | Pfeiffer H, Lima E, Lara V, et al. Thermokinetic study of the rehydration process of a calcined MgAl-layered double hydroxide[J]. Langmuir, 2010, 26(6):4 074-4 079. |
| [55] | Griffin J M, Clark L, Seymour V R, et al. Ionothermal 17O enrichment of oxides using microlitre quantities of labelled water[J]. Chemical Science, 2012, 3(7):2 293-2 300. |
| [56] | Zhao L, Qi Z, Blanc F, et al. Investigating local structure in layered double hydroxides with 17O NMR spectroscopy[J]. Adv Funct Mater, 2014, 24(12):1 696-1 702. |
| [57] | Sahoo P, Ishihara S, Yamada K, et al. Rapid exchange between atmospheric CO2 and carbonate anion intercalated within magnesium rich layered double hydroxide[J]. ACS Appl Mater Interfaces, 2014, 6(20):18 352-18 359. |
| [58] | Kozlova S G, Gabuda S P, Isupov V P, et al. Using NMR in structural studies of aluminum hydroxide intercalation compounds with lithium salts[J]. J Struct Chem, 2003, 44(2):198-205. |
| [59] | Velu S, Suzuki K, Okazaki M, et al. Synthesis of new Sn-incorporated layered double hydroxides and their thermal evolution to mixed oxides[J]. Chem Mater, 1999, 11(8):2 163-2 172. |
| [60] | Ay A N, Zümreoglu-Karan B, Temel A, et al. Layered double hydroxides with interlayer borate anions:A critical evaluation of synthesis methodology and ph-independent orientations in nano-galleries[J]. Appl Clay Sci, 2011, 51(3):308-316. |
| [61] | Fujii K, Hayashi S, Kodama H. Synthesis of an alkylammonium/magnesium phyllosilicate hybrid nanocomposite consisting of a smectite-like layer and organosiloxane layers[J]. Chem Mater, 2003, 15(5):1 189-1 197. |
| [62] | Wang G A, Wang C C, Chen C Y. The disorderly exfoliated LDHs/PMMA nanocomposite synthesized by in situ bulk polymerization[J]. Polymer, 2005, 46(14):5 065-5 074. |
| [63] | Reinholdt M X, Babu P K, Kirkpatrick R J. Preferential adsorption of lower-charge glutamate ions on layered double hydroxides:An NMR investigation[J]. J Phys Chem C, 2009, 113(9):3 378-3 381. |
| [64] | Pisson J, Morel-Desrosiers N, Morel J P, et al. Tracking the structural dynamics of hybrid layered double hydroxides[J]. Chem Mater, 2011, 23(6):1 482-1 490. |
| [65] | Sasaki S, Aisawa S, Hirahara H, et al. Synthesis of p-sulfonated calix [4] arene-intercalated layered double hydroxides and their adsorption properties for organic molecules[J]. J Eur Ceram Soc, 2006, 26(4):655-659. |
| [66] | Li Q, Kirkpatrick R J. Organic anions in layered double hydroxides:An experimental investigation of citrate hydrotalcite[J]. Am Mineral, 2007, 92(2-3):397-402. |
| [67] | Ishihara S, Sahoo P, Deguchi K, et al. Dynamic breathing of CO2 by hydrotalcite[J]. J Am Chem Soc, 2013, 135(48):18 040-18 043. |
| [68] | Hou X Q, Kirkpatrick R J, Yu P, et al. 15N NMR study of nitrate ion structure and dynamics in hydrotalcite-like compounds[J]. Am Mineral, 2000, 85(1):173-180. |
| [69] | Park A Y, Kwon H, Woo A J, et al. Layered double hydroxide surface modified with (3-aminopropyl) triethoxysilane by covalent bonding[J]. Adv Mater, 2005, 17(1):106-109. |
| [70] | Oh J M, Choi S J, Lee G E, et al. Inorganic drug delivery nanovehicle conjugated with cancer cell specific ligand[J]. Adv Funct Mater, 2009, 19(10):1 617-1 624. |
| [71] | Tao Q, Zhu J X, Frost R L, et al. Silylation of layered double hydroxides via a calcination-rehydration route[J]. Langmuir, 2010, 26(4):2 769-2 773. |
| [72] | Dong L, Ge C, Qin P, et al. Immobilization and catalytic properties of candida lipolytic lipase on surface of organic intercalated and modified MgAl-LDHs[J]. Solid State Sci, 2014, 31:8-15. |
| [73] | Hou X Q, Bish D L, Wang S L, et al. Hydration, expansion, structure, and dynamics of layered double hydroxides[J]. Am Mineral, 2003, 88(1):167-179. |
| [74] | Kirkpatrick R J, Yu P, Hou X Q, et al. Interlayer structure, anion dynamics, and phase transitions in mixed-metal layered hydroxides:Variable temperature 35Cl NMR spectroscopy of hydrotalcite and Ca-aluminate hydrate (hydrocalumite)[J]. Am Mineral, 1999, 84(7-8):1 186-1 190. |
| [75] | Hou, Kalinichev A G, Kirkpatrick R J. Interlayer structure and dynamics of Cl-LiAl2-layered double hydroxide: 35Cl NMR observations and molecular dynamics modeling[J]. Chem Mater, 2002, 14(5):2 078-2 085. |
| [76] | Hou X, Kirkpatrick R J. Interlayer structure and dynamics of ClO4- layered double hydroxides[J]. Chem Mater, 2002, 14(3):1 195-1 200. |
| [77] | Hou X Q, Kirkpatrick R J. Solid-state 77Se NMR and xrd study of the structure and dynamics of seleno-oxyanions in hydrotalcite-like compounds[J]. Chem Mater, 2000, 12(7):1 890-1 897. |
 2015, Vol. 32
2015, Vol. 32




