文章信息
- 赵保路
- ZHAO Bao-lu
- 利用电子自旋共振(ESR)技术研究一氧化氮自由基在心脑血管疾病和健康中的"双刃剑"作用
- "Double Edge" Effects of Nitric Oxide Free Radical in Cardio-Brain-Vascular Diseases and Health Studied by ESR
- 波谱学杂志, 2015, 32(2): 195-207
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 195-207
- http://dx.doi.org/10.11938/cjmr20150205
-
文章历史
- 收稿日期:2015-04-22
- 收修改稿日期:2015-05-12
The incidence and mortality of cardio-brain-vascular disease are the highest among all disease in China and all over the world, and are still increasing with the ever rising living standards. To find effective treatment and prevention of the cardio-brain-vascular disease, it is important to thoroughly understand the pathobiology of the disease. Increasing evidences have shown that reactive oxygen species (ROS) and reactive nitrogen species (RNS) play important roles in the development of cardio-brain-vascular health and disease[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. Generation of ROS and RNS are normally tightly regulated. At low and moderate concentrations, ROS/RNS mediate signal transduction cascades involved in a variety of cellular and physiological functions, and are important for defense against infectious agents. In contrast, overproduction of ROS/RNS results in oxidative stress, a deleterious process that causes damages to cell structures including lipids, proteins, and DNA, which can eventually lead to cellular senescence and apoptosis[13, 14]. Oxidative stress has been detected during myocardial ischemia-reperfusion while antioxidants protect heart from ischemia-reperfusion injury. Therefore, the potential of using antioxidants, especially natural antioxidants, in prevention and treatment of heart disease has attracted enormous interest[15, 16, 17, 18, 19, 20]. In this paper we review the progress made in understanding the “double edge” effects of nitric oxide in myocardial and brain ischemia-reperfusion and the cardio-brain-vascular protective effects of natural antioxidants against ischemia-reperfusion injury studied by ESR. 1 “Double edge” effects of NO free radicals
At low and moderate concentrations, NO as a major signaling molecule mediate signal transduction cascades involved in a variety of cellular and physiological functions, and are important for defense against infectious agents. In contrast, overproduction NO or superoxide free radical will react to form ONOO- resulting in oxidative stress, a deleterious process that causes damages to cell structures including lipids, proteins, and DNA, which can eventually lead to cellular senescence and apoptosis, especially for ischemia-reperfused injury in myocardium and brain[13, 14] (Fig. 1).
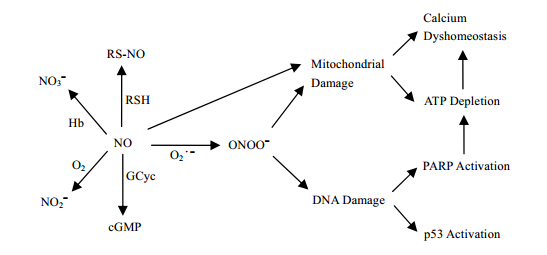
|
| Fig. 1 “Double edge” effects of nitric oxide |
Electron paramagnetic resonance (EPR) spectroscopy can be applied to measure free radical directly. To determine the free radical generation during ischemia-reperfusion, we used two different spin trip methods, hemoglobin (Hb) subunits and NOFe2+(DETC)2 complex.
The binding of NO to hemoglobin (Hb) subunits specifically at low temperature allows the measurement of NO using EPR spectroscopy. In the EPR spectrum, NO bound to a-subunit of Hb exhibits peaks at g = 2.078 and g = 2.01 with a splitting of 17.5×10-4 T, while the signal with a peak at g = 2.04 and a valley at g = 2.015 is the characteristic of NO bound to β-subunit of Hb[21, 22] [Fig. 2(a)].
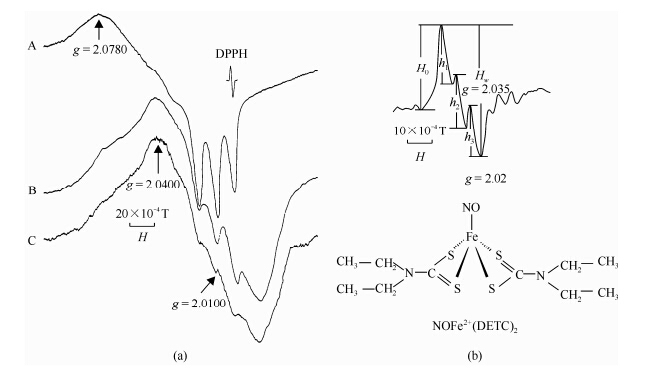
|
| Fig. 2 (a) ESR spectra of NO binding to hemoglobin (Hb) subunits; (b) ESR spectra of NOFe2+(DETC)2. ESR conditions: X-band, 100 kHz modulation with amplitude 3.2×10-4 T, microwave power 10 mW, time constant 0.128 s, central magnetic field 0.325 T, sweep width 0.05 T. Hw: = h1+h2+h3 |
NO has a high affinity to ferrous-containing protein. It can form a stable paramagnetic mononitrosyl iron complex-[NOFe2+(DETC)2] with diethyldithiocarbamate (DETC) and iron. This complex shows characteristic peaks at g = 2.035 and g = 2.020 with triplet hyperfine structure in EPR spectrum , therefore Fe2+(DETC)2 has been used as NO trapping reagents to detect NO specifically in tissues[23, 24]. NOFe2+(DETC)2 complex was obtained with NaNO (30 mmol/L) as a NO source. The reaction mixture containing 33 mg/mL PSA, 3.3 mmol/L Fe2+, 3.3 mmol/L DETC, and excess Na2S2O4 (2 mol/L). The complex Fe2+(DETC)2 was used as a spin trap for NO. The ESR spectrum of NOFe2+(DETC)2 in Fig. 2 is a typical three hyperfine splittings (h1, h2 and h3). The whole height of the triplet signal was found as Hw = h1+h2+h3, the intensity of which is proportional to NO concentration (correlation rate γ = 0.993 1). And the line shapes of NOFe2+(DETC)2 ESR signal do not change with different samples, therefore, the Hw was used to measure the NO concentration in the system [Fig. 2(b)]. 1.2 “Double edge” effects of NO free radicals generated during myocardium ischemia- reperfusion
We found that the EPR spectrum of normal myocardium showed existence of semiquinone free radical and transition metal cation in the rat myocardium. Two additional peaks at g = 2.04 and g = 2.03 were detected from the ischemia-reperfused myocardium in vitro: the peak at g = 2.04 was similar to that of the NO bound to β-subunit of Hb and the peak at g = 2.03 was tentatively attributed to oxygen and alkyl peroxide radicals. The signal at g = 2.04 could be decreased by the addition of NG-nitro-L-arginine methyl ester (NAME, the inhibitor of NO synthase) in the reperfusion solution, while increased by L-arginine (substrate of NOS), indicating that this might be associated with NO generation. Both the signals increased by the addition of Fe/H2O2 or xanthine/xanthine oxidase to the reperfusion solution, indicating that they were both dependent on ROS production[12]. It was found that the NO signal increased with L-arginine, at same time the ischemia-reperfusion heart was protected in lower concentration (10 mmol/L) but damaged in higher concentration (100 mmol/L).
Using Fe2+(DETC)2, we studied the NO free radical generation in rat heart during ischemia-reperfusion in vivo. It was shown that in normal heart, there is a baseline of the EPR signals with characteristic of NOFe2+(DETC)2 complex, perhaps representing the physiological level of NO. The EPR signal intensity of NOFe2+(DETC)2 complex in rat myocardium increased remarkably after 30 min of ischemia. L-arginine increased while NG-nitro-L-arginine (NNA, inhibitor of NOS) decreased the signal intensity, indicating that the signal was originated from NO. After 10 min of reperfusion followed 30 min of ischemia, the signal intensity of NO Fe2+(DETC)2 in rat hearts was significantly reduced as compared with that in ischemia only myocardium. It appears that the NO production increases during ischemia and decreases during reperfusion. Interestingly, the signal intensity of NOFe2+(DETC)2 complex increased significantly by SOD treatment, suggesting that superoxide anions might contribute to the decrease of NO level in the ischemia-reperfusion myocardium, because SOD scavenged the superoxide free radicals and saved NO[18, 19] (Fig. 3).
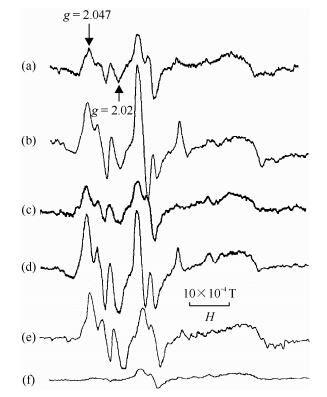
|
| Fig. 3 (a) ESR spectra of NO binding to hemoglobin (Hb) subunits; (b) ESR spectra of NOFe2+(DETC)2. ESR conditions: X-band, 100 kHz modulation with amplitude 3.2×10-4 T, microwave power 10 mW, time constant 0.128 s, central magnetic field 0.325 T, sweep width 0.05 T. Hw: = h1+h2+h3 |
Abundant evidences have shown that antioxidants, especially the natural antioxidants, have great potential in the prevention and treatment of heart diseases. Here we will review the studies on several natural antioxidants including tanshinone from salvia miltiorriza, EGb 761 from Ginkgo biloba and chinonin from traditional Chinese medicine rhizome anemarhenea in modulating and preventing the heart against ischemiarepperfusion injury. 2.1 Effects of EGb 761 on NO and ROS, myocardial damage in ischemia-reperfusion injury in vitro and in vivo
Ginkgo biloba leaf has been used in traditional Chinese medicine for thousands of years. Currently, Ginkgo biloba extract is widely used in treating cardiovascular diseases and cerebral vascular diseases in many countries. Its main ingredients are ginkgo-flavone glycosides and terpenoids. Because the chemical ingredients of Ginkgo biloba extract are complex and the extraction methods are different with different investigators, it has been hard to evaluate the biological effects of Ginkgo biloba extract. EGb 761 is a standardized extract of Ginkgo biloba consisting of 24% ginkgo-flavone glycosides (kaempferol, quercetin and isorhamnetin) and 6% terpenoid including ginkgolide A, ginkgolide B, ginkgolide C, ginkgolide M, ginkgolide J, and bilobalide B. Many studies have shown that EGb 761 protect rat hearts from ischemia-reperfusion-induced arrhythmias and functional damages, and the cardioprotective effects of EGb 761 are at least partly attributed to its antioxidant properties[16, 25, 26, 27, 28]. It was shown that EGb 761 could effectively scavenge the superoxide free radicals generated from xanthin/xanthine oxidase and hydroxyl free radicals generated from Fenton’s reaction[29, 30]. In isolated hearts subjected to ischemia and reperfusion in vitro, EGb 761 decreased the productions of both NO and ROD radicals, which is similar to SOD/catalase and L-NAME[16] in vitro. Different from in vitro, the effects of EGb 761 on NO production varied at different doses. While administration of EGb 761 at lower than 100 mg/kg increased the signal intensity of NO displayed on ESR spectrum, the administrati on of EGb 761 at 200 mg/kg the signal intensity of NO did not linearly increase (Table 1). The decrease in NO production was shown to be a result from the inhibition of iNOS expression by EGb 761 or scavenging NO at higher concentration. By lowering the ROS/RNS production, EGb 761 preserved the alpha-tocopherol storage in myocardium and reduced the prostaglandin biosynthesis during ischemia-reperfusion[31] in vitro.
| Group | n | (NO)Fe2+ (DETC)2 | TBARS | CK | TAR/% |
| N | 9 | 28.50±1.25 | 7.38±0.38 | 423±33 | 0 |
| I | 10 | 62.75±5.65a | 7.92±0.24 | 2307±122a | 10 |
| IR | 16 | 29.31±1.74 | 10.23±0.62a | 3524±254a | 43.75 |
| IR+EGb 761(25) | 6 | 40.33±3.46 | 9.95±0.31 | 3227±195 | 16.67 |
| IR+EGb 761(50) | 6 | 62.17±3.36 | 8.82±0.68 | 2342±164 | 16.67 |
| IR+EGb 761(100) | 6 | 85.17±5.96 | 8.46±0.33 | 1691±173 | 0 |
| IR+EGb 761(200) | 6 | 39.50±4.01 | 7.50±0.17 | 1720±245 | 0 |
EGb 761 also significantly reduced the leakages of LDH and CK induced by ischemia-reperfusion, protected mitochondrial structure from ischemia-reperfusion induced damage, scavenged oxygen free radicals and inhibition of lipid peroxidation[16, 28]. Consistently, the in vitro studies showed that EGb 761 inhibits lipid peroxidation as well as CK release and mitigated the incidence of ventricular arrhythmias in a dose dependent way. 2.2 Promotion effects of chinonin on NO and protective effects on cardiomyocyte in ischemia reperfusion heart in vitro and in vivo
Chinonin is a flavonoid component isolated from Chinese herb rhizoma anemarhenea, which has been used for several thousand years for treatment of heart diseases. We studied the scavenging effects of chinonin on NO and oxygen free radicals generated from ischemia-reperfusion heart in vitro[12, 33]. It was found that the ESR signals of oxygen and NO free radicals decreased simultaneously after the addition of chinonin (100 mmol/L), which is similar to the effect of SOD/catalase and L-NAME, indicating that chinonin could scavenge ROS and NO free radical generated from the ischemia-reperfusion heart in vitro. In addition, the levels of LDH and CK in the reperfusion solution decreased significantly by chinonin treatment. Chinonin was also found to protect the structure of mitochondria from the ischemia-reperfusion induced damage. These results suggested that chinonin has protective effect on myocardial ischemia-reperfusion injury and its cardioprotective effects may be attributed to its antioxidant properties.
In vivo study showed that the effects of chinonin on signal intensity of NO displayed on ESR spectrum linearly increased after administration of chinonin until 50 mg/kg. Also, chinonin affected the changes of TBARS, the release of CK, and mitigated the incidence of ventricular arrhythmias in a dose dependent way (Fig. 4, Table 2). These results indicate that chinonin has cardiovascular protective effects by means of adjusting the level of NO and inhibiting oxygen free radicals induced lipid peroxidation in myocardial ischemia-reperfusion injury in vivo[12, 33].
| Group | n | (NO)Fe2+ (DETC)2 | TBARS | CK | TAR/% |
| N | 9 | 28.50±1.25 | 7.38±0.38 | 423±33 | |
| IR | 16 | 29.31±1.74 | 10.23±0.62a | 3524±254ª | 43.75 |
| IR+ chinonin (5) | 6 | 35.34±3.55 | 7.52±0.31 | 1762±234 | 16.70 |
| IR+ chinonin (10) | 6 | 48.34±3.55 | 7.03±0.68 | 1492±232 | 0 |
| IR+ chinonin (25) | 6 | 55.55±2.76 | 7.02±0.68 | 1074±212 | 0 |
| IR+ chinonin (50) | 6 | 58.65±5.54 | 6.73±0.33 | 765±210 | 0 |
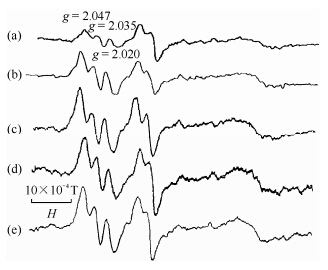
|
| Fig. 4 Effect of chinonin on ESR spectra of NO trapped by NOFe2+DETC complex in brain homogenates 1 h after 5 min ischemic damage. Spectra are from (a) sham group; (b) IR group; (c) low-; and (d) high-dose group, respectively |
In order to further elucidate the cardioprotective effects of chinonin, Shen et al. investigated the preventive effects of chinonin on the apoptotic and necrotic cell death of cardiomyocytes during hypoxia and reoxygenation process. Neonatal rat cardiomyocytes were subjected to 24 h hypoxia and 4 h reoxygenation and it was found that chinonin significantly decreased the apoptosis and necrosis induced by hypoxia and reoxygenation in cardiomyocytes. The decreased apoptosis might be caused by a down-regulation of p53 protein expression and an up-regulation of bcl-2 protein expression under the chinonin treatment. Meanwhile, chinonin treatment decreased the levels of NO-2/NO-3 and TBARS and inhibited the leakage of LDH in hypoxia-reoxygenated cardiomyocytes. These results suggest that chinonin may prevent apoptotic and necrotic cell death of cardiomyocytes during the hypoxia and reoxygenation process via reducing the production of NO and ROS and modulating the expression of bcl-2 and p53 proteins[18, 19, 20]. 2.3 Promotion effects of Crataegus flavonoids on NO and protective effects on neurons in ischemia reperfusion brain in vitro and in vivo
Crataegus (hawthorn) is one of the oldest medicinal plants and is described by many pharmacopoeias. Crataegus extract (from leaves with flowers) has been used to treat the early stages of congestive heart failure and angina pectoris. Pharmacological studies also demonstrate that Crataegus extract (from fruit and flowers) can decrease the level of cholesterol in serum and inhibit platelet conglomeration. Evidences show that Crataegus extract (from several parts of the plant including leaves) has antioxidant effects in vitro or in vivo. Crataegus flavonoids (CF) used in this study is a standard product, extracted from the leaves of the Crataegus Pinnalifida Bge (hawthorn), contained 62.35 ± 3.79% of the total flavonoids, and 27.2 ± 2.93% of proanthocyanidine as measured by HPLC.
Stroke is the third leading cause of death as dementia is a main symptom of Alzheimer’s disease. One of the important mechanisms in the pathogeny of stroke is free radical production during the reperfusion period, therefore the effects of a type of natural antioxidant, i.e. CF, on brain ischemic insults were investigated in Mongolian gerbil stroke model.
The animals were randomly divided into four groups: the IR group suffered 5 min ischemia after treatment with proper drinking water for 15 days; the sham group suffered the same treatment as the IR group except without arterial occlusion. The low- and high-dose group suffered the same treatment as IR group except that drinking water contained 0.5 mg/mL and 2.5 mg/ mL CF, respectively.
Using ESR signal intensities of 1 h following 5 min ischemia, we can deduce that ROS trapped by PBN in the brain homogenate significantly increased by about 36.89% in the IR group comparing with the sham group (Fig. 4, Table 3).
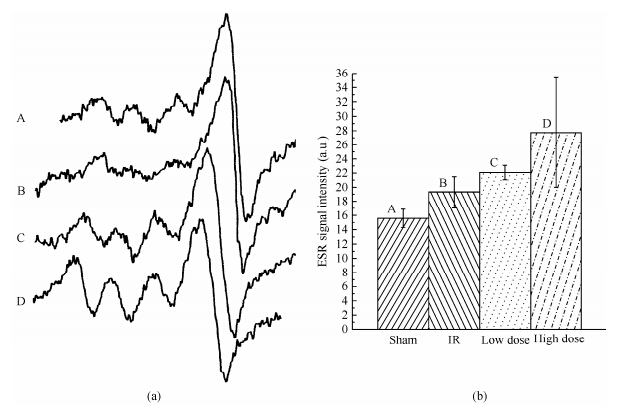
|
| Fig. 5 (a) Effects of CF on ESR spectra of NO trapped by Fe2+DETC complex in brain homogenates at 1 h after 5 min ischemic damage. Spectra are from A. sham group; B. IR group; C. low-; and D. high-dose group, respectively. (b) The statistical results from (a) |
| Group | n | ROS | NO | TBARS | Cell viability | Nitrite/nitrate |
| N | 9 | 12.2±1.5 | 19.3±2.2 | 0.78±0.13 | 270±30 | 20.30±1.30 |
| IR | 9 | 16.7±0.7 | 15.6±1.3 | 1.36±0.3a | 12±7ª | 45.11±1.7 |
| ↑36.89% | ↓19.17% | ↓74.04% | ↓95.56% | ↑122.21% | ||
| IR+ CF (0.5 mg/mL) | 9 | 13.8±0.8 | 22.1±1.1 | 1.03±0.18 | 129±64 | 36.03±1.82 |
| ↓17.37% | ↑44.67% | ↓24.15% | ↑90.70% | ↓20.136% | ||
| IR+ CF(2.5 mg/mL) | 9 | 11.5±0.9 | 27.7±0.7 | 0.72±0.14 | 254±37 | 29.50±2.21 |
| ↓31.14% | ↑77.56% | ↓47.39% | ↑95.28% | ↓34.61% |
ROS significantly decreased by about 17.37 and 31.14%, respectively, in low- and high-dose groups pretreated with CF for 15 days compared with the IR group. ROS in the high dose group were even lower than that in the sham group. Nitric oxide (NO) trapped by the Fe2+DETC complex significantly decreased by about 19.17% in the IR group compared with the sham group (Fig. 4, Table 3). Compared with the IR group, NO significantly increased about 44.67% and 77.56% in low and high-dose groups pretreated with CF for 15 days, respectively. NO of those groups were also significantly higher than the concentration of the sham group[34].
Survival of pyramidal cells in the CA1 area of hippocampus accessed by Nissl staining the CA1 region of the hippocampus is susceptible to transient ischemic insults. We assessed cell survival in the CA1 region of the hippocampus with Nissl staining. Fig. 6 represents the photographs of coronal sections containing the hippocampal CA1 region obtained 6 days after IR insult. In the CA1 region from the IR group, the pyramidal cells have almost completely disappeared as shown in Fig. 6(b) and Table 3. There were 12 ± 7 cells/mm in the CAI region from the IR group, while there were 270 ± 30 cells/mm in the CA1 region from the sham group. In contrast to the IR group, pretreatment with CF significantly increased the number of survival pyramidal cells in the CA1 region [Fig. 6(c), (d)] in a dose-dependent fashion (129 ± 64 cells/mm for low-dose group, 254 ± 35 cells/mm for high-dose group as shown in Table 3). There was no significant difference between the sham group and high-dose group[34].
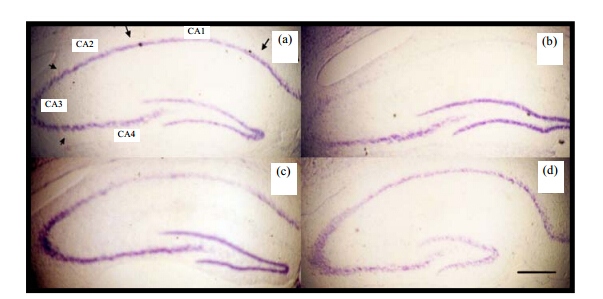
|
| Fig. 6 Nissl staining of hippocampal CA1 pyramidal neuron six days after 5 min ischemic damage. Section from (a) sham group; (b) IR group; (c) low-; and (d) high-dose group. Scar bar: 400 mm |
Consumption of tea improves vascular function and is linked to lowering the risk of cardio-brain-vascular disease. L-Theanine, first identified in 1949, is the major amino acid found in tea, which constitutes about 50 % of the plant’s total free amino acids and 1-2 % of the dry weight of tea leaves. It is the primary component of tea that contributes to the unique pleasing taste of tea. Because of its contribution to the taste, the content of theanine in tea leaves correlates highly with the quality and the price of tea[35, 36, 37].
Endothelial nitric oxide is the key regulator of vascular functions in endothelium. In this study, we establish that L-theanine, a non-protein amino-acid found in tea, promotes nitric oxide (NO) production in endothelial cells. L-theanine potentiated NO production in endothelial cells was evaluated using Griess reaction, NO sensitive electrode and a NO specific fluorescent probe (4-amino-5-methylamino-2', 7'-difluororescein diacetate). L-Theanine induced NO production was partially attenuated in the presence of L-NAME or L-NIO and completely abolished using eNOS siRNA. eNOS activation was Ca2+ and Akt independent, as assessed by fluo-4AM and immunoblotting experiments, respectively and was associated with phosphorylation of eNOS Ser 1177. eNOS phosphorylation was inhibited in the presence of ERK1/2 inhibitor, PD-98059 and partially inhibited by PI3K inhibitor, LY-294002 and Wortmanin suggesting PI3K-ERK1/2 dependent pathway. Increased NO production was associated with vasodilation in ex ovo (chorioallantoic membrane) model. These results demonstrated that L-theanine administration in vitro activated ERK/ eNOS resulting in enhanced NO production and thereby vasodilation in the artery. The results of our experiments are suggestive of L-theanine mediated vascular health benefits of tea[38]. 2.5 Cocoa polyphenols increase NO and decrease hypertension
Cocoa liquor is especially rich in a specific subclass of polyphenols, the flavanols, that have been suggested to mediate the favorable effects of cocoa products on cardiovascular health[39].
Dark chocolate but white chocolate contains antioxidants polyphenols. A randomized, controlled, investigator blinded, parallel-group trial involving 44 adults aged 56 through 73 years (24 women, 20 men) with untreated upper-range prehypertension or stage 1 hypertension without concomitant risk factors. The trial was conducted at a primary care clinic in Germany between January 2005 and December 2006 from baseline to 18 weeks to receive for 18 weeks either 6.3 g (30 kcal) per day of dark chocolate containing 30 mg of polyphenols or matching polyphenol-free white chocolate. Result show that dark chocolate intake reduced mean (SD) systolic BP by −2.9 (1.6)mmHg (P > 0.001) and diastolic BP by −1.9 (1.0)mmHg (P = 0.001) without changes in body weight, plasma levels of lipids, glucose, and 8-isoprostane Hypertension prevalence declined from 86% to 68%. The BP decrease was accompanied by a sustained increase of S-nitrosoglutathione by 0.23 (0.12) nmol/L (P < 0.001), and a dark chocolate dose resulted in the appearance of cocoa phenols in plasma. White chocolate intake caused no changes in BP or plasma biomarkers[40]. The apparent mechanism by which dark chocolate lowered BP suggests a chronic increase in the production of nitric oxide in the vascular endothelium.
The data in this relatively small sample of otherwise healthy individuals with above-optimal BP indicate that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved formation of vasodilative nitric oxide. 2.6 A formula mixture containing natural antioxidants protects cardio-brain vascular health
We selected and optimized a formula according to the above found. The experiment results show that it can generate NO, scavenge hydroxyl, superoxide, DPPH free radicals, modulate blood lipid, sugar and pressure. It can also prevent heart and lung against ischemia damage caused by Isoproterenol, and protect brain against ischemia-reperfusion damage caused by oxygen and glucose deprivation and prevent Celagent paralysis caused by Abeta in an Alzheimer’s disease model[41].
The above experiment results indicate that natural antioxidants and NO can synthetically prevent, decrease heart-brain-vascular diseases, and protect heart-brain-vascular function and health. 3 Conclusion and perspective
In conclusion, the evident studied by ESR showed the “double edge” effects of nitric oxide in myocardial and brain ischemia-reperfusion and the cardio-brain-vascular protective effects of natural antioxidants against ischemia-reperfusion injury. In the future, more effort should be spent to evaluate the efficiency of the natural antioxidants on cardio-brain-vascular diseases using clinical trials. In addition, the combination therapy using natural antioxidants and other drugs should be explored. This may allow the use of drugs at lower dosages that eliminate the cytoxicity and lead to new and powerful approaches for management of cardio-brain-vascular ischemia-reperfusion injury. It is as well important to study the molecular mechanisms of the cardio-brain-vascular effects of natural antioxidants, so that more effective medicines can be developed to fight against heart disease.
| [1] | Ferrari R, Guardigli G, Mele D, et al. Oxidative stress during myocardial ischaemia and heart failure[J]. Curr Pharm Des, 2004, 10:1 699-1 711. |
| [2] | Zweier J L, Flaherty J T, Weisfeldt M L. Direct measurement of free radical generation following reperfusion of ischemic myocardium[J]. Proc Natl Acad Sci USA, 1987, 84:1 404-1 407. |
| [3] | Zweier J L, Kuppusamy P, Williams R, et al. Measurement and characterization of postischemic free radical generation in the isolated perfused heart[J]. J Biol Chem, 1989, 261:18 890-18 895. |
| [4] | Zhao B L, Shen J G, Li M, et al. Synergic effects of NO and oxygen free radicals in ischemia-reperfusion rabbit myocardium[J]. Sci China, 1996, 26:331-338. |
| [5] | Zhao B L, Xin W J, Yang W D, et al. Direct measurement of active oxygen free radicals from ischemia-reperfusion rabbit myocardium[J]. Chin Sci Bull, 1989, 34:780-787. |
| [6] | Cheng S, Zhao B L, Xin W J, et al. Myocadium damage during ischemia-reperfusion of rat heart[J]. Chin Circ, 1990, 5:222-226. |
| [7] | Huang N, Chen Y, Zhao B L, et al. Studies on free radicals generated during ischemia-reperfusion of rat heat[J]. J Chin Med, 1990, 70:691-694. |
| [8] | Zweier J L, Rayburn B K, Flaherty J T, et al. The effect of superoxide dismutatase on free radical concentration in post ischemic myocardium[J]. Circulation, 1986, 74:371-380. |
| [9] | Sanders S P, Zweier J L, Kuppusamy P, et al. Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport[J]. J Clin Invest, 1993, 91:46-52. |
| [10] | Zweier J L, Kuppusamy P. In vivo EPR spectroscopy of free radicals in the heart[J]. Environ Health Persp, 1994, 102(Suppl 10):45-51. |
| [11] | Kuppusamy P, Chzhan M, Vij K, et al. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart:a technique for imaging tissue metabolism and oxygenation[J]. Proc Natl Acad Sci USA, 1994, 91:3 388-3 392. |
| [12] | Zhao B L, Shen J G, Li M, et al. Scavenging effect of Chinonin on NO and oxygen free radicals generated from ischemia reperfusion myocadium[J]. Biachem Biophys Acta, 1996, 1 317:131-137. |
| [13] | Afanas'ev I B. On mechanism of superoxide signaling under physiological and pathophysiological conditions[J]. Med Hypotheses, 2005, 64:127-129. |
| [14] | Linnane A W, Kios M, Vitetta L. The essential requirement for superoxide radical and nitric oxide formation for normal physiological function and healthy aging[J]. Mitochondrion, 2007, 7:1-5. |
| [15] | Zhao B L, Jiang W, Zhao Y, et al. Scavenging effect of salvia miltiorriza on free radicals and its protection for myocardial mitochondrial membrane from ischemia-reperfusion injury[J]. Biochem Mol Biol Intern, 1996, 38:1 171-1 182. |
| [16] | Shen J G, Wang J, Zhao B L, et al. Effects of EGb-761 on nitric oxide, oxygen free radicals, myocardial damage and arrhythmias in ischemia-reperfusion injury in vivo[J]. Biochim Biophys Acta, 1998, 1406:228-236. |
| [17] | Zou X L, Wan Q, Li M F, et al. Scavenging effect of green tea polyphenols on oxygen free radicals generated from ischemia-reperfused myocardium[J]. Chinese J Magn Reson, 1995, 12(3):237-244. |
| [18] | Shen J G, Guo X S, Jiang B, et al. Chinonin, a novel drug against cardiomyocyte apoptosis induced by hypoxia and reoxygenation[J]. Biochim Biophys Acta, 2000, 1500:217-226. |
| [19] | Shen J G, Li M, Xin W J, et al. Effects of Chinonin on nitric oxide free radical, myocardial damage and arrhythmia in ischemia-reperfusion injury in vivo[J]. Appl Magn Reson, 2000, 19:9-19. |
| [20] | Zhao B L, Zhou W A, Ni Y C, et al. Kinetic scavenging effects of chinonin on NO and oxygen free radicals generated from ischemia reperfusion myocardium and its protection effects on the myocardium[J]. Res Chem Intermed, 2000, 26:747-762. |
| [21] | Zhao B L, Shen J G, Li M, et al. Study on NO free radicals generated from ischemia-reperfused heart and macrophage[J]. Chinese J Magn Reson, 1997, 14(1):99-106. |
| [22] | Zhao B L, Shen J G, Tang C, et al. Analysis of EPR spectrum about NO free radicals trapped by DETCFe2+[J]. Chinese J Magn Reson, 1998, 15(3):307-311. |
| [23] | Zhang D L, Xiong J, Hu J, et al. Improved method to detect nitric oxide in biological syste[J]. Appl Magn Reson, 2001, 20:345-358. |
| [24] | Zhou G Y, Zhao B L, Hou J W, et al. Detection of nitric oxide in tissue by spin trapping EPR spectropy and triacetylglycerol extraction[J]. Biotech Tech, 1999, 13:507-511. |
| [25] | Tredici P D. Ginkgos and people-A thousand years of interactions[J]. Arnoldia, 1991, 51:2-15. |
| [26] | Tosaki A, Droy-Lefaix M T, Pali T, et al. Effects of SOD, catalase, and a novel antiarrhythmic drug, EGB 761, on reperfusion- induced arrhythmias in isolated rat hearts[J]. Free Radic Biol Med, 1993, 14:361-370. |
| [27] | Haramaki N, Aggarwal S, Kawabata T, et al. Effects of natural antioxidant ginkgo biloba extract (EGB 761) on myocardial ischemia-reperfusion injury[J]. Free Radic Biol Med, 1994, 16:789-794. |
| [28] | Shen J G, Zhao B L, Li M F, et al. Inhibitory effects of Ginkgo biloba extract (EGB761) on oxygen free radicals, nitric oxide and myocardial injury in isolated ischemic-reperfusion hearts//Proceedings of the International Symposium on Natural Antioxidants Molecular Mechanisms and Health Effects. Packer L, Traber M G, Xin W, et al. Eds.[C]. Champaign, Illinois:AOCS Press, 1996. |
| [29] | DeFeudis F V. Ginkgo biloba extract (EGb 761):pharmacological activities and clinical application[M]. Elsevier Paris:Amsterdam, 1991. |
| [30] | Varga E, Bodi A, Ferdinandy P, et al. The protective effect of EGb 761 in isolated ischemic/reperfused rat hearts:a link between cardiac function and nitric oxide production[J]. J Cardiovasc Pharm, 1999, 34:711-717. |
| [31] | Kusmic C, Basta G, Lazzerini G, et al. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart:a link between vitamin E preservation and prostaglandin biosynthesis[J]. J Cardiovasc Pharm, 2004, 44:356-362. |
| [32] | Furchgott R, Zawadzki J V. The obligatory role of the endothelium in the relaxation of arterial smooth muscle by acetycholine[J]. Nature, 1980, 288:373-376. |
| [33] | Zhao B L, Shen J G, Li M, et al. In:Chinonin Can Scavenging No Free Radicals and Protect the Myocardium Against Ischemia-Reperfusion Injury//Proceedings of the International Symposium on Natural Antioxidants Molecular Mechanisms and Health Effects. Packer L, Ttaber M G, Xin W. Eds.[C]. Champaign, Illinois:AOCS Press, 1996. |
| [34] | Zhang D L, Zhao B L. Oral administration of Crataegus extraction protects against ischemia/reperfusion brain damage in the Mongolian gerbils[J]. J Neur Chem, 2004, 90:211-219. |
| [35] | Zhao Y, Zhao B L. The neuroprotective effect of L-theanine and its inhibition on nicotine dependence[J]. Chinese Sci Bull, 2014, 59:4 014-4 019. |
| [36] | Di X, Yan J, Zhao Y, et al. L-theanine protect the APP (Swedish mutation) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway[J]. Neuroscience, 2010, 168(3):778-786. |
| [37] | Wu Z F, Zhu Y S, Cao X S, et al. Mitochondrial toxic effects of Aβ through mitofusins in the early pathogenesis of alzheimer's disease[J]. Mol Neurobiol, 2014, 50:986-996. |
| [38] | Siamwala J H, Dias P M, Majumder S, et al. L-theanine promotes nitric oxide production in endothelial cells through eNOS phosphorylation[J]. J Nutr Biochem, 2013, 24(3):595-605. |
| [39] | Hollenberg N K, Schmitz H, Macdonald I, et al. Cocoa, flavanols and cardiovascular risk[J]. Br J Cardiol, 2004, 11(5):379-386. |
| [40] | Taubert D, Roesen R, Lehmann C, et al. Effects of Low Habitual Cocoa Intake on Blood Pressure and Bioactive Nitric Oxide[J]. A Randomized Controlled Trial JAMA, 2007, 298(1):49-60. |
| [41] | Zhao B L. No free radical,natural antioxidant and cardio-brain-vascular health//The Second International Chinese Symposium on Free Radical Research[C]. Hong Kong:2014. 38. |
 2015, Vol. 32
2015, Vol. 32




