文章信息
- 彭俊辉,赵德彪,文彬,张志勇
- PENG Jun-hui, ZHAO De-biao, WEN Bin, ZHANG Zhi-yong
- 核磁共振、X射线小角散射以及计算机模拟相结合构建生物大分子复合物的结构模型
- Determining Structural Models of Biomolecular Complexes Integrating Nuclear Magnetic Resonance, Small-Angle X-ray Scattering and Computational Simulations
- 波谱学杂志, 2015, 32(2): 181-194
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 181-194
- http://dx.doi.org/10.11938/cjmr20150204
-
文章历史
- 收稿日期:2015-02-06
- 收修改稿日期:2015-05-08
Many proteins may interact with other biomolecules (proteins, DNA, or RNA, etc.) and assemble into complexes in order to accomplish a mutual biological function[1], such as cell signaling, accurate replication of DNA, cell adhesion and migration and many other important biological processes. To better understand the function of a biomolecular complex, it would be necessary to determine its three dimensional (3D) structure[2] that is often a challenging problem. X-ray crystallography and solution nuclear magnetic resonance (NMR) are the most widely used high-resolution techniques in structure determination of biomolecules. Cryo-electron microscopy (cryo-EM) is developing rapidly, and more and more biomolecular complexes have been solved by cryo-EM near atomic resolution in recent years[3]. Therefore, a straightforward way to determine the 3D structure of a complex is using one of the three techniques. However, for many biomolecular complexes, any individual technique may encounter some limitations. Crystallization would be rather difficult for large complexes with high flexibility. There is a size limit for either NMR or cryo-EM, that is, NMR is considered to be suitable for proteins with modest molecular weight and cryo-EM is generally applicable to very large complexes. Small-angle X-ray scattering (SAXS) has become an important tool in structural biology over the past decades[4, 5]. SAXS has no size limit, which is able to investigate solution structures of biomolecules from small proteins to large complexes. However, the resolution of SAXS is inherently low, so it is usually not used alone for structure elucidation.
Recently, a notion of “integrative structural biology” was proposed[6]. Since each experimental technique has its own advantage and limitation, one may try to solve the structure of a complex by integrating all the possible experimental data from different sources. For example, it would be relatively easy to solve structures of the subunits in the complex individually through X-ray crystallography or NMR. Besides its powerful usage in structure determination, NMR can also be used to obtain other structural information in the complex, such as the binding interface and orientation between the subunits. On the other hand, cryo-EM or SAXS can provide low-resolution shape information of the overall complex. Thus we may combine these complementary high and low-resolution data to determine structural model of the complex by utilizing some computational tools, such as HADDOCK[7, 8] and the IMP suite[9]. In this review, we focus on introducing these NMR experiments, SAXS data analysis, and various computational methods that combine NMR and SAXS to build models of biomolecular complexes. Finally we present a recently-published work on determining structural model of the vinculin: CAP-SH3ab complex[10]. 1 Solution nuclear magnetic resonance
Protein-protein interactions are involved in many biological processes, and structural investigations of these protein assemblies are important to elucidate the mechanism of these processes. Many protein assemblies contain disordered, flexible polypeptide segments that are difficult to crystallize. NMR spectroscopy is a powerful method to obtain structural information of the protein assemblies especially those with highly flexibilities. Chemical shift perturbation (CSP) is one of the most widely used methods to get the interfaces of protein complexes with no need of resolved 3D structures[11]. In the standard experiment, one needs an isotope labeled protein and its unlabeled partner, which can be a small molecule or another biomolecule. The 2D HSQC spectrum of the labeled protein is monitored when the unlabeled partner is titrated in. The chemical shift of the protein is very sensitive to the environmental changes caused by the interaction of the partner, and can be recorded at each titration point. The residues with significant chemical shift changes are probably located on the binding sites interacting with the partner in the case of week to intermediate binding affinity. Moreover, the dissociation constant Kd can be deduced by fitting the titration curve (chemical shift changes vs. concentration of the partner) straightforwardly[12]. As an important complementary method of crystallography, CSP provides valuable protein interaction information in the solution state[13]. However, CSP may fail to map the interface if a conformational change happens in the process of assembly of the protein complex, as the chemical shifts of many residues might be affected[12].
There is a large number of structural information in inter-nuclear magnetic dipolar coupling, but it is averaged to zero by isotropic rotation of a single molecule in solution. Dipolar couplings could be observed, if the molecule is partially aligned in oriented mediums, such as polyacrylamide gels[14] and C12E5[15]. Residual dipolar couplings (RDCs) provide long range orientational restraints that are useful in the determination of domain (or protein) orientations in multi-domain protein (or protein complexes)[16]. Once sufficient RDCs have been measured in each component of the protein complex, the orientation of molecular alignment tensors can be determined independently. If the components are in a stable complex, they experience the same alignment tensor, and the principal axes must coincide, thus providing powerful long-range geometric constraints on relative orientations of the components[17, 18]. Moreover, the inherent four-fold relative orientational degeneracy can be eliminated by using RDCs from multiple mediums that give different molecular orientations, or with additional distance restraints and geometric considerations[19].
Along with RDC, paramagnetic relaxation enhancement (PRE) and pseudocontact shift (PCS) can also provide long range structure information[20, 21]. Nitroxide radicals or metal chelators, such as EDTA-Mn2+, which contain an unpaired electron with an isotropic g-tensor, can be covalently attached to a specific site on the biomolecule and used for the observation of intramolecular or intermolecular PREs. The PRE rate for 1H-transverse magnetization (referred to as 1H-Γ2) is commonly used in the studies and measured as the difference in transverse relaxation rates between the paramagnetic and diamagnetic states of the macromolecule[22]. PRE can arise when a nucleus and the unpaired electrons of the paramagnetic center are closer than about 3.5 nm, which is very suitable for protein complex[23]. This distance range, owing to the large magnetic moment of an unpaired electron, is much larger than that of NOE, which is limited to short-range interactions less than 0.6 nm. PCS can be observed when paramagnetic metals with an anisotropic magnetic susceptibility are placed or attached into protein or protein complex. Based on this, PCS can provide distance and angular information between the paramagnetic center and the observed nucleus that are up to about 4 nm[24, 25].
The biggest barrier of NMR study in the structural investigation of the protein complexes is the size limitation while many important protein assemblies have very large molecule weights. It is a great challenge to solve these structures using traditional backbone-directed triple resonance solution NMR experiments. For biomolecular complexes larger than 25, the sensitivity and resolution of NMR experiments are degraded by the increased linewidths and the spectral overlap from large number of signals. In the past years, methyl-TROSY approaches have shed light on the structural characterization of large protein assemblies using NMR[26, 27]. Methyl groups are very excellent probes of protein structure and dynamics. As many methyl groups are found throughout the hydrophobic core regions and interfaces of protein complexes, the methyl-methyl distances can provide extremely valuable restraints in structural studies. With the development of isotope labeling strategies, proteins can be methyl-protonated in a highly deuterated background[28, 29, 30]. Then experiments utilizing a methyl-TROSY effect can be designed to obtain spectra of high resolution and sensitivity that can be used in a wide range of studies[31]. Several successful examples have proved that methyl-TROSY may provide important structural insights of protein assemblies[32, 33, 34]. 2 Small-angle X-ray scattering
Unlike those high resolution methods that may run into some aforementioned limitations when dealing with a large biomolecular complex, SAXS can be highly informative in characterizing overall structure properties of the complex in solution[35]. After rotational averaging, SAXS experiments yield an one-dimensional profile, from which shape information can be extracted, such as maximum distance (Dmax), radius of gyration (Rg), volume, molecular weight[36, 37, 38]. Since data collection and processing are very rapid, SAXS is one of the most ideal candidates to complement other experimental techniques[39]. Despite its low-resolution nature, SAXS is very useful for three-dimensional (3D) shape reconstruction, fold recognition, flexible fitting of known high resolution structures, ensemble refinement of flexible biomolecules and model construction of macromolecules and their complexes, by combining appropriate computational tools[40, 41, 42, 43, 44, 45, 46, 47] . In the following, the basic principle of SAXS and 3D shape model reconstruction are introduced. 2.1 Basic principle of SAXS
Schematic diagram of a typical SAXS experiment is shown in Fig. 1. In SAXS experiments, X-ray photons are scattered onto the sample in solution, and the scattering intensities as the function of scattering angle (typically 0.1° to 10°) are recorded on a detector. Originally, a two-dimensional image is obtained. Since the solution sample is isotropic, the two-dimensional image can be averaged into an one-dimensional profile I(q). The amplitude of the scattering vector q can be expressed as q = 4π sin(θ)/λ, where 2θ is the scattering angle and λ is the wavelength of the X-ray. The Rg value can be estimated directly from sufficient small q values using Guinier approximation[48],$I(q) = I(0)\exp ( - \frac{1}{3}R_g^2q)$ , where I(0) is the forward scattering intensity. Empirically, the q values should be smaller than 1.3/Rg to ensure a safe estimate of Rg. Further, the SAXS profile can be transformed into P(r), the pairwise distance distribution function (PDDF), using the program GNOM[49]. The PDDF can give some overall information on the shape of the biomolecule. For example, a glo bular protein has a roughly symmetric PDDF with only one peak, whereas a multi-domain or multi-subunit protein often shows a tailing shape with multiple shoulder peaks. Molecular weight can be estimated either by comparing scattering intensities with standard SAXS profiles[35] or using some other programs like SAXSMOW[50].
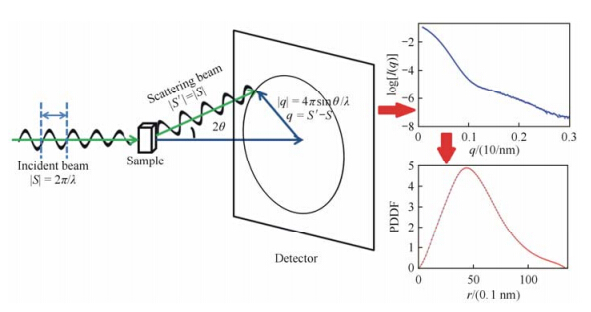
|
| Fig. 1 Basic concept of SAXS experiment |
On the other hand, theoretical SAXS profiles of biomolecules can be back-calculated through their atomic resolution structures[46, 51, 52, 53, 54, 55, 56]. For example, CRYSOL[51] uses multipole expansion to compute the scattering pattern while taking the hydration shell into account. Other approaches, such as FoXS[55] and Fast-SAXS[53], compute theoretical scattering intensities using the famous Debye formula,
where fi(q) and fj(q) are structure factors of the ith and jth atoms at the scattering vector amplitude q, and rij is the distance between the two atoms. The discrepancy between theoretical and experimental data can be given by a χ value that is expressed as
where K is the number of data points, σ(qi) is the standard deviation of the experimental data at qi, and μ is the scaling factor. A smaller χ value means better fitness between the theoretical and experimental data. Most of the computational tools that construct structure models from SAXS profiles generally use this target function to refine structure. 2.2 3D shape model reconstruction
It has been shown that the electron densities of biomolecules can be approximated by bead models or dummy atom models[57]. Based on this innovation, a 3D shape model of the biomolecule can be derived from its PDDF. DAMMIN can use low-angle portions of the scattering profile to restore a dummy atom model[40]. DAMMIF is a computationally faster version of DAMMIN, but with a little loss of accuracy[41]. GASBOR needs to incorporate high-angle portions of the SAXS profile to compute a dummy atom chain-like model[42], which has a higher resolution than the DAMMIN/DAMMIF model. Since phase and orientation information is averaged in the SAXS profile, alternative reconstruction solutions could exist[35] . Thus, one needs to compare these structure models among different runs of reconstruction and finally compare to the known atomic structures to assess the quality of the shape model[35]. Normalized spatial discrepancy (NSD) can be used to estimate the differences between two shape models or between shape models and atomic structures using the program SUPERCOMB[58]. Meanwhile, this program can be used to dock the atomic structure to its corresponding shape model, which can also be accomplished by other programs like Situs[59]. 2.3 Computational tools to construct atomic structural models
For a large protein complex, conventional high-resolution methods such as X-ray crystallography and NMR may have difficulties in determining its atomic structure. However, structures of subunits and interface information may be obtained through these experiments or homology modeling. In this case, low-resolution information obtained from SAXS would be very useful. A structural model of the complex could be directly obtained by docking individual subunits of the complex into the shape model[60].
Due to the low-resolution restriction of SAXS, many algorithms directly refine the structural model against the one dimensional SAXS intensity. In general, two strategies are applied, one is refining while sampling, and the other is screening after sampling[36]. The SASREF program in the ATSAS package[61] belongs to the first category, which is used to determine the positions and orientations of subunits within the biomolecular complex by fitting the SAXS data. SASREF combines rigid body modeling and ab initio method, where each subunit is treated by a rigid body and dummy residues are implemented to model missing regions in the complex. Simulated annealing (SA) minimization is used to translate and rotate the rigid bodies and optimize the local conformation of the dummy residues to minimize discrepancy between the theoretical SAXS curve of structure model and the experimental SAXS data. The program allows the incorporation of restraints from other experimental methods, such as orientation restraints from residual dipolar coupling (RDC) experiments, inter residue contact restraints from cross-linking experiments and inter residue distance restraints from FRET experiments. The rigid body modeling and SA are also used in other programs like Xplor-NIH[56] to refine the biomolecular complex using NMR and SAXS.
In order to optimize the interface between subunits, there are some methods that combine rigid body global searching algorithm and local interface complementary minimizations (energy based scoring functions), such as pyDockSAXS[62] and FoXsDock[63] for the docking of subunits. Another program named IMP[9, 64] (integrative modeling platform) gathers various experimental restraints, such as RDC, cross-linking, FRET and mutational analysis in combination with SAXS and computational interface prediction methods[65] together to guide the modeling procedure.
Rigid body modeling methods are widely used since they are fast and efficient by treating subunits in the complex as rigid bodies. However, the subunits are often flexible and may have conformational changes when forming the complex. In these cases, flexible fitting methods should be applied. Monte Carlo (MC) algorithms are one of the commonly used methods. In each MC step, the theoretical scattering from the resulting model is compared with the experimental SAXS profile, until the results are in convergence. These methods are mainly different in how to make MC moves. For example, Zheng et al. applied a coarse-grained elastic network model for flexible fitting of atomic structure model to SAXS profile[46], and Gorba et al. used normal modes to guide the fitting procedure[66].
For a very flexible complex, theoretical scattering profile of any individual structure cannot fit the experimental SAXS data well, so an ensemble of conformations should be used to characterize the complex in solution[45]. The average scattering profile of the ensemble can be calculated as, $I(q) = \sum\limits_{i = 1}^{{N_e}} {{p_i}{I_i}(q)} $ , where Ne is the ensemble size, Ii(q) is the theoretical scattering profile of the conformation i, and pi is its relative population. The discrepancy between the ensemble and the experimental SAXS profile can also be described by the χ value [Eq. (2)]. In this case, the refining while sampling approaches can also be applied. For example, the ensemble refinement algorithm used in Xplor-NIH refines several models in parallel[56], and the ensemble size should be optimized to best reproduce the SAXS data.
However, these screening after sampling approaches are popularly used for flexible systems. In this category of approaches, a pool of structure models is produced, and then the structures are screened out of the pool to best fit the SAXS data. The structural pool can be generated by rigid body modeling[45], molecular dynamics simulations[67], enhanced sampling methods[47], or coarse grained simulations[68, 69]. The discrepancy χ value [Eq. (2)] between the theoretical scattering and the experimental SAXS profile is used to optimize the structural ensembles. Different algorithms are applied in the screening procedure. In ensemble optimization method (EOM)[45] and amplified collective motion method in combination with SAXS (ACM-SAXS)[47], genetic algorithm (GA) is applied, and thus each conformation in the pool has the same opportunity to be screened out. In minimal ensemble search (MES)[67], GA is also used, but the ensemble size (usually 2 to 5) is smaller than that of EOM (often 10 to 20). In the basis-set-supported SAXS (BSS-SAXS) reconstruction[68] and ensemble refinement of SAXS (EROS) method[69], a slightly different algorithm is used. The conformations in the pool are classified into different structural clusters first, and then Bayesian-based Monte Carlo or maximum entropy method is implemented to compute the relative population of each cluster. 3 Case study: structure model of the vinculin: CAP-SH3ab complex
Vinculin is important in regulation of cell adhesion and migration[70]. A crystal structure of human vinculin has been determined (PDB entry 1TR2) with a resolution of 0.285 nm[71]. The full-length vinculin consists of five domains (called D1-D5). The first four domains D1 to D4 constitute the globular head and D5 is the vinculin tail. The vinculin head and tail are linked by a flexible proline-rich loop that is missing in the crystal structure. CAP is a cytoskeletal adaptor protein with three SH3 domains at its C-terminus, which is important in regulating turnover of adhesion[72]. The binding of CAP with vinculin is important for its function[73], and it has been reported that CAP interacts with the proline-rich loop through its first two SH3 domains (named as SH3a and SH3b, respectively)[74]. Conventional methods may have difficulties in studying the vinculin: CAP-SH3ab complex. The molecular weight of the complex is about 134 k, which is large for NMR and small for Cryo-EM. On the other hand, the proline-rich loop is too flexible to be crystallized. Therefore, to gain the structural and functional view of the complex, NMR and SAXS, together with computational simulations are used to construct a structure model of the complex[10]. High resolution solution structures of the two SH3 domains are obtained by NMR spectroscopy. ITC experiments indicate that the SH3a domain binds to residues 858-867 in the proline-rich loop of vinculin (called prr1) and the SH3b domain binds to residues 870-879 (called prr2). Chemical shift perturbation studies tell us which residues are involved in the binding interface. SAXS experiments are conducted for the vinculin: CAP-SH3ab complex.
Having established the knowledge above, the modeling procedure is described as follows. The workflow is shown in Fig. 2 and computer programs utilized are shown in Fig. 3. First, the missing proline-rich loop in the crystal structure of vinculin was built by homology modeling using Modeller[75]. Then we picked a structure of vinculin with extended proline-rich loop, and docked the SH3a domain to prr1 and the SH3b domain to prr2, respectively, by HADDOCK server[7, 8] using interface restraints obtained through CSP experiments. The structural model with the lowest HADDOCK score was selected and the linker between the SH3a and SH3b domains was also built by homology modeling[75]. Starting from this initial model, a 100 ns MD simulation was then carried out to further generate a pool of candidate structure models of the vinculin:CAP-SH3ab complex. From the MD trajectory, the conformation with the smallest discrepancy [Eq. (2)] with the experimental SAXS profile was screened out as the final model. A 3D shape model of vinculin: CAP-SH3ab was constructed by DAMMIN[40]. SUPCOMB[58] is used to dock the final structure model to the shape model with a NSD value of 1.4. The final structural model of the vinculin: CAP-SH3ab complex is in fairly agreement with the experimental SAXS data.
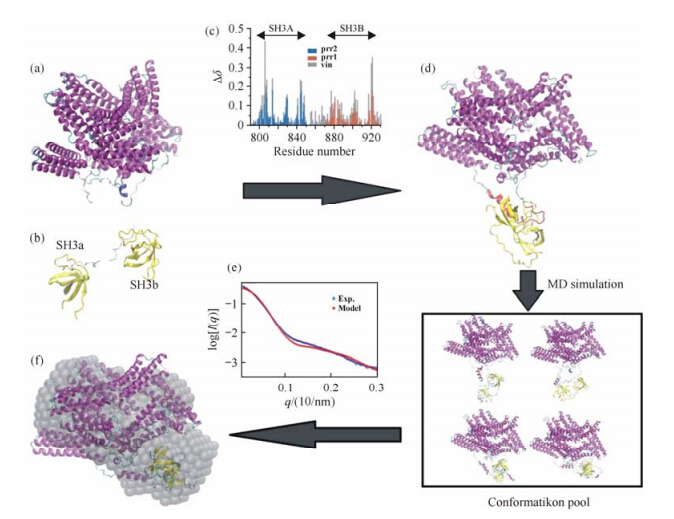
|
| Fig. 2 XRDOverall workflow of the model construction of vinculine:CAP-SH3ab complex. In this figure, (a), (b) and (c) are the experimental restraints, which are crystal structure of vinculine, NMR structures of the tandem SH3ab domains and chemical shift perturbution experiment. Based on (a), (b) and (c), an initial complex model (d) can be obtained through homology modeling of the missing loop and docking the tandem SH3ab domains to vinculine by HADDOCK. Further, a long MD simulation is carried out. By minimizing the discrepancy between the experimental (blue) and theoretical (red) SAXS profiles as shown in (e), the conformation with the smallest χ value (f) is screened out from the conformation pool as the final model |
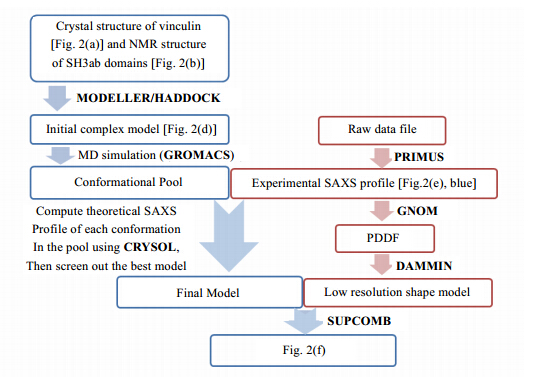
|
| Fig. 3 Flow chart of computer programs utilized in the model construction procedure |
Nowadays, SAXS technique has become more and more popular among structural biologists[5]. In some aspects, SAXS is an excellent complementary to NMR. Structures of relatively small proteins may be directly obtained by satisfying experimental NOE restraints. For larger biomolecules, experiments such as RDC can be conducted. From RDC experiments, one can tell relative orientations between subunits within a complex. However, structure models constructed from only RDC data could be ambiguous sometimes. One promising approach would be to incorporate SAXS, where global shape information is extracted, to refine the final models[52]. There are some well-known excellent computational tools that can incorporate NMR and SAXS into the calculation of structure models, such as CNS[76] and Xpor-NIH[77].
Since the resolution of SAXS profile is relatively low, over-fitting may be a serious issue that restricts its application. To avoid over-fitting, one needs high quality scattering profile with high signal-noise ratio, efficient sampling methods, appropriate criteria to screen models, and validating between different experimental data. The improvement of beamlines for biological SAXS over the last few years and advancements in sample preparation have made high quality SAXS profile of biomolecules available for many laboratories[4, 5]. Thus, we may need to take more efforts in the improvement of computational algorithms.
Many other experiments can also offer restraints in model construction procedures, such as footprinting[78], mass spectrometry[79], proteomics[80]. Although there have been studies that combine one or more restraints from experiments to construct structure models of biomolecules assemblies[56, 81], integrating various experimental data together is still challenging. The program IMP is a good try for integrative structural modeling[9]. One alternative approach would be to construct models according to one part of restraints and then validate the models using the other part, which may a lso avoid over-fitting introduced by applying only a small portion of experimental restraints.
Acknowledgement: This work was supported by National Key Basic Research Program of China (grant 2013CB910203), the National Natural Science Foundation of China (grant 31270760), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDB08030102), the Specialized Research Fund for the Doctoral Program of Higher Education (grant 20113402120013).
| [1] | Robinson C V, Sali A, Baumeister W. The molecular sociology of the cell[J]. Nature, 2007, 450:973-982. |
| [2] | Alberts B. Molecular Biology of the Cell (4th ed)[M]. New York:Garland Science;2002. |
| [3] | Kuehlbrandt W. Cryo-em enters a new era[J]. Elife, 2014, 3. |
| [4] | Mertens H D T, Svergun D I. Structural characterization of proteins and complexes using small-angle X-ray solution scattering[J]. J Struct Biol, 2010, 172:128-141. |
| [5] | Graewert M A, Svergun D I. Impact and progress in small and wide angle X-ray scattering (saxs and waxs) [J]. Curr Opin Struc Biol, 2013, 23:748-754. |
| [6] | Ward A B, Sali A, Wilson I A. Integrative structural biology[J]. Science, 2013, 339:913-915. |
| [7] | Dominguez C, Boelens R, Bonvin A M. Haddock:A protein-protein docking approach based on biochemical or biophysical information[J]. J Am Chem Soc, 2003, 125:1 731-1 737. |
| [8] | De Vries S J, van Dijk M, Bonvin A M J J. The haddock web server for data-driven biomolecular docking[J]. Nat Protoc, 2010, 5:883-897. |
| [9] | Russel D, Lasker K, Webb B, et al. Putting the pieces together:Integrative modeling platform software for structure determination of macromolecular assemblies[J]. PLoS Biol, 2012, 10. |
| [10] | Zhao D B, Wang X J, Peng J H, et al. Structural investigation of the interaction between the tandem sh3 domains of c-cbl-associated protein and vinculin[J]. J Struct Biol, 2014, 187:194-205. |
| [11] | Zuiderweg E R P. Mapping protein-protein interactions in solution by nmr spectroscopy[J]. Biochemistry-us, 2002, 41:1-7. |
| [12] | Pellecchia M, Montgomery D L, Stevens S Y, et al. Structural insights into substrate binding by the molecular chaperone dnak[J]. Nat Struct Biol, 2000, 7:298-303. |
| [13] | Nguyen C, Haushalter R W, Lee D J, et al. Trapping the dynamic acyl carrier protein in fatty acid biosynthesis[J]. Nature, 2014, 505:427-431. |
| [14] | Chou J J, Gaemers S, Howder B, et al. A simple apparatus for generating stretched polyacrylamide gels, yielding uniform alignment of proteins and detergent micelles[J]. J Biomol NMR, 2001, 21:377-382. |
| [15] | Ruckert M, Otting G. Alignment of biological macromolecules in novel nonionic liquid crystalline media for nmr experiments[J]. J Am Chem Soc, 2000, 122:7 793-7 797. |
| [16] | Fushman D, Varadan R, Assfalg M, et al. Determining domain orientation in macromolecules by using spin-relaxation and residual dipolar coupling measurements[J]. Prog Nucl Mag Res Spectrosc, 2004, 44:189-214. |
| [17] | Dosset P, Hus J C, Marion D, et al. A novel interactive tool for rigid-body modeling of multi-domain macromolecules using residual dipolar couplings[J]. J Biomol NMR, 2001, 20:223-231. |
| [18] | Valafar H, Prestegard J H. Redcat:A residual dipolar coupling analysis tool[J]. J Magn Reson, 2004, 167:228-241. |
| [19] | Ramirez B E, Bax A. Modulation of the alignment tensor of macromolecules dissolved in a dilute liquid crystalline medium[J]. J Am Chem Soc, 1998, 120:9 106-9 107. |
| [20] | Liu Z, Tang C. Paramagnetic relaxation enhancement——A tool for visualizing transient protein structures[J]. Chinese J Magn Reson, 2011, 28(3):301-316. |
| [21] | Yang Y, Chen J L, Su X C. Paramagnetic labeling of proteins and pseudocontact shift in structural biology[J]. Chinese J Magn Reson, 2014, 31(2):155-171. |
| [22] | Iwahara J, Tang C, Clore G M. Practical aspects of 1H transverse paramagnetic relaxation enhancement measurements on macromolecules[J]. J Magn Reson, 2007, 184:185-195. |
| [23] | Clore G M, Iwahara J. Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes[J]. Chem Rev, 2009, 109:4 108- 4 139. |
| [24] | Hass M A S, Ubbink M. Structure determination of protein-protein complexes with long-range anisotropic paramagnetic nmr restraints[J]. Curr Opin Struc Biol, 2014, 24:45-53. |
| [25] | Saio T, Yokochi M, Kumeta H, et al. Pcs-based structure determination of protein-protein complexes[J]. J Biomol NMR, 2010, 46:271-280. |
| [26] | Kay L E. Solution nmr spectroscopy of supra-molecular systems, why bother? A methyl-trosy view[J]. J Magn Reson, 2011, 210:159-170. |
| [27] | Sprangers R, Velyvis A, Kay L E. Solution nmr of supramolecular complexes:Providing new insights into function[J]. Nat Methods, 2007, 4:697-703. |
| [28] | Tugarinov V, Kay L E. An isotope labeling strategy for methyl trosy spectroscopy[J]. J Biomol NMR, 2004, 28:165-172. |
| [29] | Ayala I, Sounier R, Use N, et al. An efficient protocol for the complete incorporation of methyl-protonated alanine in perdeuterated protein[J]. J Biomol NMR, 2009, 43:111-119. |
| [30] | Gans P, Hamelin O, Sounier R, et al. Stereospecific isotopic labeling of methyl groups for nmr spectroscopic studies of high-molecular-weight proteins[J]. Angew Chem Int Ed, 2010, 49:1 958-1 962. |
| [31] | Tugarinov V, Hwang P M, Ollerenshaw J E, et al. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes[J]. J Am Chem Soc, 2003, 125:10 420-10 428. |
| [32] | Shi L C, Kay L E. Tracing an allosteric pathway regulating the activity of the hslv protease[J]. Proc Natl Acad Sci, 2014, 111:2 140-2 145. |
| [33] | Velyvis A, Kay L E. Measurement of active site ionization equilibria in the 670 kda proteasome core particle using methyl-trosy NMR[J]. J Am Chem Soc, 2013, 135:9 259-9 262. |
| [34] | Velyvis A, Schachman H K, Kay L E. Application of methyl-trosy NMR to test allosteric models describing effects of nucleotide binding to aspartate transcarbamoylase[J]. J Mol Biol, 2009, 387:540-547. |
| [35] | Lipfert J, Doniach S. Small-angle X-ray scattering from rna, proteins, and protein complexes[J]. Annu Rev Biophys Biomol Struct, 2007, 36:307-327. |
| [36] | Schneidman-Duhovny D, Kim S J, Sali A. Integrative structural modeling with small angle X-ray scattering profiles[J]. BMC Struct Biol, 2012, 12. |
| [37] | Putnam C D, Hammel M, Hura G L, et al. X-ray solution scattering (SAXS) combined with crystallography and computation:Defining accurate macromolecular structures, conformations and assemblies in solution[J]. Q Rev Biophys, 2007, 40:191-285. |
| [38] | Rambo R P, Tainer J A. Characterizing flexible and intrinsically unstructured biological macromolecules by sas using the porod-debye law[J]. Biopolymers, 2011, 95:559-571. |
| [39] | Forster F, Webb B, Krukenberg K A, et al. Integration of small-angle X-ray scattering data into structural modeling of proteins and their assemblies[J]. J Mol Biol, 2008, 382:1 089-1 106. |
| [40] | Svergun D I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing[J]. Biophys J, 1999, 76(6):2 879-2 886;1999, 77(5):2 896. |
| [41] | Franke D, Svergun D I. Dammif, a program for rapid ab-initio shape determination in small-angle scattering[J]. J Appl Crystallogr, 2009, 42:342-346. |
| [42] | Svergun D I, Petoukhov M V, Koch M H J. Determination of domain structure of proteins from X-ray solution scattering[J]. Biophys J, 2001, 80:2 946-2 953. |
| [43] | Zheng W J, Doniach S. Fold recognition aided by constraints from small angle X-ray scattering data[J]. Protein Eng Des Sel, 2005, 18:209-219. |
| [44] | Petoukhov M V, Svergun D I. Global rigid body modeling of macromolecular complexes against small-angle scattering data[J]. Biophys J, 2005, 89:1 237-1 250. |
| [45] | Bernado P, Mylonas E, Petoukhov M V, et al. Structural characterization of flexible proteins using small-angle X-ray scattering[J]. J Am Chem Soc, 2007, 129:5 656-5 664. |
| [46] | Zheng W J, Tekpinar M. Accurate flexible fitting of high-resolution protein structures to small-angle X-ray scattering data using a coarse-grained model with implicit hydration shell[J]. Biophys J, 2011, 101:2 981-2 991. |
| [47] | Wen B, Peng J H, Zuo X B, et al. Characterization of protein flexibility using small-angle X-ray scattering and amplified collective motion simulations[J]. Biophys J, 2014, 107:956-964. |
| [48] | Guinier A. La diffraction des rayons X aux très petits angles:Application à l'étude de phénomènes ultramicroscopiques[J]. Ann Phys, 1939, 12:161-237. |
| [49] | Svergun D I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria[J]. J Appl Crystallogr, 1992, 25:495-503. |
| [50] | Fischer H, Neto M D, Napolitano H B, et al. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale[J]. J Appl Crystallogr, 2010, 43:101-109. |
| [51] | Svergun D, Barberato C, Koch M H J. CRYSOL-A program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates[J]. J Appl Crystallogr, 1995, 28:768-773. |
| [52] | Grishaev A, Wu J, Trewhella J, et al. Refinement of multidomain protein structures by combination of solution small-angle X-ray scattering and nmr data[J]. J Am Chem Soc, 2005, 127:16 621-16 628. |
| [53] | Yang S, Park S, Makowski L, et al. A rapid coarse residue-based computational method for X-ray solution scattering characterization of protein folds and multiple conformational states of large protein complexes[J]. Biophys J, 2009, 96:4 449-4 463. |
| [54] | Grishaev A, Guo L A, Irving T, et al. Improved fitting of solution X-ray scattering data to macromolecular structures and structural ensembles by explicit water modeling[J]. J Am Chem Soc, 2010, 132:15 484-15 486. |
| [55] | Schneidman-Duhovny D, Hammel M, Sali A. Foxs:A web server for rapid computation and fitting of saxs profiles[J]. Nucleic Acids Res, 2010, 38:W540-W544. |
| [56] | Schwieters C D, Suh J Y, Grishaev A, et al. Solution structure of the 128 kda enzyme i dimer from escherichia coli and its 146 kda complex with hpr using residual dipolar couplings and small- and wide-angle X-ray scattering[J]. J Am Chem Soc, 2010, 132:13 026-13 045. |
| [57] | Chacon P, Moran F, Diaz J F, et al. Low-resolution structures of proteins in solution retrieved from X-ray scattering with a genetic algorithm[J]. Biophys J, 1998, 74:2 760-2 775. |
| [58] | Kozin M B, Svergun D I. Automated matching of high- and low-resolution structural models[J]. J Appl Crystallogr, 2001, 34:33-41. |
| [59] | Wriggers W, Milligan R A, McCammon J A. Situs:A package for docking crystal structures into low-resolution maps from electron microscopy[J]. J Struct Biol, 1999, 125:185-195. |
| [60] | Wriggers W, Chacon P. Using situs for the registration of protein structures with low-resolution bead models from X-ray solution scattering[J]. J Appl Crystallogr, 2001, 34:773-776. |
| [61] | Konarev P V, Petoukhov M V, Volkov V V, et al. Atsas 2.1, a program package for small-angle scattering data analysis[J]. J Appl Crystallogr, 2006, 39:277-286. |
| [62] | Pons C, D'Abramo M, Svergun D I, et al. Structural characterization of protein-protein complexes by integrating computational docking with small-angle scattering data[J]. J Mol Biol, 2010, 403:217-230. |
| [63] | Schneidman-Duhovny D, Hammel M, Sali A. Macromolecular docking restrained by a small angle X-ray scattering profile[J]. J Struct Biol, 2011, 173:461-471. |
| [64] | Webb B, Lasker K, Schneidman-Duhovny D, et al. Modeling of proteins and their assemblies with the integrative modeling platform[J]. Methods Mol Biol, 2011, 781:377-397. |
| [65] | de Vries S J, Bonvin A M. Cport:A consensus interface predictor and its performance in prediction-driven docking with HADDOCK[J]. PLoS One, 2011, 6(3):e17695. |
| [66] | Gorba C, Miyashita O, Tama F. Normal-mode flexible fitting of high-resolution structure of biological molecules toward one-dimensional low-resolution data[J]. Biophys J, 2008, 94:1 589-1 599. |
| [67] | Pelikan M, Hura G L, Hammel M. Structure and flexibility within proteins as identified through small angle X-ray scattering[J]. Gen Physiol Biophys, 2009, 28:174-189. |
| [68] | Yang S C, Blachowicz L, Makowski L, et al. Multidomain assembled states of hck tyrosine kinase in solution[J]. Proc Natl Acad Sci, 2010, 107:15 757-15 762. |
| [69] | Różycki B, Kim Y C, Hummer G. Saxs ensemble refinement of escrt-iii chmp3 conformational transitions[J]. Structure, 2011, 19:109-116. |
| [70] | Carisey A, Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling[J]. Eur J Cell Biol, 2011, 90:157-163. |
| [71] | Borgon R A, Vonrhein C, Bricogne G, et al. Crystal structure of human vinculin[J]. Structure, 2004, 12:1 189-1 197. |
| [72] | Baumann C A, Ribon V, Kanzaki M, et al. Cap defines a second signalling pathway required for insulin-stimulated glucose transport[J]. Nature, 2000, 407:202-207. |
| [73] | Zhang M, Liu J, Cheng A, et al. Identification of cap as a costameric protein that interacts with filamin c[J]. Mol Biol Cell, 2007, 18:4 731-4 740. |
| [74] | Mandai K, Nakanishi H, Satoh A, et al. Ponsin/sh3p12:An 1-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions[J]. J Cell Biol, 1999, 144:1 001-1 017. |
| [75] | Eswar N, Webb B, Marti-Renom M A, et al. Comparative protein structure modeling using MODELLER:Chapter 5:Unit 5.6[M]. Curr Protoc Bioinformatics, John Wiley & Sons Inc, 2006. |
| [76] | Brunger A T. Version 1.2 of the crystallography and NMR system[J]. Nat Protoc, 2007, 2:2 728-2 733. |
| [77] | Schwieters C D, Kuszewski J J, Tjandra N, et al. The xplor-nih NMR molecular structure determination package[J]. J Magn Reson, 2003, 160:65-73. |
| [78] | Takamoto K, Chance M R. Radiolytic protein footprinting with mass spectrometry to probe the structure of macromolecular complexes[J]. Annu Rev Bioph Biom Struct, 2006, 35:251-276. |
| [79] | Vandermarliere E, Stes E, Gevaert K, et al. Resolution of protein structure by mass spectrometry[J]. Mass Spectrom Rev, 2014. |
| [80] | Lasker K, Phillips J L, Russel D, et al. Integrative structure modeling of macromolecular assemblies from proteomics data[J]. Mol Cell Proteomics, 2010, 9:1 689-1 702. |
| [81] | Alber F, Dokudovskaya S, Veenhoff L M, et al. Determining the architectures of macromolecular assemblies[J]. Nature, 2007, 450 :683-694. |
 2015, Vol. 32
2015, Vol. 32






