文章信息
- 刘长东, 朱广
- LIU Chang-dong, ZHU Guang
- 利用液体核磁共振技术解析核酸四链体结构
- Quadruplex Nucleic Acid Structure Determination by Solution NMR
- 波谱学杂志, 2015, 32(2): 150-162
- Chinese Journal of Magnetic Resonance, 2015, 32(2): 150-162
- http://dx.doi.org/10.11938/cjmr20150202
-
文章历史
- 收稿日期:2015-03-17
- 收修改稿日期:2015-05-10
Nuclear magnetic resonance spectroscopy (NMR) is an essential tool in the study of G-quadruplexes which are formed by G-rich repetitive nucleic acids through stacking of a few guanine tetrads (or G-tetrads). In G-quadruplex, each G-tetrad is made of four guanines interconnected with Hoogsteen hydrogen bonds and coordinated by cations with adjacent G-tetrad. Several experimental techniques have been widely employed to study G-quadruplexes including circular dichroism (CD) spectra, electrophoresis and mass spectrometry etc. However, only X-ray crystallography and NMR spectroscopy can provide detailed structural information of G-quadruplex at atomic resolution. In addition, NMR spectroscopy has allowed not only the determination of structures of G-quadruplexes, but also the investigations for their dynamics, stability and interactions with proteins or ligands in solution.
1 Sample preparation and NMR spectroscopyNormally, the DNA oligonucleotides are chemically synthesized and are annealed at a 0.1 mmol/L single stranded concentration by heating to 95 ℃ for 15 min, followed by slow cooling to room temperature overnight in an annealing buffer.
Standard 2D NMR experimental spectra, including NOESY, TOCSY and COSY, are collected at 5, 10 and 25 ℃ to obtain the complete proton resonance assignment[1]. The NMR experiments for samples in water solution are performed with Watergate, Jump-and-Return water suppression techniques or in D2O. All spectra can be processed by using the program NMRPipe[2, 3]. Peak assignments and integrations can be conveniently achieved by applying the software Sparky (http://www.cgl.ucsf.edu/home/sparky/).
2 Circular dichroism spectroscopy (CD)Circular dichroism spectroscopy (CD) is one of the simple methods that can be used to assess the nature of the quadruplex fold. CD spectra are very sensitive to stacking interactions among the guanosines in G-quadruplexes that are correlated with the syn/anti conformation of guanosine involved in G-tetrad core. As a result, a unique CD signature for a given topology (parallel, antiparallel or hybrid) is determined[4, 5, 6].
In general, CD spectra of the typical four stranded parallel DNA quadruplexes show absorption maxima at 264 nm and minima at 240 nm. They can be easily distinguished from single or double stranded parallel quadruplexes which exhibit an additional positive peak near 290 nm in CD spectra[7]. The appearance of an absorption maximum at 295 nm and minimum at 265 nm is the characteristic of anti-parallel DNA quadruplexes (Fig. 1). The spectrum of hybrid quadruplexes is characterized not only by dominant positive bands at 295 nm and 265 nm but also by a negative band at 240 nm. Furthermore, analysis of the temperature dependence of the CD spectra can be used to monitor the stability and determine the melting temperature of the G-quadruplex. Through modifications of the sequence and careful optimization of solution conditions, it is possible to obtain a single conformer in solution, which is essential for the study of quadruplex structures by NMR spectroscopy and X-ray crystallography.
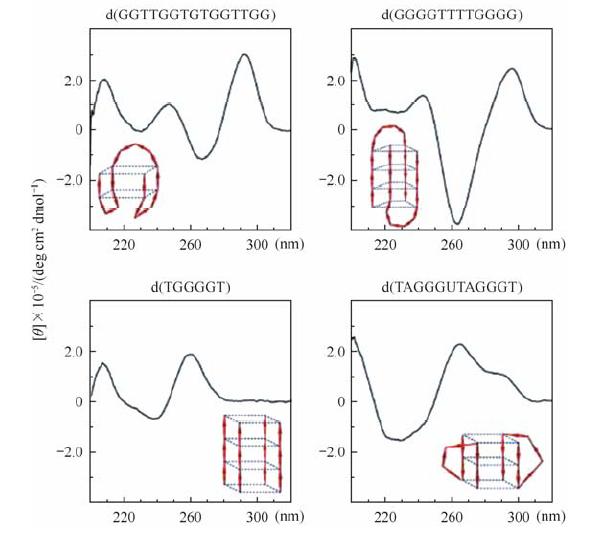 |
| Fig. 1 The CD spectra of four types of DNA G-quadruplexes and their corresponding structures are shown in each of these panels. Reproduced with permission from ref. [7] |
Once a guanine rich nucleotide sequence folds into a G-quadruplex, the guanine imino protons (H1) exhibit typical chemical shifts within the range of d 10-12, compared with d 13-14 for those involved in the Watson-Crick base pair[8, 9](Fig. 2). In order to observe all the exchangeable imino protons, it is suggested to do experiments at variable temperature even at the lowest possible temperature such as 5 ℃.
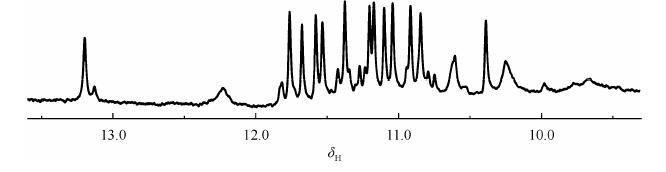 |
| Fig. 2 NMR imino proton spectrum of d[(GGGTTA)2GGGTATGGG] in K+ solution recorded at 10 ℃ |
Spectral assignment of G-quadruplexes formed by DNA and RNA sequences is the first and critical step for structural analysis by NMR methods following the classical procedure[10, 11, 12]. Classical sequential NOE connectivities H8/6(n)-H1'(n)-H8/6(n + 1) can be traced from the 5' end to the 3' end in a G-quadruplex in NOESY spectra collected at different mixing times (50-300 ms are usually suitable). However, these sequential connectivities are strongly dependent on the glycosidic conformation of guanines within the G-tetrad core. For geometric reasons, H1'(n)-H8(n+1) connections from a syn or an anti residue to a syn residue are very weak or missing. In contrast, a syn resiude to anti residue gives both H1'(n)-H8(n+1) and H1'(n+1)-H8(n) cross peaks (Fig. 3). These sequential assignments should be cross checked through the H8/6(n)-H2'/H2"(n)-H8/6(n+1) connectivities in a NOESY spectrum at 200 ms or 300 ms mixing time. Similar connectivities can also be followed through H8/6(n)-H3'/H4'(n)-H8/6(n+1). The sequential connectivities in the loops are structure dependent and lead to broken sequential connectivities. Intra-residue protons assignments are checked through strong NOE cross peaks between sugar H1' and H4' or H2"/H2' protons and between sugar H2' and H2" or H3' protons. These sugar protons should also be identified by COSY spectrum or TOCSY spectra with 40 and 80 ms mixing times. If there is a presumed G-quadruplex fold, H8-H8 and H1-H1 NOE cross peaks of guanines in the G-tetrad core should also can be identified.
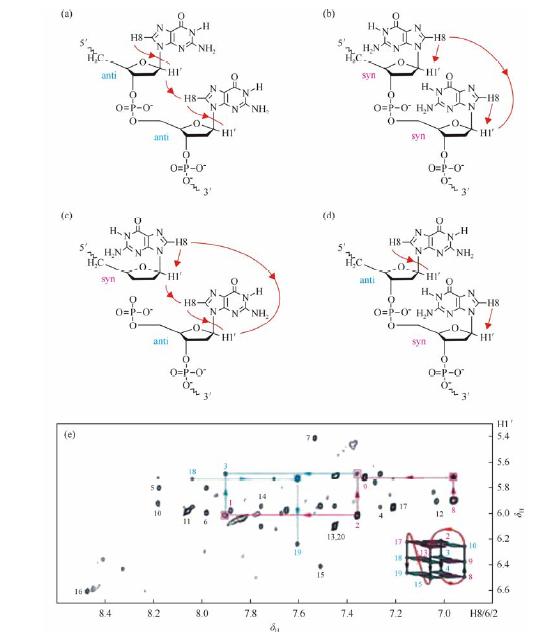 |
| Fig. 3 Sequential NOE connectivity between two adjacent residues from 5' to 3': (a) anti-anti, (b) syn-syn, (c) syn-anti and (d) anti-syn. (e) Region of NOESY spectrum from a DNA G-quadruplex (fold shown in schematic) showing cross peaks originated from anti-anti (filled cyan square), syn-syn (filled magenta square) and syn-anti (open magenta box) steps. Reproduced with permission from ref. [1] |
To some extent, spectral assignments based on NOEs are model-dependent and may lead to incorrect structural interpretation[13]. The assignments obtained from NOEs must be cross checked by using other experimental data such as COSY, TOCSY and even site-specific labeling.
4.2 Through-bond correlated approachThe experiments based on through-bond correlations can provide unambiguous determination or confirmation/verification of spectral assignments as compared to NOE-based approach. For example, the assignments of imino and aromatic protons are verified using through-bond correlations 1H-13C HMBC at natural abundance based on long range coupling between the imino and H8 protons in the same guanine via the 13C5 carbon (Fig. 4)[14]. COSY and TOCSY spectra are generally used to confirm NOE-based assignments of H2'/H2" and other sugar protons. The sugar and aromatic protons within the same residue can be discriminated based on Heteronuclear Single Quantum Correlation (1H-13C HSQC) experiment. Meanwhile, 1H-15N HSQC can also be used to correlate imino proton to its nitrogen.
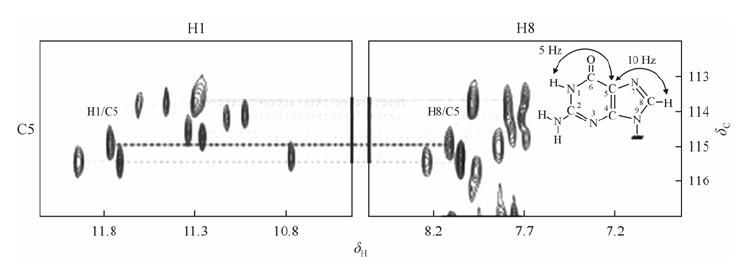 |
| Fig. 4 The imino and H8 regions of 2D HMBC spectrum showing correlation between imino proton and H8 proton within the guanosine base. Through-bond correlations between guanosine imino and H8 protons via 13C (at 5-position) at natural abundance, using long-range J couplings shown inset. Reproduced with permission from ref. [1] |
Imino protons and other resonances of nucleotides can be assigned by using the site-specific low enrichment isotope labeling approach (Fig. 5). One or more residues are site-specifically 15N, 13C-enriched to only a few percent such as 1-5%, which corresponds to several folds above the natural abundance (0.37% and 1.1% for 15N and 13C, respectively). A 1% isotopic increase in the 15N and 13C natural abundance results in fourfold isotopic increase of 15N, and twofold of 13C that can be used to enhance signals in the heteronuclear experiment[15]. In practice all resonances can now be assigned unambiguously through this simple approach.
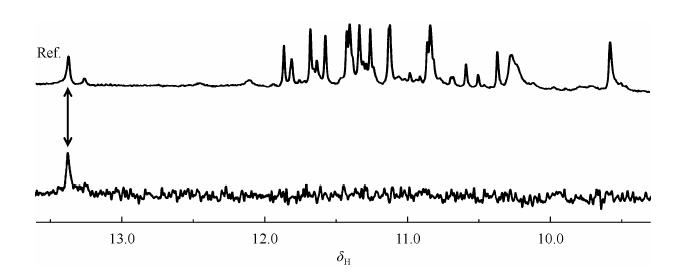 |
| Fig. 5 The imino region of 1D 15N filtered HSQC spectrum of d[GGGTTTGGGTTA GGGTTA GGG]. The sample contains site-specific low-enrichment (2%) 15N labelled oligonucleotides at indicated positions. Spectra were recorded at 800 MHz (1D 1H NMR spectrum and 1D 15N filtered HSQC spectrum) at 10 ℃ in 5% D2O, 70 mmol/LKCl, 20 mmol/L potassium phosphate buffer with pH 7.0. The 1D 1H NMR reference spectrum is on the top |
As the labeled RNA phosphoramidites are not available now, the site-specific low enrichment cannot be applied in RNA G-quadruplexes. However, site-specific RNA-to-DNA substitution is a good alternative method for spectral assignment[16, 17].
4.4 Natural abundance base substitutionsDirect assignments can be obtained through residue-specific substitution such as G-to-I, G-to-BrG and T-to-dU based on the disappearance or appearance of characteristic cross peaks in these residues[18]. In addition, the aromatic H8 of guanosine substituted for Br can be used to aid in the assignments of suspected syn residues. A substitution of 8-BrdG to a syn Guanine can also be used to test whether this syn residue exists in the corresponding wild type fold[19].
5 Topology determinationAfter the unambiguous assignments are obtained based on the NOE-based approach, through-bond correlated NMR experiments and site-specific low enrichment, the folding topology of G-quaduplex can be readily determined from specific NOE patterns of the G-tetrad core. Within a G-tetrad core, the cyclic NOE connectivities between the imino (H1) proton of a guanine and the H8 proton of the next guanine are used for establishing the G-tetrad layer (Fig. 6). These characteristic NOEs are often easily detected in a well-resolved area of the NOESY spectra with the mixing times of 200 ms, 250 ms or 300 ms. Commonly, the direct distance between these protons is ~0.47 nm. The NOEs between imino protons of neighboring guanines within the same G-tetrad layer can also be observed if these protons are well-resolved as the distance between the imino protons of the two neighboring guanines is ~0.44 nm. But in many cases these cross-peaks of imino protons are too close to the diagonal to be discriminated. The short distances between stacked G-tetrad layers can also be observed. There are two ways that two G-tetrad layers can stack on each other: same-polarity or opposite-polarity. For the latter, two main different stacking modes correspond to the syn-anti and anti-syn connection, respectively.
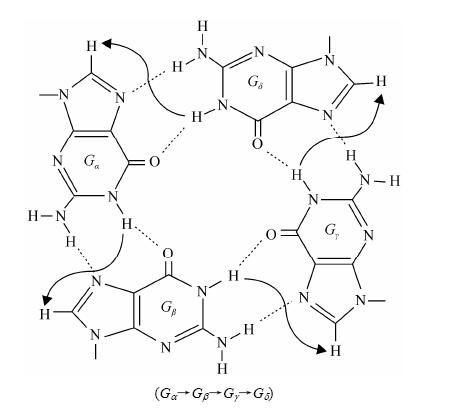 |
| Fig. 6 Characteristic guanine imino-H8 NOE connectivity patterns around a (Ga→Gb→Gg→Gd) tetrad as indicated with arrows |
High-resolution NMR structure of G-quadruplexes can be calculated based on experimentally observed NMR data. A regular protocol of structure calculation generally consists of three steps:
(i) generation of a pool of structures (simulated annealing or distance geometry protocol coupled with simulated annealing);
(ii) refinement procedure (restrained molecular dynamics refinement or a relaxation matrix density refinement);
(iii) selection of ‘‘accepted’’ structures based on energy, number of violations and convergence (RMSD).
The experimental restraints are applied during structure calculation including NOE-derived proton-proton distance, hydrogen-bond, dihedral angle and artificial restraints such as planarity (for residues involved in G-tetrad core or base pairing).
Usually several NOESY spectra with four to five mixing times ranging from 50 to 300 ms are collected. Distances are estimated from the initial build up rates of NOE curves by the approximation:
where, rij is the distance between protons i and j, rref is a reference distance, and Rij and Rref are the initial buildup rates. The interproton distances are generally estimated from the distance between CH6-CH5, (rref = 0.245 nm), H1'-H2" (rref = 0.220 nm), or from TH5-TH7 (rref = 0.299 nm) with the boundaries of ±20% up to ±30% (which include the experimental error, the spin-diffusion and the local dynamics). For exchangeable protons, averaged distances are calculated from the classification of peak volumes as weak (observed only at 200 or 300 ms mixing time), medium (weak intensity at 50 or 75 ms mixing time), and strong (medium to strong intensity at 50 or 75 ms mixing time) with approximate boundaries of ±30% to ±40%. Hydrogen-bond restraints are translated into distances restraints between the donor and the acceptor with a ~3% margin. Glycosidic dihedral angles, are restrained in anti or syn conformation, based on the intensity of the observed intraresidue NOEs between H6/H8 and H1' in NOESY spectrum with short mixing time such as 50 or 75 ms. The backbone dihedral angles (e ) can be restrained based on reported data[20, 21].
For the structure calculation of G-quadruplexes, the most critical parameters are the energy weight of NOE constraints, the cooling procedure of the simulated annealing protocol, and the length of the molecular dynamics simulation[22]. During the structure calculation, two distinct parts of a G-quadruplex can be considered: the core and the loops. The structure of the G-tetrad core usually can be well defined and the resulting RMSD (heavy atoms) of the G-quadruplex core between converged structures is generally less than 0.1 nm. The loops depending on the sequence which may contain zero, one, or several residues are usually more difficult to fold than the core. One of the possible reasons is that the residues in the loop are more flexible than the guanines in the G-tetrad core. If the hydrogen-bond restraints can be observed in the loops, this will be very helpful in converging the structures for the loops. But it is rather difficult to get information about the orientation of the bases as the labile and exposed protons cannot be detected by NMR.
7 RNA G-quadruplexRecent studies[23, 24, 25] showed that mammalian telomeres were transcribed by DNA-dependent RNA polymerase II. As these RNA sequences are rich in guanines, formation of G-quadruplex structures by these telomeric repeat-containing RNAs (TERRA) has been demonstrated. In contrast to the conformational diversity of telomeric DNA G-quadruplexes, TERRA was found to form only parallel-stranded G-quadruplexes[26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38]. However, so far in contrast to the DNA G-quadruplexes, few structural studies on RNA G-quadruplexes have been performed and knowledge about structural diversity or conservation of RNA G-quadruplexes is limited.
8 G-quadruplex-binding proteinsRecently, many proteins binding to G-quadruplexes have been identified. The human telomeric sequence d[(TTAGGG)n], is recognized by many proteins which can modulate telomerase activity such as shelterin, a complex composed of six proteins including POT1 (protection of telomeres 1), TRF1 and TRF2 (telomere repeat binding factor 1 and 2), TPP1, TIN2 (TRF1 interacting protein 2) and RAP1 (repressor activation protein 1)[39, 40, 41, 42, 43, 44, 45, 46, 47]. Sequences with the potential to form G-quadruplexes are often located in promoter regions of various oncogenes. The NHE III1 region of the c-MYC promoter has been shown to interact with nucleolin protein as well as other G-quadruplex-binding proteins, e.g., nucleolin, nucleophosmin, BRCA1 tumor suppressor, CNBP (cellular nucleic acid-binding protein), MAZ (myc-associated zinc-finger) protein and hnRNP A1[48, 49, 50, 51, 52, 53, 54]. In addition to DNA quadruplexes, the RNA quadruplex formation in telomere-repeat-containing RNA is also involved in interaction with TRF2 (telomere repeat binding factor 2) and plays key role in telomere regulation[43]. These examples suggest the importance of quadruplex structures in cell processes.
9 G-quadruplex-binding ligandsA wide range of ligands has been reported and a number of structure-activity relationships derived for small molecule binding to quadruplexes[55]. The ligands possess several common features, notably planar aromatic chromophores and positively charged side chains. Binding of ligands to quadruplexes can be straightforwardly assessed by NMR solution studies. When a ligand binds to a G-quadruplex, a titration of the ligand into the G-quadruplex can result in a broadening and/or shift of the G-quadruplex protons which are involved in or neighbored to the binding site[56]. In most cases, the interaction modes between the G-quadruplexes and ligands are mainly defined by the observation of intermolecular NOEs[57]. Another widely used technique for studying interaction between a G-quadruplex and a ligand is intermolecular saturation transfer in STD NMR experiment[58, 59, 60].
10 In-cell NMR study of G-quadruplexIn cell, biological macromolecules act in a crowded intracellular environment containing a variety of macromolecules such as nucleic acids, proteins, polysaccharides, and metabolites that occupy 30-40% of internal cellular volume[61]. Many in vitro studies indicated that factors modulating G-quadruplex structures include sequence composition, the presence of the type of counterions, and the degree of macromolecular crowding. The applications of in-cell NMR in X. laevis oocytes and targeted delivery of isotope labeled proteins by microinjection spurred in-cell NMR studies of G-quadruplex in molecular crowding enviroment[62, 63, 64]. Briefly, the synthesized isotope-labeled nucleic acids were injected into Xenopus oocytes and 1D proton or 2D 1H-15N/13C correlation experiments were recorded over ~20 h. The human telomeric repeat sequence d[GGG(TTAGGGG)3T] was the first G-quadruplex structure to be investigated by in-cell NMR[62]. Whereas the 1D 1H spectrum of d[GGG(TTAGGGG)3T] in vitro indicated the presence of the two-tetrad antiparallel basket-type G-quadruplex structure, the spectrum of in-cell NMR gave a strikingly different imino proton envelope in intact Xenopus oocytes suggesting alternative conformations inside cells. Upon oocyte lysis, mixed populations of basket- and nonbasket-type structures were detected. A similar study in crowding agents polyethylene glycol (PEG) and Ficoll showed that the structural properties of d[TAGGG(TTAGGG)3], TA-core, d[AGGG(TTAGGG)3TT] and A-core-TT G-quadruplex, were substantially different from those in Xenopus cytostatic factor egg extracts[63]. To increase spectral resolution under crowding conditions, 2D 1H-15N correlation NMR experiments and 15N, 13C isotope-labeled d[GGG(TTAGGGG)3T] were employed and demonstrated that in Xenopus cytostatic factor extracts d[GGG(TTAGGGG)3T] exhibited the hybrid-1 topology, which is prominently populated in dilute potassium solutions[64]. Recently, the SOFAST-HMQC experiment was used to probe the G-quadruplex structures established by d[(TGGGGT)4] inside living X. laevis oocytes. The results indicated that in intracellular environment d[(TGGGGT)4] preferentially forms a G-quadruplex conformation, the one found in vitro under KCl conditions[65]. The interaction between the ligand and d[(TGGGGT)4] G-quadruplex was also directly observed through 1H-15N SOFAST-HMQC experiment inside living cells. While in-cell NMR spectroscopy in intact X. laevis oocytes and egg extracts provides unprecedented insight into the structural behavior of G-quadruplexes under molecular crowding conditions, this technique has several inherent limitations such as limited to X. laevis oocytes and eggs at the moment, low resolution and sensitivity, primarily reports on local structure and “blind” to long-order changes. Undoubtedly, in-cell NMR spectroscopy of G-quadruplex has a considerable application potential. Especially, a combination of in-cell single particle FRET techniques with in-cell NMR will provide a very powerful suite of tools for solving very complex and so far unapproachable problems in G-quadruplex studies.
11 ConclusionSolution-state NMR is a powerful technique in uncovering the conformations of G-quadruplex. Complete assignments can be obtained by using the NOE-based and through-bond correlated approach routinely. In combination with isotopic labeling techniques, STD and recently developed RDC as well as PRE experiments, high-resolution structures of G-quadruplexes or bound to ligands and proteins will be yielded. The powerful NMR technique for rapid characterization of G-quadruplexes will benefit the functional study in this field.
| [1] | Adrian M, Heddi B, Phan A T. NMR spectroscopy of G-quadruplexes[J]. Methods. 2012, 57(1):11-24. |
| [2] | Zhu G, Bax A. Improved linear prediction for truncated signals of known phase[J]. J Magn Reson, 1990, 90(2):405-410. |
| [3] | Delaglio F, Grzesiek S, Vuister G W, et al. NMRPipe:a multidimensional spectral processing system based on UNIX pipes[J]. J Biomol NMR, 1995, 6(3):277-293. |
| [4] | Vorlickova M, Kejnovska I, Sagi J, et al. Circular dichroism and guanine quadruplexes[J]. Methods, 2012, 57(1):64-75. |
| [5] | Randazzo A, Spada G P, Da S M. Circular dichroism of quadruplex structures[J]. Top Curr Chem, 2013, 330:67-86. |
| [6] | Karsisiotis A I, Hessari N M, Novellino E, et al. Topological characterization of nucleic acid G-quadruplexes by UV absorption and circular dichroism[J]. Angew Chem Int Ed Engl, 2011, 50(45):10 645-10 648. |
| [7] | Paramasivan S, Rujan I, Bolton P H. Circular dichroism of quadruplex DNAs:applications to structure, cation effects and ligand binding[J]. Methods, 2007, 43(4):324-331. |
| [8] | Feigon J, Koshlap K M, Smith F W. 1H NMR spectroscopy of DNA triplexes and quadruplexes[J]. Methods Enzymol, 1995, 261:225-255. |
| [9] | Patel D J, Tonelli A E. Assignment of the proton Nmr chemical shifts of the T-N3H and G-N1H proton resonances in isolated AT and GC Watson-Crick base pairs in double-stranded deoxy oligonucleotides in aqueous solution[J]. Biopolymers, 1974, 13(10):1 943-1 964. |
| [10] | Wuthrich K. NMR of Proteins and Nucleic Acid[M]. New York:John Wiley & Sons, 1986. |
| [11] | Wijmenga S S, Mooren M W, Hilbers C W. NMR of Nucleic Acids:from Spectrum to Structure[M]. Oxford:Oxford University Press, 1993. |
| [12] | Wijmenga S S, van Buuren B N M. The use of NMR methods for conformational studies of nucleic acids[J]. Prog Nucl Magn Reson Spectrosc, 1998, 32(4):287-387. |
| [13] | Wang Y, Patel D J. Solution structure of the Oxytricha telomeric repeat d[G4(T4G4)3] G-tetraplex[J]. J Mol Biol, 1995, 251(1):76-94. |
| [14] | Phan A T. Long-range imino proton-13C J-couplings and the through-bond correlation of imino and non-exchangeable protons in unlabeled DNA[J]. J Biomol NMR, 2000, 16(2):175-178. |
| [15] | Phan A T, Patel D J. A site-specific low-enrichment 15N, 13C isotope-labeling approach to unambiguous NMR spectral assignments in nucleic acids[J]. J Am Chem Soc, 2002, 124(7):1 160-1 161. |
| [16] | Martadinata H, Phan A T. Structure of propeller-type parallel-stranded RNA G-quadruplexes, formed by human telomeric RNA sequences in K+ solution[J]. J Am Chem Soc, 2009, 131(7):2 570-2 578. |
| [17] | Phan A T, Kuryavyi V, Darnell J C, et al. Structure-function studies of FMRP RGG peptide recognition of an RNA duplex-quadruplex junction[J]. Nat Struct Mol Biol, 2011, 18(7):796-804. |
| [18] | Phan A T, Gueron M, Leroy J L. Investigation of unusual DNA motifs[J]. Methods Enzymol, 2001, 338:341-371. |
| [19] | Dias E, Battiste J L, Williamson J R. Chemical probe for glycosidic conformation in telomeric DNAs[J]. J Am Chem Soc, 1994, 116(10):4 479-4 480. |
| [20] | Clowney L, Jain S C, Srinivasan A R, et al. Geometric parameters in nucleic acids:nitrogenous bases[J]. J Am Chem Soc, 1996, 118(3):509-518. |
| [21] | Gelbin A, Schneider B, Clowney L, et al. Geometric parameters in nucleic acids:sugar and phosphate constituents[J]. J Am Chem Soc, 1996, 118(3):519-529. |
| [22] | Foloppe N, MacKerell A D Jr. All-atom empirical force field for nucleic acids:I. Parameter optimization based on small molecule and condensed phase macromolecular target data[J]. J Comput Chem, 2000, 21(2):86-104. |
| [23] | Schoeftner S, Blasco M A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II[J]. Nat Cell Biol, 2008, 10(2):228-236. |
| [24] | Azzalin C M, Reichenbach P, Khoriauli L, et al. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends[J]. Science, 2007, 318(5 851):798-801. |
| [25] | Horard B, Gilson E. Telomeric RNA enters the game[J]. Nat Cell Biol, 2008, 10(2):113-115. |
| [26] | Joachimi A, Benz A, Hartig J S. A comparison of DNA and RNA quadruplex structures and stabilities[J]. Bioorg Med Chem, 2009, 17(19):6 811-6 815. |
| [27] | Collie G W, Haider S M, Neidle S, et al. A crystallographic and modelling study of a human telomeric RNA (TERRA) quadruplex[J]. Nucleic Acids Res, 2010, 38(16):5 569-5 580. |
| [28] | Xu Y, Ishizuka T, Kimura T, et al. A U-tetrad stabilizes human telomeric RNA G-quadruplex structure[J]. J Am Chem Soc,2010, 132(21):7 231-7 233. |
| [29] | Arora A, Maiti S. Differential biophysical behavior of human telomeric RNA and DNA quadruplex[J]. J Phys Chem B, 2009, 113(30):10 515-10 520. |
| [30] | Collie G W, Parkinson G N, Neidle S, et al. Electrospray mass spectrometry of telomeric RNA (TERRA) reveals the formation of stable multimeric G-quadruplex structures[J]. J Am Chem Soc, 2010, 132(27):9 328-9 334. |
| [31] | Xu Y, Kaminaga K, Komiyama M. G-quadruplex formation by human telomeric repeats-containing RNA in Na+ solution[J]. J Am Chem Soc, 2008, 130(33):11 179-11 184. |
| [32] | Phan A T. Human telomeric G-quadruplex:structures of DNA and RNA sequences[J]. FEBS J, 2010, 277(5):1 107- 1 117. |
| [33] | Zhang D H, Fujimoto T, Saxena S, et al. Monomorphic RNA G-quadruplex and polymorphic DNA G-quadruplex structures responding to cellular environmental factors[J]. Biochemistry, 2010, 49(21):4 554-4 563. |
| [34] | Collie G W, Sparapani S, Parkinson G N, et al. Structural basis of telomeric RNA quadruplex——acridine ligand recognition[J]. J Am Chem Soc, 2011, 133(8):2 721-2 728. |
| [35] | Martadinata H, Heddi B, Lim K W, et al. Structure of long human telomeric RNA (TERRA):G-quadruplexes formed by four and eight UUAGGG repeats are stable building blocks[J]. Biochemistry, 2011, 50(29):6 455-6 461. |
| [36] | Randall A, Griffith J D. Structure of long telomeric RNA transcripts:the G-rich RNA forms a compact repeating structure containing G-quartets[J]. J Biol Chem, 2009, 284(21):13 980-13 986. |
| [37] | Martadinata H, Phan A T. Structure of propeller-type parallel-stranded RNA G-quadruplexes, formed by human telomeric RNA sequences in K+ solution[J]. J Am Chem Soc, 2009, 131(7):2 570-2 578. |
| [38] | Xu Y, Suzuki Y, Ito K, et al. Telomeric repeat-containing RNA structure in living cells[J]. Proc Natl Acad Sci USA, 2010, 107(33):14 579-14 584. |
| [39] | Biffi G, Tannahill D, Balasubramanian S. An intramolecular G-quadruplex structure is required for binding of telomeric repeat-containing RNA to the telomeric protein TRF2[J]. J Am Chem Soc, 2012, 134(29):11 974-11 976. |
| [40] | Safa L, Delagoutte E, Petruseva I, et al. Binding polarity of RPA to telomeric sequences and influence of G-quadruplex stability[J]. Biochimie, 2014, 103:80-88. |
| [41] | Ray S, Bandaria J N, Qureshi M H, et al. G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding[J]. Proc Natl Acad Sci USA, 2014, 111(8):2 990-2 995. |
| [42] | Zaug A J, Podell E R, Cech T R. Human POT1 disrupts telomeric G-quadruplexes allowing telomerase extension in vitro[J]. Proc Natl Acad Sci USA, 2005, 102(31):10 864-10 869. |
| [43] | Kelleher C, Kurth I, Lingner J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro[J]. Mol Cell Biol, 2005, 25(2):808-818. |
| [44] | Ye J Z, Hockemeyer D, Krutchinsky A N, et al. POT1-interacting protein PIP1:a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex[J]. Genes Dev, 2004, 18(14):1 649-1 654. |
| [45] | Hwang H, Buncher N, Opresko P L, et al. POT1-TPP1 regulates telomeric overhang structural dynamics[J]. Structure, 2012, 20(11):1 872-1 880. |
| [46] | Denchi E L, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1[J]. Nature, 2007, 448(7 157):1 068-1 071. |
| [47] | Wang F, Podell E R, Zaug A J, et al. The POT1-TPP1 telomere complex is a telomerase processivity factor[J]. Nature, 2007, 445(7 127):506-510. |
| [48] | Xiong J, Fan S, Meng Q, et al. BRCA1 inhibition of telomerase activity in cultured cells[J]. Mol Cell Biol, 2003, 23(23):8 668-8 690. |
| [49] | Ballal R D, Saha T, Fan S, et al. BRCA1 localization to the telomere and its loss from the telomere in response to DNA damage[J]. J Biol Chem, 2009, 284(52):36 083-36 098. |
| [50] | Scognamiglio P L, Di Natale C, Leone M, et al. G-quadruplex DNA recognition by nucleophosmin:new insights from protein dissection[J]. Biochim Biophys Acta, 2014, 1 840(6):2 050-2 059. |
| [51] | Gonzalez V, Guo K, Hurley L, et al. Identification and characterization of nucleolin as a c-myc G-quadruplex-binding protein[J]. J Biol Chem, 2009, 284(35):23 622-23 635. |
| [52] | Cogoi S, Zorzet S, Rapozzi V, et al. MAZ-binding G4-decoy with locked nucleic acid and twisted intercalating nucleic acid modifications suppresses KRAS in pancreatic cancer cells and delays tumor growth in mice[J]. Nucleic Acids Res, 2013, 41(7):4 049-4 064. |
| [53] | Paramasivam M, Membrino A, Cogoi S, et al. Protein hnRNP A1 and its derivative Up1 unfold quadruplex DNA in the human KRAS promoter:implications for transcription[J]. Nucleic Acids Res, 2009, 37(9):2 841-2 853. |
| [54] | Cogoi S, Paramasivam M, Membrino A, et al. The KRAS promoter responds to Myc-associated zinc finger and poly(ADP-ribose) polymerase 1 proteins, which recognize a critical quadruplex-forming GA-element[J]. J Biol Chem, 2010, 285(29):22 003-22 016. |
| [55] | De Cian A, Gros J, Guedin A, et al. DNA and RNA quadruplex ligands[J]. Nucleic Acids Symp Ser (Oxf), 2008, (52):7- 8. |
| [56] | Phan A T, Kuryavyi V, Gaw H Y, et al. Small-molecule interaction with a five-guanine-tract G-quadruplex structure from the human MYC promoter[J]. Nat Chem Biol, 2005, 1(3):167-173. |
| [57] | Dai J, Carver M, Hurley L H, et al. Solution structure of a 2:1 quindoline-c-MYC G-quadruplex:insights into G-quadruplex-interactive small molecule drug design[J]. J Am Chem Soc, 2011, 133(44):17 673-17 680. |
| [58] | Mayer M, Meyer B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy[J]. Angew Chem Int Ed Engl, 1999, 28(12):1 784-1 788. |
| [59] | Di Micco S, Bassarello C, Bifulco G, et al. Differential-frequency saturation transfer difference NMR spectroscopy allows the detection of different ligand-DNA binding modes[J]. Angew Chem Int Ed Engl, 2005, 45(2):224-228. |
| [60] | Martino L, Virno A, Pagano B, et al. Structural and thermodynamic studies of the interaction of distamycin A with the parallel quadruplex structure [d(TGGGGT)]4[J]. J Am Chem Soc, 2007, 129(51):16 048-16 056. |
| [61] | Zimmerman S B, Trach S O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli[J]. J Mol Biol, 1991, 222(3):599-620. |
| [62] | Hansel R, Foldynova-Trantirkova S, Lohr F, et al. Evaluation of parameters critical for observing nucleic acids inside living Xenopus laevis oocytes by in-cell NMR spectroscopy[J]. J Am Chem Soc, 2009, 131(43):15 761-15 768. |
| [63] | Hansel R, Lohr F, Foldynova-Trantirkova S, et al. The parallel G-quadruplex structure of vertebrate telomeric repeat sequences is not the preferred folding topology under physiological conditions[J]. Nucleic Acids Res, 2011, 39(13):5 768 -5 775. |
| [64] | Hansel R, Lohr F, Trantirek L, et al. High-resolution insight into G-overhang architecture[J]. J Am Chem Soc, 2013, 135(7):2 816-2 824. |
| [65] | Salgado G F, Cazenave C, Kerkour A, et al. G-quadruplex DNA and ligand interaction in living cells using NMR spectroscopy[J]. Chem Sci, 2015. DOI:10.1039/C4SC03853C |
 2015, Vol. 32
2015, Vol. 32




