Article Information
- 张岩, 赵玢, 杨中正, 申杰, 胡伟, 蓝文贤, 吴厚铭, 曹春阳
- ZHANG Yan, ZHAO Bin, YANG Zhong-zheng, SHEN Jie, HU Wei, LAN Wen-xian, WU Hou-ming, CAO Chun-yang
- 原核表达过程中非目标蛋白质识别与确认的方法:NRSF/REST蛋白功能结构域ZnF2-8原核表达过程中β-内酰胺酶的确认
- Methods for Identification and Characterization of Protein Unexpectedly Expressed inEscherichia Coli: A Case Study Involving β-Lactamase Observed during the Expression of Zinc Finger 2-8 of NRSF/REST
- 波谱学杂志, 2015, 32(1): 1-11
- Chinese Journal of Magnetic Resonance, 2015, 32(1): 1-11
- http://dx.doi.org/10.11938/cjmr20150101
-
Article History
Received date: 2014-02-11
Revised date: 2015-01-08


DOI:10.11938/cjmr20150101
Low cost production of proteins in heterologous hosts is an extensive technique in modern protein science including structural genomics and proteomics. To obtain engineered proteins utilized in medical, industrial or research areas, high-throughput methods for cloning, protein expression, and purification have been developed[1-9], and even commercialized[10-12]. However, in many cases, the target proteins are not generated as we anticipated, or the non-predicted proteins are unexpectedly overexpressed in Escherichia coli(E. coli). Especially, due to the fact that the molecular weights of non-predicted proteins are close to those of the target proteins, they could not be easily identified directly by running SDS-PAGE gel electrophoresis, so that all designed purification steps for target proteins have to be performed. Sometimes, the non-target proteins are also very useful. In those cases, it’s necessary to know how to identify non-predicted proteins. In this paper, we developed a complete method of how to identify and to characterize unintended proteins overexpressed in E. coli.
In the CNS, the neuron-restricted silencer factor/RE1-silencing transcription factor (NRSF/REST) functions as a key transcriptional repressor for neuron-specific genes in the non-neuronal cells by interaction with neuron-restricted silencer element (NRSE/RE1) dsDNA[13]. However, it was recently reported that NRSF/REST could also work as a neuron-specific gene transcriptional activator through interactions with a small non-coding NRSE/RE1 dsRNA[14-17]. NRSF/REST is a krüppel family zinc finger (ZnF) protein, containing nine ZnF motifs, one lys-rich region, one pro-rich region and two repression domains at its C-and N-termini, respectively (supplementary Fig.S1). To study the molecular mechanisms involved in the biological interactions mentioned above, it’s necessary to obtain ZnF motifs in tandem of NRSF/REST. Currently, except NRSF/REST functional ZnF2-8 motif, we successfully got successive ZnF motifs such as ZnF6-8, ZnF5-8, ZnF4-8 and ZnF3-8 in a large scale through a pET15b vector (Novogen) (supplementary Fig.S1)[18]. During the overexpression of NRSF/REST ZnF2-8 with the same vector, β-lactamase was surprisingly identified, and further characterized by two-dimensional (2D) NMR 1H-15N HSQC spectroscopy, X-ray crystallography and other biochemical assays.

|
| Fig. S1 Schematic diagrams of NRSF/REST and its fragments constructs mentioned in the paper, boxes indicating repression domains (RD1 and RD2 in both N and C termini) or pro-rich domain, standing ellipses indicating zinc fingers, lying ellipse indicating lys-rich region. The residues included in the fragments are: amino acids (aa) 300~380 for ZnF5-7, aa 300~407 for ZnF5-8, aa 271~407 for ZnF4-8, aa 243~407 for ZnF3-8 and aa 211~407 for ZnF2-8, respectively |
The NRSF/REST functional ZnF2-8 motif from mouse NRSF/REST was subcloned into a pET15b vector (Novogen) as previously described[18], and transformed into Rosetta (DE3) cells. The unlabeled protein was over-expressed in 1 liter of Luria-Bertani (LB) medium. The isotope 15N-labeled protein was produced in 1 liter of M9 minimal medium, in which isotope labeled 15NH4Cl ( > 99% 15N, CIL Inc.) and unlabeled glucose are the only sources of nitrogen and carbon. The cells were grown at 37℃. When the absorbance at 600 nm (OD600) reached 0.7~0.8, a final concentration of 0.1 mmol/L isopropyl-β-D-thiogalactopyranoside (IPTG) was used to induce the expression at 18℃ for another 18 h [Fig. 1(a)], then the cells were harvested at 6 000 g at 4℃ for 15 min, resuspended in buffer A containing 10 mmol/L Tris, pH 8.0, and lysed at highpressure. Cell debris was removed by centrifugation. The supernatant was loaded onto a Macro-prep High Q column (Bio-Rad) previously equilibrated with buffer A. The flow-through fractions were collected and loaded directly onto a DEAE column (Bio-Rad), then eluted with a linear gradient of 0~0.1 mol/L NaCl at a flow rate of 1 mL per minute. The fractions were collected, concentrated and loaded onto a HiLoad 16/60 Superdex 75 column (GE Healthcare) and then eluted with buffer A. Based on the results from the mass-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy and the SDS-PAGE gel electrophoresis [Fig. 1(b)], the identity of the protein was suggested not to be the predicted NRSF/REST functional ZnF2-8 motif. As we published before, NRSF/REST ZnF2-8 could be overexpressed through a home-modified pET15b vector containing a SUMO-tag [Fig. 1(c)][18]. For the sake of discussion, we temporarily named it as non-target protein, hereinafter.

|
| Fig. 1 Characterization of unexpectedly overexpressed protein by running SDS-PAGE gel. (a) β-lactamase unexpectedly overexpressed; lane 1, cells before IPTG induction; lane 2, total cells after IPTG induction for native ZnF2-8 overexpression; lane 3, supernatant after the cells (in lane 2) sonication; lane 4, total cells after IPTG induction for ZnF2-8 C216R mutant overexpression; lane 5, supernatant after the cells (in lane 4) sonication. (b) Purified β-lactamase (in lane 1). (c) Purified NRSF/REST ZnF2-8 (in lane 1), overexpressed by a modified pET5 vector containing SUMO-tag, as published in reference[18]. In each gel, the arrow directions indicated the molecular weights of the expressed proteins, and lane M means protein standard marker |
To investigate whether or not this non-target protein has DNA binding affinities as other NRSF/REST functional ZnF motifs (for example, ZnF4-8 and ZnF5-8) do[18], a native DNA gel shift assay was performed[18]. Each strand of NRSE/RE1 dsDNA was commercially synthesized at HPLC grade (Shanghai Sangon Biological Engineering Technology and Service Co. Ltd, China) containing the following sequences: 5'-TTC AGC ACC ACG GAC AGC GCC-3' and 5'-GGC GCT GTC CGT GGT GCT GAA-3'[17]. To form dsDNA, the equimolar mixture of these two strands was annealed by boiling for 5 min, followed by slowly cooling for 30 min at room temperature. The DNA binding assay was conducted using a fixed concentration of 75 pmol of annealed NRSE/RE1 dsDNA. The protein was added into DNA solution at different molar ratios of protein vs. NRSE/RE1 dsDNA (0:1, 1:1, 2:1, 3:1 and 4:1, respectively, as indicated from left to right in each lane of Fig. 2). The protein solution in the absence of NRSE/RE1 dsDNA was used as a control (i.e., the molar ratio of protein vs. dsDNA is equal to 1:0, as indicated in Fig. 2).
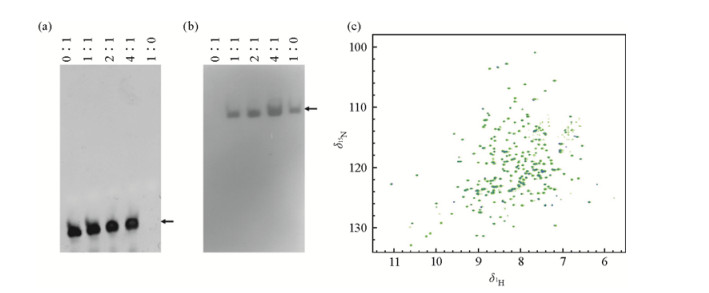
|
| Fig. 2 Investigation on the interaction between non-target protein and NRSE/RE1 dsDNA by (a, b) native DNA gel shift assay, or (c) by 2D NMR 1H-15N HSQC titration assay. (a) The gel was stained by EtBr to indicate the position of free NRSE/RE1 dsDNA. (b) The gel was stained by coomassie blue to point out the position of the non-target protein. On the top of the gel, the mole ratios of protein vs. DNA were indicated. (c) Overlay of 2D NMR 1H-15N HSQC spectra of non-target protein in the absence of (derk grey) and in the presence of NRSE/RE1 dsDNA (light grey) |
The mixed solution was incubated for 30 min at room temperature in the final volume of 20 μL in the buffer of 10 mmol/L Tris, pH 7.0, 0.1 mmol/L ZnCl2. Then the native DNA PAGE gels (5% polyacrylamide contained) were run, and further stained with ethidium bromide (EtBr) [Fig. 2(a)] or coomassie blue [Fig. 2(b)] to indicate the interaction results. No gel shifts were observed in either case, implying that the expressed protein does not interact with NRSE/RE1 dsDNA.
1.3 2D NMR 1H-15N HSQC binding assayTo confirm that this non-target protein does not bind to NRSE/RE1 dsDNA, 2D NMR 1H-15N HSQC spectra were acquired at 20℃ on its 15N labeled sample in the absence of and in the presence of NRSE/RE1 dsDNA (at the molar ratio of 1:1) [Fig. 2(c)]. The NMR sample was concentrated to about 0.5 mmol/L in NMR buffer (20 mmol/L Tris-d6, 100 mmol/L NaCl, 1 mmol/L ZnCl2, 1 mmol/L NaN3, 10% D2O, pH 7.0), which was measured by using its absorbance at 280nm (A280) and extinction coefficient value. The NMR experiments were run on a Varian Unity Inova 600 spectrometer (with cryo-probe) equipped with three channels and pulse-field gradient. In both experiments, the sweeping width in 1H direct dimension is 14 centered at δ4.82 and that in 15N indirect dimension is 40 centered at δ118. The 1H dimension was collected with 1 024 complex points, while the 15N dimension was collected with 512 complex points. The scanning number in both cases was set as 64.
1.4 Electrospray ionization mass spectrometric peptide mapping assayTo obtain the right amino acid sequence of the non-target protein, the electrospray ionization mass spectrometric peptide mapping assay was performed as described before[19], by Shanghai Applied Protein Technology Co. Ltd. The amino sequences of several fragments from the non-target protein with matched calculated molecular weights and the observed m/z values were given in Table 1. Then, we analyzed these sequences by blasting them in the protein sequence databases about three different organisms (EST_mouse, E. coli and Mus_musculus) in NCBI website (http://www.ncbi.nlm.nih.gov/protein/). Based on these analyses, the non-target protein was suggested to be β-lactamase.
| Calc. Mass | Obs. Mass | Sequence |
| 1087.6293 | 1087.6277 | VLLCGAVLSR |
| 1275.6732 | 1275.7086 | SALPAGWFIADK |
| 1286.7678 | 1286.7737 | LLTGELLTLASR |
| 1351.7693 | 1351.7961 | GIIAALGPDGKPSR |
| 1740.8486 | 1740.8914 | ELTAFLHNMGDHVTR |
| 1783.8895 | 1783.9344 | IVVIYTTGSQATMDER |
| 2083.1006 | 2083.147 | QQLIDWMEADKVAGPLLR |
| 2096.0044 | 2096.199 | LDRWEPELNEAIPNDER |
In the meanwhile, initial crystallization tests of non-target protein were carried out with crystal screen kit, grid screen kit, natrix kit, and index kit (Hampton) in 96-well plates. The crystals were finally obtained by the sitting-drop through mixing the non-target protein (~10 mg/mL) with the same volume of reservoir solution containing 0.2 mol/L MgCl2, 0.1 mol/L Bis-Tris pH 6.5, 25% PEG 3 350 (w/v). Crystals were equilibrated in a cryo-protectant buffer containing reservoir buffer plus 4% glycerol (v/v) and were flash-frozen in liquid nitrogen. X-ray diffraction data were collected on the beamline 3W1A of Beijing Synchrotron Radiation Facility (BSRF, China) using a MAR CCD M-165 detector. The wavelength of the radiation was 0.126 nm. The data were indexed, integrated and scaled using the software HKL-2000 suite[20]. The structure was determined by molecular replacement with the program PHASER in the CCP4 suite. The atomic coordinates of TEM1 β-lactamase (PDB code: 1ERO) were used as the initial search model. After the model was automatically built by using ARP/wARP classic, the remaining peptides and waters were introduced manually using the program Coot[21]. The final model was refined against the high-resolution native data by using PHENIX[22]. The figures were generated using Pymol (http://www.pymol.org) (Fig. 3). The statistics of the structure refinement and the quality of the final structural model were summarized in Table 2.
| Data collection | |
| Wavelength/(0.1 nm) | 1.26 |
| Unit cell/(0.1 nm) | a=47.0, b=72.8, c=72.7 |
| Space group | P212121 |
| Resolution/(0.1 nm) | 50 -1.91 (1.98~1.91) |
| Unique reflection | 37743 |
| Completeness | 98.1 (89.3) |
| Rmergea | 0.087 (0.425) |
| Data redundancy | 13.1 (10.9) |
| Average I/σ | 43.8 (7.4) |
| Structure refinement | |
| Resolution/(0.1 nm) | 34.9 -1.91 (1.98~1.91) |
| Rcryst / Rfree(%)b | 16.3 (23.3) / 19.7 (29.6) |
| No. of reflection working | 36758 |
| No. of reflection test set | 3600 |
| Total No. of atoms | 2026 / 315 protein/water |
| Completeness of data | 96.3 (87.0) |
| R. M. S. D bond length/(0.1 nm) | 0.007 |
| R. M. S. D bond angle( ) | 1.016 |
| Ramachandran plot | |
| most favored regions (%) | 92.5 |
| additionally allowed (%) | 7.0 |
| generously allowed (%) | 0.4 |
| disallowed (%) | 0.0 |
| Numbers in parentheses represent the value for the highest resolution shell. a. Rmerge=Σ|Ii -Im|/ΣIi, where Ii is the intensity of the measured reflection and Im is the mean intensity of all symmetry related reflections. b. Rcryst=Σ||Fobs| -|Fcalc||/Σ|Fobs|, where Fobs and Fcalc are observed and calculated structure factors; Rfree=ΣT||Fobs|-|Fcalc||/ΣT|Fobs|, where T is a test data set of about 5% of the total reflections randomly chosen and set aside prior to refinement | |
To investigate whether or not NRSF/REST functional ZnF-2 motif enhances β-lactamase overexpression in E. coli, the mutation from Cys216 to Arg216 in ZnF-2 of NRSF/REST ZnF2-8 was carried out with a QuikChange site-directed mutagenesis kit (Stratagene Inc). In ZnF-2, Cys216 is one of residues bound to zinc ion. The plasmid was transformed into Rosetta (DE3) cells. The mutant was overexpressed in 1 liter of LB medium. The expression of the mutant protein was examined by running SDS-PAGE gel electrophoresis (Fig. 1), implying that β-lactamase overexpression was still induced by 0.1 mmol/L IPTG during the expression of NRSF/REST ZnF2-8 C216R mutant.
2 Results and discussion 2.1 A brief description of the method to analyze unexpectedly expressed proteinThe first step is to run SDS-PAGE gel electrophoresis after the protein expression is completed by IPTG induction, to indicate whether or not the molecular weight of the expressed protein is close to that of the target protein. Sometimes for some unknown reasons, although the molecular weight of the produced protein is not similar to that of the target protein, SDS-PAGE gel electrophoresis might still demonstrate that they are identical. Thus, this step is just preliminary. The second step is to analyze the conditions used in the purification step. In general, most of the members in a family are usually purified through an almost similar procedure. For example, HP heparin column is specific for purification of nucleic acid binding proteins. Therefore, if the expressed protein cannot be purified by heparin column, it should not be a DNA binding protein. The third step is to analyze the known properties of the protein with some biochemical assays including NMR chemical shift perturbation assay, fluorescence anisotropy binding studies, etc. The final step is to try to get the exact information of the molecular weight and the amino acid sequence of the unexpectedly expressed protein by performing MALDI-TOF mass spectroscopy and electrospray ionization mass spectrometric peptide mapping assay. Among them, each step is helpful, but sometimes performing only one step is good enough for us to analyze whether or not the expressed protein is the target protein.
2.2 NRSF/REST ZnF2-8 was not produced from current pET15b plasmidSince NRSF/REST ZnF3-8, ZnF4-8 and ZnF5-8 had already been obtained in a large quantity with similar pET15b plasmid[18], we initially believed that we could also produce NRSF/REST ZnF2-8 in the same way. However, firstly, to remove the impurities, we tried to purify the non-target protein through HP Heparin column (G E Healthcare), as we did before upon purifying NRSF/REST ZnF motifs including ZnF3-8, ZnF4-8, ZnF5-8 and ZnF6-8. We surprisingly found that this non-target protein could not bind to Heparin column, which is always used to purify the nucleic acid binding proteins. Secondly, the results from MALDI-TOF mass spectroscopy (data not shown) indicated that this non-target protein has a molecular weight (MW) of 28 889.8, much higher than that (MW=24 586.0) of NRSF/REST ZnF2-8 [Fig. 1(b) and 1(c)]. Thirdly, we could not detect the single-wavelength anomalous dispersion (SAD) signal belonging to heavy atom zinc in the beamline 3W1A of BSRF, further suggesting that no zinc ion is located in this non-target protein. Fourthly, 2D NMR 1H-15N HSQC spectrum of the non-target protein displayed well-dispersed cross peaks, but did not show chemical shift changes upon mixing with NRSE/RE1 dsDNA, which indicated that this non-target protein does not bind to this dsDNA, which is consistent with the results from native DNA gel shift assay (Fig. 2). Finally, the electrospray ionization mass spectrometric mapping assay demonstrated the strongest evidences that the amino acid sequence of the non-target protein is not identical to that of NRSF/REST functional ZnF2-8 motif. In a word, NRSF/REST ZnF2-8 was not produced from the current pET15b plasmid.
2.3 β-lactamase was unexpectedly expressed instead of NRSF/REST ZnF2-8Since the expressed protein was not our previously predicted target protein, we then switched our interest into the investigation on what it was, where it came from and how it could be generated. The electrospray ionization mass spectrometric peptide mapping assay indicated that the amino acid sequences of the fragments are mostly identical to those found in E. coli β-lactamase (Table 1). The β-lactamases are widely spread bacterial enzymes that can effectively cleave and inactivate the classic β-lactam families of antibiotics (penicillins and cephalo sporins)[23, 24]. As we know, the pET15b vector has anti-ampicillin activity, while bacterial Rossetta (DE3) cell does not. So, we assumed that, in our case, the β-lactamase expression might be resulted from pET15b plasmid. The full-length β-lactamase encoded from the gene contained in pET15b vector was reported as TEM-1 β-lactamase, containing 284 amino acids. But its molecular weight (MW=31 314.9) is obviously much higher than the result (m/z: 28 889.8) from the MALDI-TOF mass spectroscopy. Thus, β-lactamase might have been degraded in either its N-terminus or C-terminus during its expression or purification. Further amino acid sequence analysis indicated that the N-terminus of β-lactamase including 25 amino acids is a signal peptide, which is always cut off by some enzyme when the protein expression is completed. The calculated molecular weight of this N-terminal 25 amino acids truncated β-lactamase was found to be 28 907, much close to the observed m/z value (28 889.8) in the MALDI-TOF mass spectroscopy. Based on these analyses, we determined the X-ray three-dimensional (3D) structure of this β-lactamase by using this truncated amino acid sequence and the reported TEM-1 β-lactamase 3D structure (pdb code: 1ERO) as a searching model. The final refined structure is almost identical to that of TEM-1 β-lactamase, because they can be superimposed with a root-mean-square deviation (RMSD) value of 3.5×10-2 nm over all backbone Cα atoms (Fig. 3).

|
| Fig. 3 Non-target protein crystallization and its X-ray three-dimensional structure. (a) Crystals of non-target protein. (b) The ribbon representation of non-target protein structure. (c) Overlay of the non-target protein structure (dark grey) with that of previously published β-lactamase (PDB code: 1ERO) (light grey) |
Then, we tried to probe how the accidental expression of β-lactamase was happened. Since there were no problems to obtain NRSF/REST functional motifs such as ZnF3-8, ZnF4-8 and ZnF5-8 through a regular pET15b vector[18], β-lactamase was overexpressed only during ZnF2-8 expression, suggesting that NRSF/REST ZnF-2 might be helpful to enhance β-lactamase expression. So, we decided to destroy ZnF-2 in ZnF2-8 by performing site-directed mutation from Cys216 to Arg216. This mutation can wreck the tetra-coordination of zinc ion in NRSF/REST ZnF-2 motif. Out of our expectation, the SDS-PAGE gel electrophoresis (Fig. 1) revealed that β-lactamase was still overexpressed in E. coli. Therefore, the ZnF-2 gene might result in the errors of NRSF/REST ZnF2-8 heterologous expression, and stimulate the overexpression of β-lactamase. These errors might come either from hungry codon syndrome, or from the potential interactions with chaperone proteins, or from other unknown interactions. The detailed mechanism for how these errors were generated is too complicated, which is needed to be carefully studied in the future.
3 Data depositionThe coordinates for the β-lactamase have been deposited in the Protein Data Bank with accession code 4GKU and RCSB ID RCSB074309.
Acknowledgement: This work was supported by the National Basic Research Program of China (2009CB918600, 2011CB966300), by the National Natural Science Foundation of China (30800176, 20921091), and by National New Drug Design Program from Ministry of Health of China (2011ZX09506). We thank Prof. Yuhui Dong and other members of his group at BSRF (China) for assistance in data collection. The authors are indebted to Prof. Roland Riek in the Laboratory of Physical Chemistry, ETH Zurich, Switzerland, and Prof. Fred H Gage in the laboratory of Genetics, the Salk Institute for Biological Studies, La Jolla, California, USA, for kindly providing pET15b plasmid containing full length NRSF/REST gene.| [1] | Berrow N S, Alderton D, Sainsbury S et al . A versatile ligation-independent cloning method suitable for high-throughput expression screening applications[J]. Nucleic Acids Res , 2007, 35 : e45 DOI:10.1093/nar/gkm047 |
| [2] | Cabrita L D, Dai W W, Bottomley S P . A family of E-coli expression vectors for laboratory scale and high throughput soluble protein production[J]. BMC Biotechnol , 2006, 6 : 12 DOI:10.1186/1472-6750-6-12 |
| [3] | Engler C, Kandzia R, Marillonnet S . A one pot, one step, precision cloning method with high throughput capability[J]. PLoS ONE , 2008, 3 : e3647 DOI:10.1371/journal.pone.0003647 |
| [4] | Gileadi N A, Burgess-Brown S M, Colebrook G et al . High Throughput Production of Recombinant Human Proteins for Crystallography//Kobe B, Guss M, Huber T, Eds. Structural Proteomics: High Throughput Methods[M]. New York: Humana Press, 2008 : 221 -246. |
| [5] | Graslund S, Nordlund P, Weigelt J et al . Protein production and purification[J]. Nat Methods , 2008, 5 : 135-146 DOI:10.1038/nmeth.f.202 |
| [6] | Scheich C, Kummel D, Soumailakakis D et al . Vectors for co-expression of an unrestricted number of proteins[J]. Nucleic Acids Res , 2007, 35 : e43 DOI:10.1093/nar/gkm067 |
| [7] | Cormier C Y, Mohr S E, Zuo D et al . Protein structure initiative material repository: an open shared public resource of structural genomics plasmids for the biological community[J]. Nucleic Acids Res , 2010, 38 : D743-D749 DOI:10.1093/nar/gkp999 |
| [8] | Blommel P G, Martin P A, Wrobel R L et al . High efficiency single step production of expression plasmids from cDNA clones using the Flexi Vector cloning system[J]. Protein Expr Purif , 2006, 47 : 562-570 DOI:10.1016/j.pep.2005.11.007 |
| [9] | Li M Z, Elledge S J . Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC[J]. Nat Methods , 2007, 4 : 251-256 DOI:10.1038/nmeth1010 |
| [10] | Walhout A J M, Temple G F, Brasch M A et al . GATEWAY recombinational cloning: Application to the cloning of large numbers of open reading frames or ORFeomes[J]. Methods Enzymol , 2000, 328 : 575-592 DOI:10.1016/S0076-6879(00)28419-X |
| [11] | Stols L, Gu M Y, Dieckman L et al . A new vector for high-throughput, ligation-independent cloning encoding a tobacco etch virus protease cleavage site[J]. Protein Expr Purif , 2002, 25 : 8-15 DOI:10.1006/prep.2001.1603 |
| [12] | Chanda P K, Edris W A, Kennedy J D . A set of ligation-independent expression vectors for co-expression of proteins in Escherichia coli[J]. Protein Expr. Purif , 2006, 47 : 217-224 DOI:10.1016/j.pep.2005.10.019 |
| [13] | Ballas N, Mandel G . The many faces of REST oversee epigenetic programming of neuronal genes[J]. Curr Opin Neurobiol , 2005, 15 : 500-506 DOI:10.1016/j.conb.2005.08.015 |
| [14] | Eddy S R . Non-coding RNA genes and the modern RNA world[J]. Nat Rev Genet , 2001, 2 : 919-929 DOI:10.1038/35103511 |
| [15] | Fire S, Xu M K, Montgomery S A et al . Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans[J]. Nature , 1998, 391 : 806-811 DOI:10.1038/35888 |
| [16] | Pasquinelli A E . MicroRNAs: deviants no longer[J]. Trends Genet , 2002, 18 : 171-173 DOI:10.1016/S0168-9525(01)02624-5 |
| [17] | Kuwabara T, Hsieh J, Nakashima K et al . A small modulatory dsRNA specifies the fate of adult neural stem cells[J]. Cell , 2004, 116 : 779-793 DOI:10.1016/S0092-8674(04)00248-X |
| [18] | Zhang Y, Hu W, Shen J et al . Cysteine 397 plays important roles in the folding of the neuron-restricted silencer factor/RE1-silencing transcription factor[J]. Biochem Bioph Res Commun , 2011, 414 (2) : 309-314 DOI:10.1016/j.bbrc.2011.09.045 |
| [19] | Chowdhury S K, Katta V, Chait B T . Electrospray ionization mass spectrometric peptide mapping: A rapid, sensitive technique for protein structure analysis[J]. Biochem Bioph Res Commun , 1990, 167 (2) : 686-692 DOI:10.1016/0006-291X(90)92080-J |
| [20] | Otwinowski Z, Minor W . Processing of X-ray diffraction data collected in oscillation mode[J]. Method Enzymol , 1997, 276 : 307-326 DOI:10.1016/S0076-6879(97)76066-X |
| [21] | Emsley P, Cowtan K . Developments in the CCP4 molecular-graphics project[J]. Acta Crystallogr D Biol Crystallogr , 2004, 60 : 2 126-2 132 DOI:10.1107/S0907444904019158 |
| [22] | Afonine P V, Mustyakimov M, Grosse-Kunstleve R W et al . Joint X-ray and neutron refinement with phenix.refine[J]. Acta Crystallogr D Biol Crystallogr , 2010, 66 : 213-221 DOI:10.1107/S0907444909052925 |
| [23] | Abraham E P, Chain E B . An enzyme from bacterial able to destroy penicillin[J]. Nature , 1940, 146 : 837-837 |
| [24] | Frere J M . β-lactamase and bacterial resistance to antibiotics[J]. Molec Microbiol , 1995, 16 : 385-395 DOI:10.1111/mmi.1995.16.issue-3 |
 2015, Vol. 32
2015, Vol. 32



