文章信息
- 黄桂坤, 邱莉, 焦杨, 袁经权, 谢集照, 缪剑华
- HUANG Gui-kun, QIU Li, JIAO Yang, YUAN Jing-quan, XIE Ji-zhao, MIAO Jian-hua
- 三七中2个微量人参皂苷的NMR信号全归属
- NMR Assignments of Two Minor Ginsenosides Extratced from Panax notoginseng (Burk.) F. H. Chen
- 波谱学杂志, 2014, 31(3): 415-425
- Chinese Journal of Magnetic Resonance, 2014, 31(3): 415-425
-
文章历史
收稿日期: 2013-10-09
收修改稿日期: 2014-07-19


2. 广西药用植物研究所,广西 南宁 530023;
3. 暨南大学 中药及天然药物研究所,广东 广州 510632
2. Guangxi Institute of Medical Plant, Nanning 530023, China;
3. Institute of Traditional Chinese Medicine and Natural Products, Jinan University, Guangzhou 510632, China
三七为五加科人参属植物Panax notoginseng (Burk.) F. H. Chen,具有活血化瘀、消肿定痛功效.三七的药用历史悠久,因其显著的临床功效和药理活性,自上世纪70年代后得到了深入细致的研究[1].现代药理学研究表明,三七总皂苷具有保护心脑血管系统、止血、抗氧化、降血脂、保肝、保护肾脏和免疫等作用.达玛烷型人参皂苷类化学成分是三七的主要化学成分及药效成分[2].本文从三七根的80%乙醇提取物中分离鉴定了2个微量达玛烷型人参皂苷类化合物,分别为6-O-(β-D-glucopyranosyl)-20-O-(β-D-xylopyranosyl)-3β,6α,12β,20(S)-tetrahydroxydammar-24-ene (1)和notoginsenoside U (2). 2011年第一次报道了化合物1在氘代吡啶溶液中13C NMR数据以及部分1H NMR数据[3],但在化合物1的结构解析过程中,我们发现化合物1结构中连接在C-20位木糖基的13C NMR数据与文献[3]报道存在较大的差异,对此提出探讨与论证,并采用MS,1H NMR,13C NMR,1H-1H COSY,HSQC,HMBC以及化学方法,对化合物1的结构进行详细的解析及确证,并完善了化合物1的1H NMR归属.化合物2为三七中的一种微量的皂苷,仅见使用氘代二甲基亚砜(DMSO-d6)为NMR实验溶剂的波谱数据报道[4],鉴于文献对人参皂苷类化合物的实验溶剂绝大多数为氘代吡啶(C5D5N)的情况,本实验在2D NMR的基础上对化合物2在C5D5N溶液中的1H NMR和13C NMR数据进行全归属,为以后的结构鉴定工作提供一定的参考.

|
| 图 1 化合物1和2的结构 Fig. 1 Structures of compounds 1 and 2 |
NMR谱采用Bruker ARX-600型NMR波谱仪测定.溶剂为C5D5N,TMS为内标. 1H和13C NMR的工作频率为600.17 MHz和150.91 MHz,谱宽分别为12 315.1 Hz和36 057.7 Hz.二维谱包括2D梯度场1H-1H COSY,HSQC,HMBC谱,均采用标准脉冲程序. HSQC和HMBC的F1 (1H)和F2 (13C)二维谱的谱宽分别为9 014.4 Hz和31 694.9 Hz. HSQC采样数据点阵t2×t1=512×256,HMBC采样数据点阵t2×t1=512× 256. HR-ESI-MS采用Waters API QSTAR Pular-1质谱仪,ESI-MS采用Agilent 1100-LC/MSD TrapSL质谱仪,LC-MS采用Agilent GC-MS/MS 7000B三重四极杆气质联用仪.制备液相色谱仪SHIMADZU LC-8A(岛津,日本),检测器为UV SPD 10A(岛津,日本);制备型色谱柱:YMC Prep-ODS 20 mm×250 mm, 10 μm(YMC,日本);R-1001N型旋转蒸发仪(上海亚荣生化仪器厂);电子分析天平(德国赛多利斯股份有限公司).
1.2 药材三七药材购于广西玉林市药材市场,由广西壮族自治区食品药品检验所韦家福主任中药师鉴定为三七[Panax notoginseng (Burk.) F. H. Chen]的干燥根,药材标本保存在广西医科大学天然药物化学实验室.
1.3 提取与分离三七根粗粉9 kg,用8倍80%乙醇回流提取3次,每次回流2 h,减压浓缩回收乙醇,最后得浸膏1 800 g.将浸膏混悬于适量水中,依次用等体积的二氯甲烷、正丁醇萃取,各萃取三次,减压回收有机溶剂后分别得二氯甲烷层SC (72 g)、正丁醇层SB (1 517 g)、水层SW (686 g). SB部分经大孔树脂,以乙醇-水梯度洗脱得到5个馏分(SB1~SB5),SB3再次经大孔树脂柱色谱分离后得到2个馏分(SB3-1~SB3-2),SB3-2经硅胶柱色谱二氯甲烷-甲醇洗脱得到5个馏分(SB3-2-1~SB3-2-5),SB3-2-5经开放ODS C18柱色谱以甲醇-水梯度洗脱得到5个馏分(SB3-2-5-1~SB3-2-5-5),SB3-2-5-4馏分经Sephadex LH-20凝胶柱色谱分离后通过反相制备液相分离分别得到化合物1 (79 mg)和化合物2 (53 mg).
1.4 人参皂苷的酸水解,糖腈乙酸酯衍生化以及GC-MS分析0.05 mmol标准糖与8 mg盐酸羟胺均匀混合,加入0.5 mL的吡啶,90 ℃水浴加热1 h,生成糖醇.反应物冷却至室温后,加入0.3 mL乙酸酐,90 ℃水浴加热1 h,即生成糖腈乙酸酯衍生物对照品溶液.
取样品约1 mg,加入2 mL的浓度为10%的HCl,90 ℃水浴加热4 h,反应物冷却至室温后,用等体积氯仿萃取两次,合并萃取液,得到样品苷元部分,水层减压蒸干,加入2 mg盐酸羟胺及1 mL的吡啶,90 ℃水浴加热1 h,生成糖醇.再加入0.5 mL乙酸酐,90 ℃水浴加热1 h,即得糖腈乙酸酯衍生物溶液,所得样品即可用GC-MS分析,并与标准糖衍生物对照[5].
2 结果与讨论 2.1 化合物1白色粉末,易溶于甲醇、乙醇. Liebermann-Burchard反应阳性,化合物经酸水解后糖腈乙酸酯衍生化,GC-MS分析,并与标准糖的糖腈乙酸酯衍生物比较,确定分子中含有葡萄糖和木糖,提示为三萜皂苷类化合物. HR-ESI-MS m/z:793.470 9 [M+Na]+ (calcd. for C41H68O12Na,793.471 4).结合1H、13C NMR数据,推测分子式为C41H70O13.
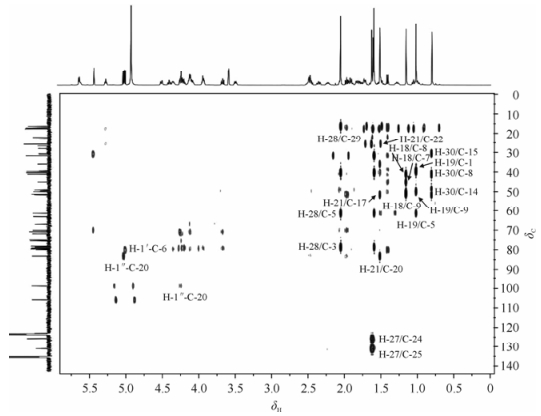
在1H NMR (600 MHz,C5D5N)谱中,髙场区有8个角甲基质子信号:δ 0.80 (3H,s,H-30),1.02 (3H,s,H-19),1.15 (3H,s,H-18),1.51 (3H,s,H-21),1.69 (3H,s,H-29),1.61 (3H,s,H-26),1.63 (3H,s,H-27),2.05 (3H,s,H-28);低场区有一个烯氢信号δ 5.28 (1H,t,J=7.1 Hz);另外δ 5.04 (1H,d,J=7.6 Hz),5.02 (1H,d,J=7.8 Hz)为2个糖残基的端基质子信号,根据其偶合常数,推测两个糖残基的端基构型均为β构型.
在13C NMR (150 MHz,C5D5N)谱中,给出41个碳信号,结合HSQC谱中给出的碳氢相关信息,可知其中有8个甲基碳信号;2个烯碳信号δ 131.1,125.8;2个糖的端基碳信号δ 105.9,98.84.结合碳谱、氢谱数据,推测化合物1为达玛烷三萜皂苷类化合物,依据δ 61.38 (C-5)进一步推测其为达玛烷型原人参三醇皂苷[6].仔细分析化合物1的核磁数据,发现除了连接在C-20位的糖链数据外,化合物1的碳谱数据与人参皂苷Rg1的非常相似[7].
在HMBC谱中(图 2),质子信号δ 0.80 (H-30)与碳信号δ 30.63 (C-15),41.10 (C-8),51.33 (C-14)有远程相关;质子信号δ 1.02 (H-19)与碳信号δ 39.41 (C-1),49.98 (C-9),61.38 (C-5)有远程相关;质子信号δ 1.15 (H-18)与碳信号δ 41.10 (C-8),45.11 (C-7),49.98 (C-9)有远程相关;质子信号δ 1.51 (H-21)与碳信号δ 35.99 (C-22),51.64 (C-17),83.20 (C-20)有远程相关;质子信号δ 1.69 (H-29)与碳信号δ 31.72 (C-28),40.33 (C-4),61.38 (C-5),78.62 (C-3)有远程相关;质子信号δ 1.61 (H-26)与碳信号δ 17.69 (C-27),125.8 (C-24),131.1 (C-25)有远程相关;质子信号δ 1.63 (H-27)与碳信号δ 25.72 (C-26),125.8 (C-24),131.1 (C-25)有远程相关;质子信号δ 2.05 (H-28)与碳信号δ 16.33 (C-29),40.33 (C-4),61.38 (C-5)有远程相关;质子信号δ 1.41 (H-5)与碳信号δ 31.72 (C-28),45.11 (C-7),39.66 (C-10),80.09 (C-6),78.62 (C-3)有远程相关;质子信号δ 1.96 (H-13)与碳信号δ 30.63 (C-15),41.10 (C-8),51.64 (C-17),83.20 (C-20)有远程相关;质子信号δ 5.28 (H-24)与碳信号δ 131.1 (C-25),25.72 (C-26),17.69 (C-27)有远程相关.由此可进一步确定该化合物为原人参三醇皂苷,结合1H-1H COSY,HSQC,HMBC即可对苷元母核1H,13C NMR数据进行归属.
|
| 图 2 化合物1的HMBC谱 Fig. 2 HMBC spectrum of compound 1 |
糖基部分归属[8, 9],在1H-1H COSY谱中,质子信号δ 4.51 (H-6′a)与δ 4.35 (H-6′b),δ 4.35 (H-6′)与δ 3.94 (H-5′),δ 3.94 (H-5′)与4.22 (H-4′),δ 4.22 (H-4′)与δ 4.24 (H-3′)相关,质子信号δ 4.24 (H-3′)与δ 4.09 (H-2′),δ 4.09 (H-2′)与δ 5.02 (H-1′)相关,为葡萄糖残基的质子信号.质子信号δ 3.67 (H-5”b)与δ 4.27 (H-5”a),δ 4.27 (H-5”)与δ 4.13 (H-4”),δ 4.13 (H-4”)与δ 4.12 (H-3”)相关,质子信号δ 4.12 (H-3”)与δ 3.96 (H-2”),δ 3.96 (H-2”)与δ 5.04 (H-1”)相关,此为木糖的质子信号.结合HSQC谱,即可对糖的1H,13C NMR信号进行归属.通过HMBC谱,可进一步确定糖的连接位置及连接方式.在HMBC谱中,可观察到质子信号δ 5.02 (H-1′)与δ 80.09 (C-6)碳信号远程相关,质子信号δ 4.41 (H-6)与碳信号δ 105.9 (C-1′)远程相关,结合C-6位的化学位移向低场移至δ 80.09,可确定葡萄糖残基连接在苷元的C-6位.同时,还可观察到质子信号δ 5.04 (C-1”)与碳信号δ 83.20 (C-20)远程相关,由此可确定木糖残基连接在苷元的C-20位.至此,可得到化合物1的平面结构.
结合文献总结的达玛烷型三萜皂苷C-20位差向异构体的位移规律[6, 10]:人参皂苷中C-20位碳的立体结构多数为S型,在吡啶溶液中,20 (R)和20 (S)差向异构体的C-12,17,21和C-22化学位移有明显的差异,特别是C-12位具有-OH的化合物,由于12-β-OH与20-OH间形成分子内氢键,导致g -旁氏效应(g -gauche)增强.该化合物的C-12,17,21和C-22的化学位移分别在δ 70.12,51.64,22.11,35.99,故确定C-20位的构型为S型.
综上所述,该化合物鉴定为:6-O-(β-D-glucopyranosyl)-20-O-(β-D-xylopyranosyl)-3β,6α,12β,20(S)-tetrahydroxydammar-24-ene.但该化合物数据与文献[3]中相应结构报道数据有较大差异,主要差别在连接于苷元C-20位的木糖的化学位移不同.结合GC-MS分析结果和文献[11, 12, 14-17]数据的比较,确定化合物1中连接在其C-20位的糖为木糖.结合1H NMR,13C NMR,HSQC,HMBC,1H-1H COSY对化合物1的NMR数据进行全归属,并对文献[3]报道数据差异部分进行了修正,为人参皂苷类结构鉴定提供参考,见表 1.
| Position | δH(J/Hz) | δC | HSQC | 1H-1H COSY | HMBC | Ref.[3] | |
| δC | δH(J/Hz) | ||||||
| 1 | 0.99(1H, m); 1.72(1H, m) | 39.41 | + | H-2 | C-5, C-9, C-19 | 39.4 | / |
| 2 | 1.82(1H, m); 1.91(1H, m) | 27.91 | + | H-1, H-3 | C-4, C-10 | 27.8 | / |
| 3 | 3.50(1H, m) | 78.62 | + | H-2 | C-4, C-28, C-29 | 78.6 | / |
| 4 | / | 40.33 | / | / | / | 40.3 | / |
| 5 | 1.41(1H, d, 10.5) | 61.38 | + | H-6 | C-3, C-6, C-7, C-10, C-29 | 61.4 | / |
| 6 | 4.41(1H, td, 10.5, 3.3) | 80.09 | + | H-5, H-7 | C-5, C-1′ | 80.0 | / |
| 7 | 1.92(1H, m); 2.48(1H, m) | 45.11 | + | H-6 | C-5, C-6, C-8, C-9, C-18 | 45.1 | / |
| 8 | / | 41.10 | / | / | / | 41.1 | / |
| 9 | 1.49(1H, m) | 49.98 | + | / | C-5, C-11, C-12, C-14 | 49.9 | / |
| 10 | / | 39.66 | / | / | / | 39.6 | / |
| 11 | 1.52(1H, m); 1.65(1H, m) | 30.97 | + | H-12 | C-9 | 30.9 | / |
| 12 | 4.11(1H, m) | 70.12 | + | H-11, H-13 | / | 69.5 | / |
| 13 | 1.96(1H, m) | 49.14 | + | H-12 | C-8, C-15, C-17, C-20 | 49.1 | / |
| 14 | / | 51.33 | / | / | / | 51.5 | / |
| 15 | 1.05(1H, m); 1.64(1H, m) | 30.63 | + | H-16 | C-8, C-30 | 30.6 | / |
| 16 | 1.27(1H, m); 1.74(1H, m) | 26.59 | + | H-15, H-17 | C-14, C-20 | 26.5 | / |
| 17 | 2.47(1H, m) | 51.64 | + | H-16 | C-13, C-15, C-16, C-20 | 51.3 | / |
| 18 | 1.15(3H, s) | 17.52 | + | / | C-7, C-8, C-9 | 17.5 | / |
| 19 | 1.02(3H, s) | 17.50 | + | / | C-1, C-5, C-9 | 17.5 | / |
| 20 | / | 83.20 | / | / | / | 83.0 | / |
| 21 | 1.51(3H, s) | 22.11 | + | / | C-17, C-20, C-22 | 22.1 | / |
| 22 | 1.79(1H, m); 2.36(1H, m) | 35.99 | + | H-23 | C-21, C-23, C-24 | 36.0 | / |
| 23 | 2.23(1H, m); 2.49(1H, m) | 23.14 | + | H-22, H-24 | C-24, C-25 | 23.1 | / |
| 24 | 5.28(1H, t, 7.1) | 125.8 | + | H-23 | C-22, C-26, C-27 | 125.8 | 5.24(1H, t) |
| 25 | / | 131.1 | / | / | / | 131.0 | / |
| 26 | 1.61(3H, s) | 25.72 | + | / | C-24, C-25, C-27 | 25.7 | / |
| 27 | 1.63(3H, s) | 17.69 | + | / | C-24, C-25, C-26 | 17.7 | / |
| 28 | 2.05(3H, s) | 31.72 | + | / | C-3, C-4, C-5, C-29 | 31.7 | / |
| 29 | 1.69(3H, s) | 16.33 | + | / | C-3, C-4, C-5, C-28 | 16.3 | / |
| 30 | 0.80(3H, s) | 17.14 | + | / | C-8, C-14, C-15 | 17.2 | / |
| 6-Glc | |||||||
| 1′ | 5.02(1H, d, 7.8) | 105.9 | + | H-2′ | C-6 | 106.9 | 4.96(1H, d, 7.8) |
| 2′ | 4.09(1H, m) | 75.45 | + | H-1′, H-3′ | C-1′ | 75.4 | / |
| 3′ | 4.24(1H, m) | 79.62 | + | H-2′, H-4′ | C-2′, C-4′, C-5′ | 79.5 | / |
| 4′ | 4.22(1H, m) | 71.85 | + | H-3′, H-5′ | C-3′, C-6′ | 71.8 | / |
| 5′ | 3.94(1H, m) | 78.09 | + | H-4′, H-6′ | C-3′, C-4′ | 78.0 | / |
| 6′ | 4.51(1H, d, 11.6); 4.35(1H, m) | 63.08 | + | H-5′ | C-3′ | 63.1 | / |
| 20-Xyl | |||||||
| 1” | 5.04(1H, d, 7.6) | 98.84 | + | H-2” | C-20 | 98.6 | 4.93(1H, d, 7.2) |
| 2” | 3.96(1H, m) | 74.97 | + | H-1”, H-3” | C-1”, C-3” | 72.6 | / |
| 3” | 4.12(1H, m) | 79.14 | + | H-2”, H-4” | C-4”, C-5” | 75.1 | / |
| 4” | 4.13(1H, m) | 70.92 | + | H-3”, H-5” | C-3” | 70.1 | / |
| 5” | 4.27(1H, m); 3.67(1H, dd, 11.1, 9.8) | 67.03 | + | H-4”, H-5” | C-3”, C-5” | 66.8 | / |
| *为文献[3]中与本文差别较大的信号 | |||||||
白色粉末,易溶于甲醇、吡啶. Liebermann-Burchard反应阳性,化合物经酸水解糖腈乙酸酯衍生化,GC-MS分析,并与标准糖的糖腈乙酸酯衍生物比较,确定分子中只含有葡萄糖,提示为三萜皂苷类化合物. ESI-MS m/z:823 [M+Na]+,839 [M+K]+,799 [M-H]-,835 [M+Cl]-,845 [M+HCOO]-.结合1H、13C NMR数据,推测分子式为C42H72O14.
1H NMR (600 MHz,C5D5N)谱中,髙场区给出8个角甲基质子信号:δ 0.96 (3H,s,H-30),1.00 (3H,s,H-19),1.09 (3H,s,H-18),1.44 (3H,s,H-29),1.59 (3H,s,H-26),1.64 (3H,s,H-21),1.65 (3H,s,H-27),1.97 (3H,s,H-28);低场区有1个烯氢信号δ 5.31 (1H,t,J=6.7 Hz);另外,δ 5.11 (1H,d,J=7.7 Hz),5.09 (1H,d,J=7.8 Hz)为2个糖残基的端基质子信号,根据其偶合常数,推测2个糖残基的端基构型均为β型.
13C NMR (150 MHz,C5D5N)谱中给出42个碳信号,结合HSQC谱可知,其中有8个甲基碳信号;2个烯碳信号δ 131.0,125.9;2组葡萄糖的碳信号δ 98.04,74.84,79.26,71.61,77.02,70.24;δ 105.3,75.24,78.36,71.70,78.33,62.81,结合氢谱,推测化合物2结构为也为人参皂苷类化合物.与原人参二醇相比[19],C-6位羟基的存在使C-5位碳信号向低场移动5~6,而依据δ 61.72 (C-5)可确定化合物2为原人参三醇皂苷[6],此为原人参三醇类化合物的特征碳信号,与化合物1的母核结构相同.此外,苷元C-20位苷化后向低场移至δ 83.38,可推出糖基与苷元在C-20位结合.
在HSQC谱中可以观察到δ 5.11 (H-1′)与δ 98.04 (C-1′)有相关,δ 5.09 (H-1”)与δ 105.3 (H-1”)有相关,可确定2个糖的端基1H、13C NMR数据.在HMBC谱中,一糖端基质子信号δ 5.11 (H-1′)与碳信号δ 83.38 (C-20)有远程相关,证明一个葡萄糖残基连接在C-20位,为内侧糖.另一糖端基质子信号δ 5.09 (H-1”)与碳信号δ 70.24 (C-6′)远程相关,同时质子信号δ 4.72 (H-6′)与碳信号δ 105.3 (C-1”)有远程相关,与人参皂苷F1[20]相比,该化合物内侧糖的C-6′位向低场移至δ 70.24,可以确定2个葡萄糖以(1→6)苷键相连,并连接在苷元的C-20位,至此得到化合物2的平面结构.结合1H-1H COSY,HMBC谱,对糖残基的质子信号进行归属.在1H-1H COSY谱,从端基质子信号出发,可以观察到δ 5.11 (H-1′)与δ 3.91 (H-2′)相关,δ 3.91 (H-2′)与δ 4.15 (H-3′)相关,同时从C-6′位的质子信号出发,可以观察到δ 4.31 (Hb-6′)与δ 4.72 (Ha-6′)相关,δ 4.72 (H-6′)与δ 4.05 (H-5′)相关,结合HMBC谱中质子信号δ 4.04 (H-4′)与碳信号δ 79.26 (C-3′)有远程相关,即可对内侧糖的1H、13C NMR信号进行归属. 1H-1H COSY谱中,还可观察到δ 5.09 (H-1”)与δ 4.03 (H-2”)相关,由于信号的重叠,还需从C-6”位的质子信号开始,可观察到δ 4.34 (Hb-6”)与δ 4.51 (Ha-6”)相关,δ 4.34 (H-6”)与δ 3.92 (H-5”)相关,δ 3.92 (H-5”)与δ 4.20 (H-4”)相关,结合HMBC谱中质子信号δ 5.09 (H-1”)与碳信号δ 78.36 (C-3”)有远程相关,也可对外侧葡萄糖的1H、13C NMR信号进行归属.
根据人参皂苷类化合物的化学位移规律,化合物2的C-12,C-17,C-21,C-22的化学位移分别为δ 70.11,51.58,22.34和36.16,故确定苷元C-20位立体构型为S构型[6, 8].
结合文献[4]报道数据,将此化合物鉴定为(20S)-protopanaxatriol 20-O-β-D-glucopy-ranosyl-(1→6)-β-D-glucopyranoside,即notoginsenoside U.
文献[4]中该化合物的NMR实验溶剂为DMSO-d6,而本文实验溶剂为C5D5N,考虑到人参皂苷类化合物多数文献都是使用C5D5N,溶剂一致不但可以消除由于溶剂效应带来的化学位移差别和峰形的差别,而且便于与文献数据比较,C5D5N另一个优点是的1H和13C NMR溶剂残余信号都出现在较低场,与皂苷类化合物的绝大部分信号不发生重叠.特此,结合1H、13C NMR以及1H-1H COSY,HSQC,HMBC等2D NMR数据,对化合物2的NMR信号进行全归属,见表 2.
| Position | dH(J /Hz) | dC | HSQC | 1H-1H COSY | HMBC | Ref.[4] | |
| dC | dH(J /Hz) | ||||||
| 1 | 0.99(1H, m); 1.71(1H, m) | 39.33 | + | H-2 | C-3, C-9, C-10, C-19 | 39.4 | 0.94(α), 1.55(β) |
| 2 | 1.87(1H, m); 1.92(1H, m) | 28.11 | + | H-3, H-1 | / | 26.8 | 1.92(α), 1.84(β) |
| 3 | 3.50(1H, m) | 78.46 | + | H-2 | / | 78.0 | 2.92(1H, dd, 4.5, 10.4) |
| 4 | / | 40.30 | / | / | / | 40.2 | / |
| 5 | 1.20(1H, d, 10.4) | 61.72 | + | H-6 | C-4, C-6, C-7, C-9, C-10, C-19, C-28, C-29 | 61.4 | 0.74(1H, 8.6) |
| 6 | 4.40(1H, m) | 67.70 | + | H-5, H-7 | / | 67.4 | 3.87(1H, 10.6) |
| 7 | 1.86(1H, m); 1.95(1H, m) | 47.48 | + | H-6 | C-5, C-6, C-9, C-14, C-18 | 47.3 | 1.38(α), 1.51(β) |
| 8 | / | 41.18 | / | / | / | 41.3 | / |
| 9 | 1.55(1H, m) | 49.90 | + | / | C-1, C-12 | 49.8 | 1.27 |
| 10 | / | 39.36 | / | / | / | 40.0 | / |
| 11 | 1.56(1H, m); 1.57(1H, m) | 30.71 | + | H-12 | C-8, C-12, C-13 | 31.1 | 1.63(α), 1.05(β) |
| 12 | 4.21(1H, m) | 70.11 | + | H-13, H-11 | / | 70.0 | 3.56 |
| 13 | 2.00(1H, m) | 49.14 | + | H-12 | C-8, C-15, C-17, C-20, C-30 | 49.1 | 1.51 |
| 14 | / | 51.30 | / | / | / | 51.6 | / |
| 15 | 2.08(1H, m); 0.98(1H, m) | 30.82 | + | H-16 | C-14 | 30.9 | 1.45(α), 0.92(β) |
| 16 | 1.82(1H, m); 1.31(1H, m) | 26.59 | + | H-17, H-15 | / | 28.0 | 1.75(α), 1.24(β) |
| 17 | 2.56(1H, m) | 51.58 | + | H-16 | C-16, C-20 | 51.6 | 2.17(1H, dd, 10.8, 7.6) |
| 18 | 1.09(3H, s) | 17.61 | + | / | C-7, C-9, C-14 | 18.2 | 0.94(s) |
| 19 | 1.00(3H, s) | 17.42 | + | / | C-1, C-5, C-9 | 18.0 | 0.83(s) |
| 20 | / | 83.38 | / | / | / | 83.3 | / |
| 21 | 1.64(3H, s) | 22.34 | + | / | C-20, C-22, C-17 | 22.7 | 1.25(s) |
| 22 | 1.83(1H, m); 2.40(1H, m) | 36.16 | + | H-23 | C-20, C-21, C-23, C-24 | 36.4 | 1.76(α), 1.45(β) |
| 23 | 2.38(1H, m); 2.61(1H, m) | 23.17 | + | H-24, H-22 | C-22, C-24 | 23.3 | 2.02(α), 1.93(β) |
| 24 | 5.31(1H, d, 6.7) | 125.9 | + | H-23 | C-23, C-26, C-27 | 126.2 | 5.06(1H, t, 7.4) |
| 25 | / | 131.0 | / | / | / | 131.3 | / |
| 26 | 1.59(3H, s) | 25.76 | + | / | C-24, C-25, C-27 | 26.6 | 1.63(s) |
| 27 | 1.65(3H, s) | 17.91 | + | / | C-24, C-25, C-26 | 18.8 | 1.56(s) |
| 28 | 1.97(3H, s) | 31.95 | + | / | C-3, C-4, C-5, C-29 | 32.1 | 1.20(s) |
| 29 | 1.44(3H, s) | 16.46 | + | / | C-3, C-4, C-5, C-28 | 16.8 | 0.83(s) |
| 30 | 0.96(3H, s) | 17.42 | + | / | C-8, C-14, C-15 | 17.9 | 0.83(s) |
| 20-Glc | |||||||
| 1' | 5.11(1H, d, 7.7) | 98.04 | + | H-2' | C-20, C-3', C-5' | 97.5 | 4.45(1H, d, 9.0) |
| 2' | 3.91(1H, m) | 74.84 | + | H-1', H-3' | C-1', C-3', C-4' | 74.6 | 2.93 |
| 3' | 4.15(1H, t, 8.5) | 79.26 | + | H-2' | C-1', C-4' | 78.1 | 3.16 |
| 4' | 4.04(1H, m, overlapped) | 71.61 | + | / | C-3' | 71.1 | 3.10 |
| 5' | 4.05(1H, m, overlapped) | 77.02 | + | H-6' | C-1' | 76.2 | 3.31 |
| 6' | 4.31(1H, dd, 11.5, 5.6); 4.72(1H, m) | 70.24 | + | H-5' | C-5', C-4', C-1” | 69.7 | 3.94 |
| 6'-Glc | |||||||
| 1” | 5.09(1H, d, 7.8) | 105.3 | + | H-2” | C-6', C-3” | 104.4 | 4.23(1H, d, 7.69) |
| 2” | 4.03(1H, m) | 75.24 | + | H-1” | C-1”, C-3” | 74.8 | 2.87 |
| 3” | 4.20(1H, m, overlapped) | 78.36 | + | / | C-5” | 77.8 | 3.04 |
| 4” | 4.20(1H, m, overlapped) | 71.70 | + | H-5” | C-6”, C-3” | 71.1 | 3.03 |
| 5” | 3.92(1H, m) | 78.33 | + | H-6”, H-4” | C-6”, C-4”, C-3” | 77.7 | 3.10 |
| 6” | 4.34(1H, dd, 11.8, 5.4); 4.51(1H, m) | 62.81 | + | H-5” | C-5”, C-4” | 63.1 | 3.64(dd, 2.5, 11.8, α-H); 3.40(β-H) |
通过1H-1H COSY,HSQC及HMBC等二维技术,对从三七中分离得到的两个微量原人参三醇皂苷6-O-(β-D-glucopyranosyl)-20-O-(β-D-xylopyranosyl)-3β,6α,12β,20(S)-tetrahydroxydammar-24-ene (1)和notoginsenoside U (2)的结构进行鉴定,并对其NMR数据进行全归属,为今后的结构鉴定工作提供一定的参考.
| [1] | Zeng Jiang(曾江), Cui Xiu-ming(崔秀明), Zhou Jia-ming(周家明) et al . Studies on chemical constituents from rhizomes of Panax notoginseng(三七根茎的化学成分研究)[J]. Journal of Chinese Medicinal Materials(中药材) , 2007, 30 (11) : 1388-1391 |
| [2] | Ng T B . Pharmacological activity of sanchi ginseng (Panax notoginseng)[J]. J Pharm Pharmacol , 2006, 58 : 1007-1019 DOI:10.1211/jpp.58.8.0001 |
| [3] | Liu Li-min(刘利民), Zhang Xiao-qi(张晓琦), Wang Hao(汪豪) et al . Minor saponins constituents from Panax notoginseng tap root(三七主根的微量皂苷类成分研究)[J]. Journal of China Pharmaceutical University(中国药科大学学报) , 2011, 42 (2) : 115-118 |
| [4] | Sun H X, Ye Y P, Pan Y J . Immunological-adjuvant saponins from the roots of Panax notoginseng[J]. Chem Biodivers , 2005, 2 : 510-515 DOI:10.1002/(ISSN)1612-1880 |
| [5] | Liang C, Zhang X D, Wei L P et al . Investigation of the molecular ion structure for aldononitrile acetate derivatized muramic acid[J]. J Microbiol Meth , 2011, 86 : 224-230 DOI:10.1016/j.mimet.2011.05.007 |
| [6] | Tanaka O . Application of 13C-nuclear magnetic resonance spectrometry to structural studies on glycosides: saponins of Panax spp. and natural sweet glycosides[J]. Yakugaku Zasshi , 1985, 105 : 323-351 |
| [7] | Matsuura H, Kasai R, Tanaka O et al . Further studies on dammarane-saponins of sanchi-ginseng[J]. Chem Pharm Bull , 1983, 31 (7) : 2281-2287 DOI:10.1248/cpb.31.2281 |
| [8] | Teng Rong-wei(滕荣伟), Li Hai-zhou(李海舟), Wang De-zu(王德祖) et al . NMR complete assignments of three protopanaxadiol bisdesmosides(三七皂苷NMR研究-3个原人参二醇型双糖链配糖体的NMR信号全归属)[J]. Chinese J Magn Reson(波谱学杂志) , 2002, 19 (1) : 1-8 |
| [9] | Wu Chun-hua(吴春华), Li Chun-mei(李春梅), Chen Feng(陈凤) et al . NMR characterization of two monoterpene glycosides isolated from Boshniakia rossica Fedtsh et Flerow(草苁蓉中2个单萜苷类化合物的NMR研究)[J]. Chinese J Magn Reson(波谱学杂志) , 2013, 30 (2) : 247-255 |
| [10] | Wu Li-jun(吴立军) . Practical Spectral Analysis of Organic Compounds(实用有机化合物光谱解析)[M]. Beijing(北京): People's Health Publishing House(人民卫生出版社), 2009 . |
| [11] | Wang Jin-hui(王金辉). Studies on the chemical constitutions and bioactivities of leaves and stems of Panax quinquefolium L. (西洋参茎叶化学成分和生物活性的研究)[D]. Shenyang(沈阳): Shenyang Pharmaceutical University(沈阳药科大学), 1999. http://www.oalib.com/references/17034312 |
| [12] | 上海吉友医药科技有限公司. 20-O-糖基及其原人参二醇类化合物及其制备方法:中国, 200910201258.7. [P], 2010-07-21. http://www.oalib.com/references/17034313 |
| [13] | Chen J, Zhao R, Zeng Y M et al . Three new triterpenoid saponins from the leaves and stems of Panax quinquefolium[J]. J Asian Nat Prod Res , 2009, 11 (3) : 195-201 DOI:10.1080/10286020802682734 |
| [14] | Liu Chang-da(刘昌达). Research on the chemieal constituents of flower buds of Panax quinquefolium L. (西洋参花蕾化学 成分的研究)[D]. Shenyang(沈阳): Shenyang Pharmaceutical University(沈阳药科大学), 2008. http://cdmd.cnki.com.cn/article/cdmd-10163-2008101195.htm |
| [15] | Cui X M, Jiang Z Y, Zeng J et al . Two new dammarane triterpene glycosides from the rhizomes of Panax notoginseng[J]. J Asian Nat Prod Res , 2008, 10 (9) : 845-849 DOI:10.1080/10286020802144776 |
| [16] | Kun Zou, Shu Zhu, Meselhy R M et al . Dammarane-type saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH Cells[J]. J Nat Prod , 2002, 65 : 1288-1292 DOI:10.1021/np0201117 |
| [17] | Yang T R, Kasai R, Zhou Jun et al . Dammarane saponins of leaves and seeds of Panax notognseng[J]. Phytochenistry , 1983, 22 (6) : 1473-1478 DOI:10.1016/S0031-9422(00)84039-X |
| [18] | 中国科学院大连化学物理研究所.一种催化热解制备低极性人参皂苷及其苷元的方法:中国, 1508147. [P], 2004-06-30. |
| [19] | Nguyen M D, Kasai R, Ohtani K et al . Saponins from vietnamese ginseng, Panax vietnamensis HA et Grushv. collected in central vietnam. Ⅱ.[J]. Chem Pharm Bull , 1994, 42 (1) : 115-122 DOI:10.1248/cpb.42.115 |
| [20] | Song Jian-ping(宋建平), Zheng Jiang(曾江), Cui Xiu-ming(崔秀明) et al . Studies on chemical constituents from rhizomes of Panax notoginseng(Ⅱ)(三七根茎的化学成分研究(Ⅱ))[J]. Journal of Yunnan University(Natural sciences)(云南大学学报(自然科学版)) , 2007, 29 (3) : 287-290 |
 2014, Vol. 31
2014, Vol. 31



