Article Information
- 沈伊民, 郑伟丽, CHENG Yu-Chung N, 丁玉川, HIGASHIDA Tetsuhiro, 李杰, 叶永泉, RAYNAUD Jean-Sebastien, HAACKE E Mark
- SHEN Yi-min, ZHENG Wei-li, CHENG Yu-Chung N, DING Yu-chuan, HIGASHIDA Tetsuhiro, LI Jie, YE Yong-quan, RAYNAUD Jean-Sebastien, HAACKE E Mark
- 大鼠中风模型的超小氧化铁粒子神经血管成像
- USPIO High Resolution Neurovascular Imaging in a Rat Stroke Model of Transient Middle Cerebral Artery Occlusion
- 波谱学杂志, 2014, 31(1): 20-31
- Chinese Journal of Magnetic Resonance, 2014, 31(1): 20-31
-
Article History
Received date: 2013-10-16
Revised date: 2013-11-12



 , ZHENG Wei-li1, CHENG Yu-Chung N1, DING Yu-chuan2, HIGASHIDA Tetsuhiro2, LI Jie2, YE Yong-quan1, RAYNAUD Jean-Sebastien3, HAACKE E Mark1,4
, ZHENG Wei-li1, CHENG Yu-Chung N1, DING Yu-chuan2, HIGASHIDA Tetsuhiro2, LI Jie2, YE Yong-quan1, RAYNAUD Jean-Sebastien3, HAACKE E Mark1,4
2. Department of Neurological Surgery, Wayne State University School of Medicine, Detroit 48201, USA;
3. Guerbet Research, Aulnay-Sous-Bois 93600, France;
4. Department of Biomedical Engineering, Wayne State University, Detroit 48201, USA

 , 郑伟丽1, CHENG Yu-Chung N1, 丁玉川2, HIGASHIDA Tetsuhiro2, 李杰2, 叶永泉1, RAYNAUD Jean-Sebastien3, HAACKE E Mark1,4
, 郑伟丽1, CHENG Yu-Chung N1, 丁玉川2, HIGASHIDA Tetsuhiro2, 李杰2, 叶永泉1, RAYNAUD Jean-Sebastien3, HAACKE E Mark1,4
2. 美国韦恩州立大学 医学院神经外科系,底特律 48201,美国;
3. 法国格尔伯实验室, 欧奈苏布瓦 93600, 法国;
4. 美国韦恩州立大学 生物医学工程系,底特律 48201,美国
Stroke is a major cause of severe long-term disability and is characterized by a sudden loss of motor, sensory, or cognitive function, or some combination thereof. Ischemic stroke (e.g., arterial occlusion) accounts for most cases. Treating stroke to protect underperfused tissue is an important area of research today. Along these lines, Sildenafil (an angiogenesis inducing agent) treatment has been shown to promote angiogenesis around the ischemic core in stroke recovery. Magnetic resonance imaging (MRI) provides a method to evaluate the effectiveness of such treatment[1, 2]. Specifically, susceptibility weighted imaging (SWI)[3] and T2* weighted imaging are sensitive to the venous blood vessel volume and have been demonstrated to be clinically useful in noninvasive imaging of angiogenesis in terms of area and T2* ratio change[1, 2].
Iron based agents such as ultra-small super-paramagnetic particles of iron oxide (USPIO) have been shown to be clinically useful in MR angiography (MRA) and as macrophage detectors. This has led to the following clinical applications of such agents: MRA, studying vascular effects in aging, stroke, multiple sclerosis and assessing the vascular and lymphatic systems. Iron based contrast agents have three properties of interest in MRI. First, at low concentrations, they act as T1 reducing agents. Second, at higher concentrations, they work as T2 and T2* reducing agents since the susceptibility effects are predominant. And third, at any concentration, they can also create a local susceptibility effect and therefore create a bulk phase shift; this is ideal for imaging methods such as SWI and susceptibility mapping[3, 4] Our hypotheses are: (1) USPIO along with SWI can be used to detect the development of new vessels post stroke. (2) Because of its sensitivity to both heme and non-heme iron, SWI can be used to detect both arteries and veins with different echo times using USPIO. The purpose of this stage of the work was to determine if MRI at 7 T with a USPIO iron based contrast agent would sufficiently aid in visualization of collateral vessels in the penumbral area of lesions in a rat stroke model of transient middle cerebral artery occlusion (tMCAO).
1 Materials and MethodsAll animal experiments were approved by the Wayne State University Animal Investigations Committee and were conducted according to the Guide for the Care and Use of Laboratory Animals.
1.1 Animal Model and Experimental ProtocolThe intraluminal filament middle cerebral artery (MCA) occlusion stroke model that closely mimics the clinical situation was used in this study[5]. Clinically, the MCA is the most frequently embolized artery and reperfusion occurs as a result of recanalization spontaneously, surgically, or pharmacologically[6-8]. The intraluminal filament model was based on the technique developed by Koizumi, et al.[9], and Longa, et al.[10]. Briefly, animals were anesthetized and maintained. A length of about 19 mm filaments (with 0.2 mm diameter) was inserted into the right external carotid artery via an arteriotomy with temporary occlusion of the common carotid artery. The filament was then passed up the lumen of the internal carotid artery into the intracranial circulation. The filament lodged in the narrow proximal anterior cerebral artery and blocked the MCA at its origin. The animals awoke 5~10 min after anesthesia was withdrawn. Two hours after MCA occlusion, animals were re-anesthetized, and reperfusion was established by withdrawal of the filament.
Sildenafil (Viagra®, Pfizer Inc., New York, NY, USA), a phosphodiesterase type 5 (PDE5) enzyme inhibitor, was administrated subcutaneously initialed after 24 h post tMCAO for a total of seven days at a daily dose of 10 mg/kg with concentration of 1 mg/mL.
A total of 15 Sprague-Dawley rats (200~225 g, male) were studied. Three animals were used in low dose USPIO experiments for T1 effect on MRA only. The other 12 animals were subjected to tMCAO surgeries for stroke. Before the surgeries, two animals were used to optimize dosage for T2* effect on SWI. After the surgeries, the sildenafil treatment was randomly assigned to half of the animals. Animals were grouped as stroke with sildenafil treatment rats (n=6), stroke no treatment (n=5), and no stroke (n=1) (sham surgery without tMCAO). For each animal, the MRI scans without/with USPIO were performed at four time points: a week before tMCAO (baseline), 24 h, two weeks and four weeks post tMCAO. Animals were finally anesthetized and transcardially perfused with 10% neutral-buffered formalin. The brain was removed and immersed in formalin for further studies.
1.2 MRIThe MRI scans were performed on a 7 T horizontal magnet (ClinScan; Bruker, Karlsruhe, Germany). Prior to image acquisition, anesthesia was induced by isoflurane (2% v/v) with air to sedate the animals. The T2w, T1w, SWI or MRA scans were done first without USPIO. Then all the scans were repeated after the USPIO was administrated via tail vein injection by 24 G needle.
The MRA data were acquired with the following parameters: one average, TR/TE=40/4.05 ms, flip angle FA=50°, field of view FOV=32×32×16 mm3, matrix size=768×768×64, resulting in a spatial resolution of 41.6 μm×41.6 μm×250 μm. Acquisition time TA=16 min and 23 s. The SWI data were acquired using a T2* weighted 3D gradient multi-echo sequence: two averages, TR=50 ms, TE=5.37, 9.27, 15.12 ms, FA=20°, the same FOV, matrix size and resolution as MRA scans used, the pixel bandwidths were 260 Hz for the first and the second echo, and 130 Hz for the third echo, TA=41 min.
The USPIO contrast agent used in this work was P904 (Laboratory Guerbet, Aulnay, France) with a potential for plaque macrophage targeting. P904 consists of an iron oxide core and a hydrophilic coat with a mean hydrodynamic diameter of 21 nm[11]. The P904 dose used in this study was 100 μmol Fe/kg (0.2 mL/kg, the P904 molar concentration was 0.5 mol/L).
Both magnitude and phase images were processed using the house-built software Signal Processing in NMR (SPIN)(http://www.mrc.wayne.edu/). MR phase images were first filtered by a 128×128 high pass filter and then were used to generate SWI images as a weighted multiplication of phase and magnitude images. A variety of maximal/minimum intensity projections for various thicknesses were created. T2* maps were generated from SWI three-echo magnitude images. T2* values are presented as mean±standard error (number of pixels). The high pass filtered SWI phase images were processed further by susceptibility weighted imaging mapping (SWIM) technique[4] in the SPIN software and finally generated the susceptibility maps. Blood vessel width was measured by half-width at full-maximum.
2 RESULTS 2.1 Using a low dose of USPIO to enhance MRA via a T1 shortening effectThe effect of USPIO at a low dose was studied. For a low dose of 5 μmol Fe/kg, a normal rat MCA in MRA was enhanced by 1.44 times immediately after P904 injection. For the dose of 10 μmol Fe/kg, no obvious enhancement was seen right away but was seen between 1.5 and 2 hours later (Fig. 1). After 1.5 h, the MCA (arrow 1) and its branch (arrow 2) were enhanced 1.84 and 2.14 times respectively. Meanwhile the small anterior striate arteries (arrow 3) showed up as well in the image. The superior sagittal sinus was enhanced after 2 h [Fig. 1(c) arrow 4]. In the other anatomic slice, previously invisible small longitudinal hippocampal arteries also became visible after 1.5 h [Fig. 1(e) arrow 5]. After P904 entering blood steam, its intravascular concentration gradually reduced to a value appropriate for T1 shortening effects to dominate. Therefore, the right dose for maximum enhanced MRA signal in this study likely lies in the range of 5 to 10 μmol Fe/kg.

|
| Fig. 1 Temporal MRA (pre injection baseline (a, d), 1.5 h (b, e), 2 h (c)) on two anatomic slices (top and bottom rows) for a normal rat at a low dose of 10 μmol Fe/kg USPIO. Enhanced vessels: MCA (arrow 1), its branches (arrow 2), small anterior striate arteries (arrow 3), the superior sagittal sinus (arrow 4); longitudinal hippocampal arteries (arrow 5). Image in-plane resolution was 41.6 μm×41.6μm. Image thickness was 1 mm projection over 4 slices for the purpose of demonstration |
The first two animals in stroke groups were used to optimize USPIO dose for T2* effect before tMCAO surgeries. The USPIO dose effect on phase images is shown in Fig. 2. An initial high dose of 300 μmol Fe/kg was used on the first rat. Overall blood vessels were dramatically darkened in magnitude images (data not shown) and bright in phase images [Fig. 2(b)]. At this dose, there was severe dephasing to prevent proper visualization of the big vessels. The width of a small blood vessel in phase image changed from about two pixels (83.4 μm) to about six pixels (250 μm) [Fig. 2(a), (b)]. When the dose was reduced to 100 μmol Fe/kg on the second rat, overall blood vessels were partially dark in the magnitude images. USPIO magnified another small visible vessel of the second rat by a factor of roughly two. The visibility of the smallest vessel was dependent on imaging resolution (41.6 μm×41.6 μm here) and USPIO dose. Therefore, any vessel with half voxel size (21 μm) became visible in SWI with USPIO at this dose. Clearly USPIO at the larger dose aids in visualization of many smaller vessels in phase image and SWI image.

|
| Fig. 2 USPIO magnification effect (b, d) on vessel size from phase images for 2 rats at different doses 300 and 100 μmol Fe/kg (top and bottom rows), TE=5.37 ms, single slice. (a), (c) are phase images before injection. Clearly many smaller vessels are visible or enhanced with the larger dose (b) |
For the first rat with high dose of 300 μmol Fe/kg, the susceptibility of the marked vessel changed from 37±20 parts per billion (ppb) (number of pixels n=60) to 352±130 ppb (n=103). For the second rat with a reduced dose of 100 μmol Fe/kg, the susceptibility of the other marked vessel changed from 67±38 ppb (n=84) to 117±85 ppb (n=119). The post USPIO susceptibility of the vessel in the second rat was about one third of that in the first rat. It approached to the ratio of the two USPIO doses for the two rats. This analysis indicates that USPIO dominated intravascular susceptibility instead of deoxyhemoglobin.
The cerebral vasculature of a normal rat is commonly seen in phase images, SWI images and SWIM images [Fig. 3(d)~(f)]. Many smaller vessels became visible or much enhanced by USPIO [Fig. 3(a)~(c)]. The moderate USPIO dose for T2* effect was finalized at 100 μmol Fe/kg in this study. It worked well for long echo time 9.27 ms than short 5.37 ms for phase contrast being proportional to echo time.
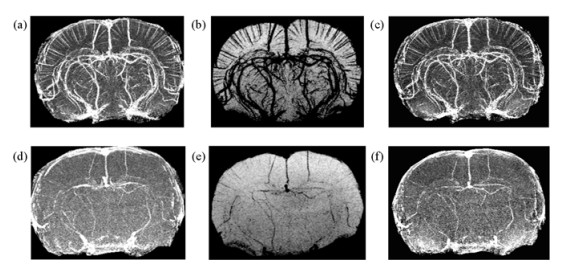
|
| Fig. 3 USPIO increases the visibility of the cerebral vasculature (top row) in phase images (left column), SWI images (center column) and SWIM images (right column) compared to baseline (bottom row) even with a moderate dose of 100 μmol Fe/kg for a normal rat. TE=9.27 ms, 4 mm projection for the purpose of demonstration |
Induction of stroke was observed by relevant behaviors such as paresis of the tail and the hind paws and the tendency to walk in circles. The ischemic core, the so-called penumbra, was easily seen as obvious hyper-intensity cerebral areas in T2 weighted images. There were four severe and two mild stroke rats in the treated group, and two severe and three mild stroke rats in the untreated group. Major cerebral hemorrhages were found in ischemic core for all severe stroke rats by SWI. Each hemorrhage was often near a big vessel or at vessel junctions. Two cases of angiogenesis were found in the severe ischemia stroke brains in the treated group (Fig. 4 and 5), and no MRI evidence for angiogenesis was found in the treated but with mild stroke, untreated and sham surgery groups.

|
| Fig. 4 SWI shows the presence of angiogenesis (arrows) of a representative Sildenafil treated rat after two weeks post tMCAO (center column) compared with baseline before tMCAO (left column). The vessel grows to a bigger size after four weeks (right column). TE=5.37 ms, 1mm projection images for the purpose of demonstration. P904 dose was 100μmol Fe/kg |
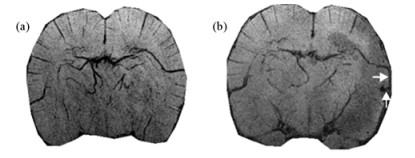
|
| Fig. 5 SWI images (1 mm projection) show a newly developed vessel in the second case after four weeks post tMCAO (b) which does not exist before tMCAO (a). The new vessel (upwards arrow) is connected to a preexisting vessel (rightwards arrow). P904 dose was 100 μmol Fe/kg |
In Fig. 4, SWI showed the presence of newly developed vessels near the periphery of the ischemic core in a Sildenafil treated severe stroke rat after two and four weeks post tMCAO. The new vessel was seen as hyperintensity in phase images and hypointensity in SWI images. It was MRI visible after two weeks post tMACO and grew to a bigger size after four weeks. For evaluation of the T2*s in the vessel, the region of interest (ROI) was drawn around the vessel in a 0.25 mm slice. With P904 injection, the average T2* values of this vessel were reduced from 25±9 (n=16) ms to 18±6 (n=17) ms after two weeks post tMCAO and were reduced from 24±12 (n=21) ms to 18±3 (n=24) ms after four weeks. Changes in T2* values of this new formed vessel by USPIO provided the evidence of active blood flow through it and therefore differentiated it from hemorrhage.
The other case of angiogenesis was found from the Sildenafil treated group as well. SWI of a severe stroke rat showed three abnormal spots in the ischemic core after two and four weeks post tMCAO (Fig. 5). The T2* value of one spot after four weeks remained unchanged about 17±2 (n=74)/17±3 (n=72) ms after USPIO injection. With USPIO aided in vessel enhancement, the other two spots (Fig. 5) were not only connected to each other (upwards arrow) but also connected to a preexisting vessel (rightwards arrow). Their T2* values after four weeks post tMCAO were reduced from 32±5 (n=66) ms to 17±3 (n=43) ms, and 22±3 (n=96) ms to 12±1 (n=106) ms respectively after USPIO injection. Therefore, they were identified as functioning new vessels.
We observed a new spot (arrows) in SWI [Fig. 6(a), Fig. 6(b)] after four weeks post tMCAO for the same rat showing angiogenesis in Fig. 4. It was within right thalamus and away from the ischemic core. The spot showed a higher susceptibility over usual small vessels from its phase wrapping, the dark phase with a white rim [(c) and (d)]. The average T2* values of this area pre/post USPIO were 12.5±2.4 (n=36)/12.4±2.6 (n=36) ms. Almost unchanged T2* value means that there was no USPIO arrival by active blood flow. The shorter T2* values indicate that it was most likely a hemorrhage rather than a new vessel. This characteristic of phase and T2* behaviors provided the evidence of hemorrhage in this case and differentiated it from angiogenesis.

|
| Fig. 6 SWI shows a hemorrhage (arrows) away from the ischemia core after four weeks post tMACO, in the same Sildenafil treated rat as in Fig.4. (a) and (b): SWI magnitude image without and with USPIO. (c) and (d): corresponding Phase images. The hemorrhage is located at a junction of vessels in the right thalamus. TE=5.37 ms, single slice, thickness=0.25 mm. P904 dose was 100 μmol Fe/kg |
Iron-based contrast agents USPIO ( < 50 nm) or SPIO ( < 100 nm) are usually used as T2*-shortening contrast agents while gadolinium-based contrast agents, e.g. Dotarem, are only used as T1-shortening contrast agents to detect disruption of brain blood barrier. USPIO P904 particles have inherent T1-shortening properties as well as strong T2* and susceptibility enhancing properties. Recent studies reported that P904 proved to be superior to Gd-DOTA for time-resolved MRA[12]. SPIO agents loaded in cells with low concentration have also been used as a positive MR contrast with a special MRI method[13]. Our low dose MRA experimental results demonstrated the feasibility for low dose USPIO P904 to be used as a T1 reducing contrast agent. Vascular enhancement by USPIO depended strongly on the dose used. A dose of roughly 5 to 10 μmol Fe/kg was expected to give the best results.
Moderate dose USPIO experiments demonstrated P904 as an effective T2* agent. Multi-echo SWI provided the flexibility of investigating vessels over a wide range at a moderate dose of 100 μmol Fe/kg. When aliasing occurs on big vessels at longer echo times, we resorted to using a shorter echo time of 5.37 ms for susceptibility mapping. When a small vessel was of interest, a longer echo time of 15.12 ms could be used. The T2* approach makes it possible to see different vessel sizes with T2* depending on TE. USPIO P904 SWI images with dramatic vascular contrast demonstrated tremendously small and previously invisible next order vessels (Fig. 3). For major arteries, the original magnitude images at the shortest echo time could be used to generate MRA images via maximum intensity projection. Finally, T2* maps (data not shown) could also be calculated from the same multi-echo SWI magnitude images without extra data collection.
Many large hemorrhages were observed inside the ischemic core post tMCAO surgery at the 24 h mark. These remained unchanged over time which agrees with previous investigations in a rat embolic stroke model[1, 2]. In one case, we observed a hemorrhage outside the ischemic core after four weeks post tMCAO (Fig. 6). The SWI resolution of 41.6 μm×41.6 μm×250 μm=0.43 (100 μm)3 in this work was 36-fold times better than previous quoted resolutions of 125 μm×125 μm×1 000 μm[2]. However, it still does not reach capillary level. Therefore angiogenesis is not seen for every stroke and treated rat. Our finding of angiogenesis at visible vessel level after two weeks in Sildenafil treated animals indicated that Sildenafil promoted angiogenesis which agreed with previous results as well[2]. In summary, using high resolution SWI made it possible to visualize angiogenesis and micro hemorrhages in greater detail (Fig. 6).
Foreign nanoparticles are usually taken up by macrophages in the liver, spleen, bone marrow or lymph nodes. In the case of iron oxide nanoparticles with the same coating material, USPIO particles are less prone to liver uptake due to their small size and hence have a longer circulation time and are good for macrophage imaging[14-24]. There is usually a 24 h scanning delay in USPIO macrophage imaging so that free USPIO particles are cleared from the circulation and all signal alterations arise from USPIO particles captured by phagocytotic cells[24]. Previous longitudinal investigations in a rat model of tMCAO showed that USPIO was observed in vessels at 24 h, within the infarct hemisphere on day 2 with maximal signal enhancement and disappeared between day 4 and day 7[17]. In the present study, we were in focus on USPIO enhancement on vasculature rather than tracking macrophage activity, therefore the MRI data were collected immediately after systemic administration of USPIO. We had four-time administrations of USPIO for each animal with one or two-week time intervals. In our baseline scans, intact blood-brain-barrier did not allow USPIO particles efflux from the vascular lumen to brain tissue. Therefore, no more residual USPIO from previous injection could affect next time point scans. There were also some challenges of in vivo high resolution SWI with USPIO. During the 41-minute scan, it is suggested to carefully keep the weak stroke animal alive and also avoid any head movement in order to obtain high quality images. Future high speed methods may make this goal easier to achieve. In addition, a non-human primate brain MCAO stroke model may be considered to use in P904 preclinical study for being structurally and functionally similar to the human brain[25].
In conclusion, at a lower dose between 5~10 μmol Fe/kg, arteries are enhanced by a factor of two and appear more clearly in an MRA. At the dose of 300 μmol Fe/kg, although USPIO reduces the intensities of blood vessels and contributes negative contrast in the magnitude image, it dramatically increases blood vessel phase contrast and contributes to positive contrast in phase images. Practically, multi-echo SWI provides the flexibility to investigate the vessels of various sizes at different echo times with a moderate dose of 100 μmol Fe/kg. High resolution multi-echo SWI with the injection of USPIO provides a noninvasive and in vivo way to visualize angiogenesis in a rat stroke model of transient MCAO and differentiate it from hemorrhage.
Acknowledgements: This work was supported by Guerbet Research, Aulnay-Sous-Bois, France. USPIO P904 was provided by Guerbet Research. Sildenafil was provided by the Research Division of Pfizer Inc. W.Z. was supported by the Canadian Institutes of Health Research (CIHR) /Heart and Stroke Foundation of Canada (HSFC) Synchrotron Medical Imaging Team Grant # CIF 99472. The authors thank Drs. Quan Jiang and Guang-liang Ding for their helpful discussion. The authors acknowledge assistance received from co-workers Lisa Brownchidle, Yang Xuan, Ching-yi Hsieh, Jing Jiang, Waqar Raza and Robin Roberts in the MR research facility in Wayne State University School of Medicine, Detroit, Michigan. The author (Y.S.) contributes this work as a memorial of his father Professor Lian-fang Shen.| [1] | Jiang Q, Zhang Z, Ding G et al . Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI[J]. Neuroimage , 2005, 28 : 698-707 DOI:10.1016/j.neuroimage.2005.06.063 |
| [2] | Ding G, Jiang Q, Li L et al . Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI[J]. Stroke , 2008, 39 : 1563-1568 DOI:10.1161/STROKEAHA.107.502146 |
| [3] | Haacke E M, Xu Y, Cheng Y C et al . Susceptibility weighted imaging (SWI)[J]. Magn Reson Med , 2004, 52 : 612-618 DOI:10.1002/(ISSN)1522-2594 |
| [4] | Haacke E M, Tang J, Neelavalli J et al . Susceptibility mapping as a Means to visualize veins and quantify oxygen saturation[J]. J Magn Reson Imaging , 2010, 32 : 663-676 DOI:10.1002/jmri.v32:3 |
| [5] | Ding Y, Zhou Y, Lai Q et al . Impaired motor activity and motor learning function in rat withmiddle cerebral artery occlusion[J]. Behavioural Brain Research , 2002, 132 : 29-36 DOI:10.1016/S0166-4328(01)00405-3 |
| [6] | Hunter A J, Green A R, Cross A J . Animal models of acute ischaemic stroke: can they predict clinically successful neuroprotective drugs?[J]. Trends Pharmacol Sci , 1995, 16 : 123-128 DOI:10.1016/S0165-6147(00)88999-3 |
| [7] | Mohr J P, Gautier J C, Hier D, Stein R W . Middle cerebral artery//Barnett HJM, Stein B M, Mohr J P, Yatsu F M. Editor Stroke: Pathophysiology, Diagnosis and Management[M]. New York: Churchill Livingstone, 1986 : 377 -450. |
| [8] | Saito I, Segawa H, Shiokawa Y et al . Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome[J]. Stroke , 1987, 18 : 863-868 DOI:10.1161/01.STR.18.5.863 |
| [9] | Koizumi J, Nakazawa T. Reperfusable brain infarction model in rat (abstratct). Proceedings of the 10th Meeting of the Japanese Stoke Society[C]. Kyoto, 1985. 4-18. |
| [10] | Longa E Z, Weinstein P R, Carlson S et al . Reversible middle cerebral artery occlusion without craniectomy in rats[J]. Stroke , 1989, 20 : 84-91 DOI:10.1161/01.STR.20.1.84 |
| [11] | Meier R, Henning T D, Boddington S et al . Breast cancers: MR imaging of FolateReceptor expression with the folatespecific nanoparticle P1133[J]. Radiology , 2010, 255 (2) : 527-535 DOI:10.1148/radiol.10090050 |
| [12] | Kinner S, Maderwald S, Parohl N et al . Contrastenhanced magnetic resonance angiography in rabbits: Evaluation of the gadoliniumbased agent P846 and the ironbased blood pool agent P904 in comparison with gadoterate meglumine[J]. Invest Radiol , 2011, 46 : 524-529 DOI:10.1097/RLI.0b013e31821ae21f |
| [13] | Cunningham C H, Arai T, Yang P C et al . Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles[J]. Magn Reson Med , 2005, 53 : 999-1005 DOI:10.1002/(ISSN)1522-2594 |
| [14] | Schroeter M, Jander S, Huitinga I et al . Phagocytic response in photochemically induced infarction of rat cerebral cortex[J]. Stroke , 1997, 28 : 382-386 DOI:10.1161/01.STR.28.2.382 |
| [15] | Schroeter M, Jander S, Witte O et al . Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion[J]. J Neuroimmunol , 1994, 55 : 195-203 DOI:10.1016/0165-5728(94)90010-8 |
| [16] | Rausch M, Sauter A, Frohlich J et al . Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage[J]. Magn Reson Med , 2001, 46 : 1018-1022 DOI:10.1002/mrm.1290 |
| [17] | Rausch M, Baumann D, Neubacher U et al . In vivo visualization of phagocytotic cells in rat brains after transient ischemia by USPIO[J]. NMR Biomed , 2002, 15 : 278-283 DOI:10.1002/(ISSN)1099-1492 |
| [18] | Sbarbati A, Reggiani A, Nicolato E et al . Correlation MRI/ultrastructure in cerebral ischemic lesions: application to the interpretation of cortical layered areas[J]. Magn Reson Imaging , 2002, 20 : 479-486 DOI:10.1016/S0730-725X(02)00528-3 |
| [19] | Schroeter M, Franke C, Stoll G et al . Dynamic changes of magnetic resonance imaging abnormalities in relation to inflammation and glial responses after photothrombotic cerebral infarction in the rat brain[J]. Acta Neuropathol , 2001, 101 : 114-122 |
| [20] | Kleinschnitz C, Bendzus M, Frank M et al . In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging[J]. J Cereb Blood Flow Metab , 2003, 23 : 1356-1361 |
| [21] | Saleh A, Wiedermann D, Schroeter M et al . Central nervous system inflammatory response after cerebral infarction as detected by magnetic resonance imaging[J]. NMR Biomed , 2004, 17 : 163-169 DOI:10.1002/(ISSN)1099-1492 |
| [22] | Schroeter M, Saleh A, Wiedermann D et al . Histochemical detection of ultrasmall superparamagnetic iron oxide (USPIO) contrast medium uptake in experimental brain ischemia[J]. Magn Reson Med , 2004, 52 : 403-406 DOI:10.1002/(ISSN)1522-2594 |
| [23] | Weber R, Wegener S, RamosCabrer P et al . MRI detection of macrophage activity after experimental stroke in rats: new indicators for late appearance of vascular degradation?[J]. Magn Reson Med , 2005, 54 : 59-66 DOI:10.1002/(ISSN)1522-2594 |
| [24] | Dousset V, Gomez C, Petry K G et al . Dose and scanning delay using USPIO for central nervous system macrophage imaging[J]. MAGMA , 1999, 8 : 185-189 DOI:10.1007/BF02594597 |
| [25] | Zhang X D . Magnetic resonance imaging of nonhuman primate ischemic stroke models[J]. Chinese J Magn Reson , 2010, 27 (4) : 548-561 |
 2014, Vol. 31
2014, Vol. 31

