2. Shenzhen Research Institute of Xiamen University, Shenzhen 518057, China
2. 厦门大学 深圳研究院, 广东 深圳 518057
Nuclear magnetic resonance (NMR) spectroscopy has been a powerful tool in numerous research fields[1-5]. As an important parameter in NMR spectroscopy, scalar coupling and plays an important role in molecular structure elucidation by providing useful information about molecular structure. However, the measurement of the coupling constant (J) often is not an easy task because of complex peak splitting and signal overlap in a NMR spectrum.
Recently, many novel methods[6] have been proposed to study scalar coupling networks and measure J coupling constants. Selective refocusing (SERF) experiment opened the door of measuring specific proton-proton interactions from spectra[7]. However, using this method, only the coupling constant between two selected protons can be measured at one time, which results in long experiment time to measure all J coupling constants. Subsequently, the gradient-encoded homonuclear selective refocusing spectroscopy (G-SERF) method[8] was proposed to resolve a coupling network of a specific proton in one experiment and to measure the coupling constants in this network. The G-SERF method relies on selective pulses, and thus great limitations exist in those samples with crowded peaks. The pure shift yielded by chirp excitation to deliver individual couplings (PSYCHEDELIC)[9] was proposed to provide a general method for obtaining J coupling constants even in crowded NMR spectra. The selective constant-time (SECT) method is a selective constant time method for resolving a coupled network of a selected proton at one time and measuring the J coupling constants[10]. Because of the removal of slice selection, a higher signal-to-noise ratio (SNR) can be obtained by optimizing the constant time (∆). However, the signal of the SECT method is modulated by relaxation decay during ∆. The method of simultaneous multi-slice selective J-resolved spectroscopy (SMS-SEJRES) aims at simultaneously measuring J values of all coupling networks in a molecule within one experiment[11]. However the SNR of spectra obtained using the SMS-SEJRES method is limited by Zangger-Sterk (ZS) and pure shift yielded by chirp excitation (PSYCHE) modules[12, 13]. Here, we propose a method of simultaneous multi-slice selective constant-time J-resolved spectroscopy (SMS-SECTJRES) for analyzing the coupling network of all protons in a molecule in one experiment and measuring the J coupling constants with higher sensitivity.
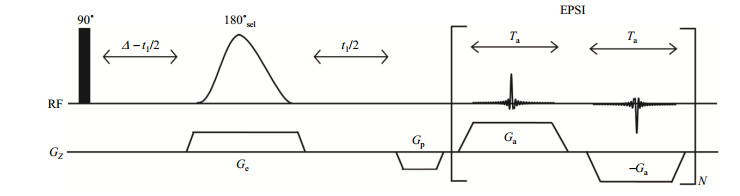
1 Experimental 1.1 SMS-SECTJRES pulse sequenceThe proposed sequence of SMS-SECTJRES is shown in Fig. 1. The first hard 90° pulse excites all magnetization vectors from the z direction to the xy plane. After the 90° hard pulse, a fixed constant time (∆) is set before sampling. A selective 180° soft pulse applied simultaneously with the weak spatial frequency encoding gradient along the z direction moves between the 90° hard pulse and the sampling period as the indirect dimensional evolution time (t1) increases. With the action of the spatial frequency encoding gradient, the selective 180° soft pulse flips different protons at different spatial positions. The spatial frequency encoding gradient produces a frequency range that is slightly larger than the spectral width of the one-dimensional spectrum of the sample, so that the selective 180° pulse can act on all of the protons in the sample and eliminate the edge effects. In a certain layer, the selective 180° soft pulse flips the spin A, then in this layer, chemical shifts and scalar couplings of other spins evolve for a fixed constant time ∆, except that those scalar coupling involving spin A evolve for t1. For the entire sample, it means performing SECT experiments on different spins in different layers. The echo planar spectroscopic imaging (EPSI) sampling module is used to simultaneously obtain both chemical shift and spatial position information, and thus separates signals from different layers. The gradient Gp is used to compensate for the dispersion caused by the spatially encoded gradient Ge and to adjust the center of the echo to the middle of the sampling window. In a certain layer, couplings involving the spin which is inverted by the 180° soft pulse can be obtained, and these couplings are present as splitting peaks in the indirect dimension. Thus J coupling constants can be measured from these splitting peaks. In the SMS-SECTJRES method, the constant time (∆) should be optimized to satisfy the signal-to-noise ratio and resolution requirements. The maximal evolution time (t1) of the SMS-SECTJRES method is 2∆, so the digital resolution (v1) of the F1 dimension is
|
Fig. 1 The pulse sequence of SMS-SECTJRES. The filled rectangle and gauss-shape pulse represent 90° hard pulse and 180° selective pulse, respectively. The gradient Gp is used to compensate for the dispersion caused by the spatially encoded gradient Ge and to adjust the center of the echo to in the middle of the sampling window. Sampling gradients Gas are used to spatial frequency coding |
All experiments were performed on a Varian 500 MHz spectrometer using a detection probe with a z-direction gradient at 298 K. The duration and power of 90° RF pulse were 9.8 μs and 58 dB respectively. All experimental samples were purchased from Aladdin whose website is https://www.aladdin-e.com/.
The first sample was 1 mol/L n-butyl bromide dissolved in CDCl3. The constant time (∆) was 0.4 s, the indirect dimension spectral width (SW1) was 50 Hz, and the indirect dimension sampling number was 40. The selective 180° pulse was set to 11.8 ms (corresponding to an excitation bandwidth of approximately 132 Hz) and its excitation center frequency was set to the center frequency of the one-dimensional spectrum of the sample. The spatially encoded gradient Ge was 0.34×10-4 T/cm, allowing the action of a selective pulse to cover all peaks of the sample. Gp was -1.75×10-3 T/cm with the duration of 500 μs. The sampling time (Ta) was 250 μs and Ga was 37.8×10-4 T/cm. The number of transients (Nt) was 8, and the total experimental time was 42 min.
The second sample used was 1 mol/L propylene carbonate dissolved in CDCl3. The constant time (∆) was 0.32 s, the spectral width (SW1) in indirect dimension was 50 Hz, and the numbers of t1 increments was 32. The duration of selective 180° pulse was 15.2 ms (corresponding to an excitation bandwidth of approximately 103 Hz) and its excitation center frequency was set to the center frequency of the one-dimensional spectrum of the sample. The spatially encoded gradient Ge was 0.38×10-4 T/cm, allowing the action of a selective pulse to cover all peaks of the sample. Gp was -30.0×10-4 T/cm with duration of 500 μs. The sampling time Ta was 250 μs and Ga was 37.8×10-4 T/cm. The number of transients (Nt) was 8, and the total experimental time was 32 min.
The third sample used was 0.1 mol/L L-menthol dissolved in CDCl3. The constant time (∆) was 0.4 s, the spectral width in the indirect dimension (SW1) was 50 Hz, and the numbers of t1 increments was 40. The duration of selective 180° pulse was 22 ms (corresponding to an excitation bandwidth of approximately 70.9 Hz) and its excitation center frequency was set to δH 1.8 in the one-dimensional spectrum of the sample. The spatially encoded gradient (Ge) was 0.11×10-4 T/cm, allowing the selective pulse to cover all peaks of the sample. Gp was -9.4×10-4 T/cm with duration of 500 μs. The sampling time (Ta) was 250 μs and Ga was 37.8×10-4 T/cm. The number of transients (Nt) was 32, and the total experimental time was 137 min.
The data collected under positive gradients Ga were rearranged into a two-dimensional matrix, and thus a three-dimensional data was obtained considering the t1 dimension. After zero-filling and a weighting function of t1, a three-dimensional Fourier transform was performed to obtain a three-dimensional spectrum, and the three dimensions correspond to J coupling, chemical shift, and spatial position, respectively. Different two-dimensional spectra can be obtained by extracting from the three-dimensional spectrum according to the spatial dimension. The selective J spectrum of the coupling network of all spins can be found from these two-dimension spectra. And for different spins, their order in spatial dimension is consistent with that in chemical shift dimension. In this way, the J spectra of the coupled networks of all the spins were obtained to measure all the J coupling constants at one time.
2 Results and discussionFig. 2 shows the experimental results of n-butyl bromide dissolved in CDCl3 using SMS-SECTJRES method. Four selective J-resolved spectra [Fig. 2(b)~(e)] can be extracted from different spatial layers, corresponding to coupled networks of four different spins. Fig. 2(b) is extracted from the layer in which the spin H1 is flipped by the selective 180°pulse. A triplet appears at the position of chemical shift of the spin H2, meaning that there is a coupling relationship between the spins H-1 and H-2, and the scalar coupling constant is 6.9 Hz. Because spin H-1 has two hydrogen nuclei in the same chemical environment, spin H-2 splits into triplets due to scalar coupling with H-1. Similarly, Fig. 2(c) shows the coupling relationships between H-2 and H-1, H-3. Fig. 2(d) shows the coupling relationship between H-3 and H-2, H-4. Fig. 2(e) shows the coupling relationship between H-4 and H-3. The coupling network of the sample can be resolved in one experiment and all JH-H values can be measured.

|
Fig. 2 The result of n-butyl bromide dissolved in CDCl3 using SMS-SECTJRES method. (a) Conventional one-dimensional 1H NMR spectrum; (b)~(e) Two-dimensional spectra of the coupling networks of protons H-1, H-2, H-3, H-4, respectively, and the J coupling constants are marked |
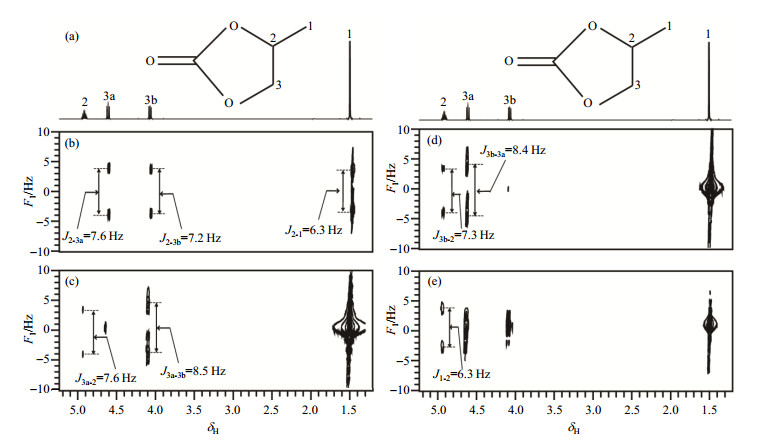
Fig. 3 shows the experimental results of propylene carbonate dissolved in CDCl3 using SMS-SECTJRES method. Fig. 3 (a) shows the planar structure of the propylene carbonate molecule and its conventional one-dimensional 1H NMR spectrum. And Fig. 3(b)~(e) present two-dimensional spectra of the coupling networks corresponding to the protons of H-2, H-3a, H-3b, H-1, respectively, where scalar coupling constants can be accurately measured. Since each of H-2, H-3a, H-3b, and H-1 contains only one hydrogen nucleus, and all peaks split into doublets.
|
Fig. 3 The result of propylene carbonate dissolved in CDCl3 using SMS-SECTJRES method. (a) Conventional one-dimensional 1H NMR spectrum; (b)~(e) Two-dimensional spectra of the coupling networks of protons H-2, H-3a, H-3b, H-1, respectively, and the corresponding J coupling constants are marked |
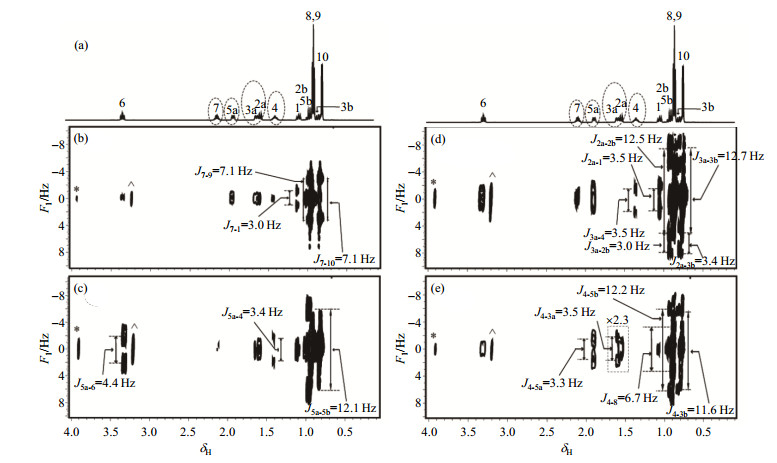
Fig. 4 shows the experimental results of L-menthol dissolved in CDCl3 using SMS-SECTJRES method. Fig. 4 (a) shows the conventional one-dimensional 1H NMR spectrum of L-menthol, and the peaks attracting us are circled with dashed line. The peaks of H-3a and H-2a are so close that cannot be separated. Fig. 4(b)~(e) present two-dimensional spectra of the coupling networks corresponding to the protons of H-7, H-5a, H-3a & H-2a, H-4, respectively, where scalar coupling constants can be accurately measured. Since each of the measured peaks only has one hydrogen nucleus, all peaks coupled with the measured proton split into doublets.
|
Fig. 4 The result of L-menthol dissolved in CDCl3 using SMS-SECTJRES method. (a) Conventional one-dimensional 1H NMR spectrum, the peaks attracting us are circled with dashed line; (b)~(e) Two-dimensional spectra of the coupling networks of protons H-7, H-5a, H-3a & H-2a, H-4, respectively with J coupling constants indicated. Peaks marked with * and ^ in (b)~(e) denote peaks of TMS and solvent due to aliasing |
In SMS-SECTJRES experiment, it should be noted that the bandwidth of selective pulse needs to be smaller than the smallest chemical shift difference of the sample, that is, the excitation bandwidth should be smaller than the smallest chemical shift difference in conventional proton spectrum. In this way, selective 180° pulse separately excites different signals at different layers. Furthermore, contradiction exists between the resolution of the spatial dimension (F3 dimension) and the spectral width of the chemical dimension (F2 dimension). In theory, the spatial resolution is ∆L = 1/(γ·Ta·Ga), which should be able to distinguish different layers. While the spectral width of the F2 dimension is SW2 = 1/(2Ta). From their expressions we can see that increasing Ta can improve the spatial resolution of the F3 dimension, but will reduce the spectral width SW2 of the F2 dimension. For samples with wide chemical shift ranges, aliasing can be introduced deliberately in the F2 dimension to alleviate this problem, but this will also increase spectral complexity.
The signal attenuation in the SMS-SECTJRES experiment mainly comes from spatially selective inversion, relaxation in the constant time, and J coupling modulation. The signal attenuation introduced by the spatially selective inversion module is related to the selected layer thickness [(BW / (γ·Ge), where BW is the excitation bandwidth of the selective pulse, γ is the gyromagnetic ratio of hydrogen, and Ge is the magnitude of the spatial encoding gradient)]. For samples with the crowded peaks, the bandwidth of the selective 180° pulse needs to be narrow enough to separately invert different signals, which aggravates signal attenuation. The chemical shift range of the sample determines the amplitude of spatially encoded gradient Ge, which also affects the spectral signal-to-noise ratio. The relaxation attenuation in constant time is
In summary, the SMS-SECTJRES pulse sequence is proposed based on selective constant-time evolution, spatial encoding and EPSI sampling module to reveal the coupled networks of all protons in a molecule and to measure all J coupling constants. It allows extracting different selective J-edited spectra in different spatial positions from the result obtained by SMS-SECTJRES. It facilitates molecular structure analysis and promotes NMR application in chemistry.
| [1] |
DONG J Y, XU L, CAO H T, et al. A new data processing method for metabonomic and its application in a study of diabetes[J].
Chinese J Magn Reson, 2007, 24(4): 381-393.
董继扬, 徐乐, 曹红婷, 等. 代谢组学数据分析方法及在糖尿病研究中的应用[J]. 波谱学杂志, 2007, 24(4): 381-393. DOI: 10.3969/j.issn.1000-4556.2007.04.001. |
| [2] |
ZHANG F, LIN D H. Interaction of proteins with metal ions studied by NMR techniques: A review[J].
Chinese J Magn Reson, 2009, 26(1): 136-149.
张芳, 林东海. 用核磁共振方法研究金属离子与蛋白质的相互作用[J]. 波谱学杂志, 2009, 26(1): 136-149. DOI: 10.3969/j.issn.1000-4556.2009.01.016. |
| [3] |
CHAI X, SUN P, YUAN B, et al. Fast detection of choline containing compounds in dairy products using NMR[J].
Chinese J Magn Reson, 2018, 35(2): 178-187.
柴鑫, 孙鹏, 袁斌, 等. 乳制品中胆碱及其衍生物的快速核磁共振检测[J]. 波谱学杂志, 2018, 35(2): 178-187. |
| [4] |
ZHOU Z M, LIU M L, ZHANG X, et al. Two effective methods for improving NMR sensitivity and resolution in studies of biomacromolecules: TROSY and CRINEPT[J].
Chinese J Magn Reson, 2004, 21(3): 371-383.
周志明, 刘买利, 张许, 等. 提高生物大分子NMR分辨率和灵敏度的有效方法: TROSY和CRINEPT[J]. 波谱学杂志, 2004, 21(3): 371-383. DOI: 10.3969/j.issn.1000-4556.2004.03.015. |
| [5] |
SUN Y, CHEN Y K, LI J P, et al. Efficiency of double cross polarization in magic-angle spinning solid-state nmr studies on membrane proteins[J].
Chinese J Magn Reson, 2017, 34(3): 257-265.
孙毅, 陈艳可, 李建平, 等. 固体核磁共振中膜蛋白双交叉极化效率与动力学参数相关的定量分析[J]. 波谱学杂志, 2017, 34(3): 257-265. |
| [6] | LIN Y Q, ZENG Q, LIN L J, et al. High-resolution methods for the measurement of scalar coupling constants[J]. Prog Nucl Magn Reson Spectrosc, 2018, 109: 135-159. DOI: 10.1016/j.pnmrs.2018.08.003. |
| [7] | FACKE T, SERF B S. A new method for H, H spin-coupling measurement in organic chemistry[J]. J Magn Reson, 1995, 113(1): 114-116. |
| [8] | GIRAUD N, BEGUIN L, COURTIEU J, et al. Nuclear magnetic resonance using a spatial frequency encoding: application to J-edited spectroscopy along the sample[J]. Angew Chem Int Ed Engl, 2010, 49(20): 3481-3484. DOI: 10.1002/anie.200907103. |
| [9] | SINNAEVE D, FOROOZANDEH M, NILSSON M, et al. A general method for extracting individual coupling constants from crowded 1H NMR spectra[J]. Angew Chem Int Ed Engl, 2016, 55: 1090-1093. DOI: 10.1002/anie.201508691. |
| [10] | LIN L J, WEI Z L, LIN Y Q, et al. Measuring JH-H values with a selective constant-time 2D NMR protocol[J]. J Magn Reson, 2016, 272: 20-24. DOI: 10.1016/j.jmr.2016.08.019. |
| [11] | ZENG Q, LIN L J, CHEN J Y, et al. A simultaneous multi-slice selective J-resolved experiment for fully resolved scalar coupling information[J]. J Magn Reson, 2017, 282: 27-31. DOI: 10.1016/j.jmr.2017.07.001. |
| [12] | ZANGGER K, STERK H. Homonuclear broadband-decoupled NMR spectra[J]. J Magn Reson, 1997, 124: 486-489. DOI: 10.1006/jmre.1996.1063. |
| [13] | FOROOZANDEH M, ADAMS R W, MEHARRY N J, et al. Ultrahigh-resolution NMR spectroscopy[J]. Angew Chem Int Ed Engl, 2014, 53(27): 6990-6992. DOI: 10.1002/anie.201404111. |
 2019, Vol. 36
2019, Vol. 36 
