上世纪以来,全球工业化导致重金属的释放量急剧增加,铜矿开采导致矿区周边土壤的铜含量极高[1]。铜是植物必需营养元素,但过量时会干扰植物光合作用,增大细胞膜透性,减缓细胞分裂,抑制其他养分吸收,甚至引起死亡[2]。人体摄入铜过量会引起低血压等铜代谢疾病[3]。
植物提取是去除土壤重金属的有效方法,具有成本低、去除彻底和景观美学作用,是一种应用前景广阔的重金属修复手段[4]。已发现的铜超积累植物有海州香薷 (Elsholtzia splendens)、鸭跖草 (Commelina communis)、矮石蕊 (Cladonia humilis) 等[5–6],它们普遍生物量小、生长缓慢,吸收积累铜量有限,收获物经济价值不高。
蓖麻是重要的工业原料[7],同时具有较高的土壤重金属污染修复价值,对Cu、Cd、Zn等重金属有较高的耐性[8],能在高浓度铜污染土壤上正常生长[9]。Huang等[10]水培发现,在750 μmol/L铜处理下,蓖麻根部铜浓度可达14587 mg/kg,Olivares等[11]发现蓖麻生长在金属矿区土壤上,籽粒产油率更高,具有修复土壤铜污染的潜力。但作为土壤铜污染修复植物,蓖麻与其他耐性植物一样,仍具有一定的局限性,如铜的转运系数较低、生长周期慢等。
施肥既可促进植物生长,又可影响土壤性质 (如酸碱性) 和重金属的形态,提高植物修复效率[12]。研究发现,NH4+-N能显著增加土壤中Pb、Cd的生物有效性[13],NO–3-N能促进Pb从鱼腥草地下部向地上部转移[14]。磷肥可促进植物早期根系的形成和生长,增强植物抗性[15],在含铜100 mg/kg的土壤中施用80 kg/hm2 P2O5可使油芥菜生物量增大31%[16],施磷可增加水稻对镉的吸收但缓解毒性症状[17],黄化刚等[18]研究表明4种磷肥 [KH2PO4、Ca(H2PO4)2、NaH2PO4和NH4H2PO4] 对东南景天吸收重金属均有促进作用。
氮、磷肥对植物吸收积累铜的影响的试验多在人工添加铜土壤上进行,对原位重金属污染土壤的研究较少。氮、磷肥施用在农作物增产领域研究较多,但在植物修复领域研究较少。本试验希望找到既促进蓖麻生长、又增加蓖麻吸收积累铜量的最佳施肥组合,并探索施肥对蓖麻吸收铜的影响及作用机制,为土壤铜污染修复提供科学依据。
1 材料与方法 1.1 试验方法 1.1.1 试验材料供试植物为蓖麻 (Ricinus communis L.),种子采自湖北省大冶市铜绿山镇铜矿坑周边,在华中农业大学温室培育并开展盆栽试验。供试土壤采自矿坑,风干、磨碎、装盆。土壤基本理化性质为:pH 8.36、有机质9.74 g/kg、全氮0.57 g/kg、碱解氮22.34 mg/kg、速效钾19.23 mg/kg、有效磷0.43 mg/kg、土壤总铜含量1249.7 mg/kg,其中弱酸提取态3.4 mg/kg、可还原态371.8 mg/kg、可氧化态195.1 mg/kg、残渣态 (铜总量减去其他形态铜含量) 679.4 mg/kg。
1.1.2 盆栽试验供试肥料为NH4NO3和NaH2PO4,分析纯试剂 (国药集团化学试剂有限公司)。设置N 0、75、150、300 mg/kg 4个水平 (用N0、N75、N150、N300表示),P 0、20、80、200 mg/kg 4个水平 (用P0、P20、P80、P200表示),进行双因素4水平完全随机设计,施肥处理包括N0P0、N75~N300、P20~P200以及9个氮磷配施组合。将肥料溶解后均匀喷洒到1.5 kg土壤中,充分混匀后装盆 (直径上口15.5 cm、底部10.5 cm、高13.4 cm),另取部分土壤不种植蓖麻,其余管理 (施肥、水分、温度等) 与植株培养相同。每盆3棵植株,每个处理3次重复。
1.1.3 植株种植与收获在云母基质 (添加有机物35 g/kg) 中育苗,待植株长出2片真叶后,挑选长势一致的幼苗用蒸馏水洗净、移栽。将移栽盆放于温室中 (湿度40%) 培养,设置白天14 h,30℃;夜间10 h,20℃。每日定时浇水至田间持水量的60%,2个月后收获。用不锈钢剪刀将植物根、茎、叶分开,蒸馏水洗净、吸干,分别装入牛皮纸袋中,105℃杀青30 min,65℃烘至恒重。烘干植株样粉碎,用于Cu含量的测定。采集种植和未种植的全部土壤,研磨过20目和100目尼龙筛,分别用于测定基本理化性质和Cu含量。
1.2 测定方法 1.2.1 植株生长指标及Cu含量收获前2天对植株的6片成熟叶片距叶基部1/2处使用叶绿素仪 (SPAD-502) 测定,取平均值作为该株的最终SPAD值。植物Cu含量采用HNO3–HClO4消煮法,称取0.2000 g植株样品于消化管中,加入10 mL混合酸 (7.5 mL HNO3 + 2.5 mL HClO4),在远红外消煮炉上390℃消煮至溶液澄清,超纯水定容,过滤后用原子吸收分光光度计 (Varian AA-240FS) 测定[19]。
1.2.2 土壤基本理化性质及Cu形态分级土壤基本理化性质测定参照《土壤农化分析》 [20]。土壤pH采用pH计测定,土水比1∶2.5。土壤有机质用重铬酸钾氧化-外加热法测定。Cu总量用王水消化,原子吸收分光光度计 (Varian AA-240FS) 测定。用BCR形态分级法提取不同形态Cu [21],即弱酸提取态用0.11 mol/L HOAc浸提,可还原态用0.5 mol/L盐酸羟胺浸提,可氧化态用H2O2–NH4OAc浸提,提取液用原子吸收分光光度计 (Varian AA-240FS) 测定Cu含量。
质量控制:在样品分析过程中进行方法空白、基质加标、平行样以及加标回收测定。添加标准物质灌木枝叶 (GBW07603) 进行植物Cu含量质量控制。
1.3 数据分析及制图采用EXCEL2016进行数据统计及处理,采用SPSS20.0进行数据分析,利用Duncan法进行平均数间差异显著性检验,采用Origin9.0作图。
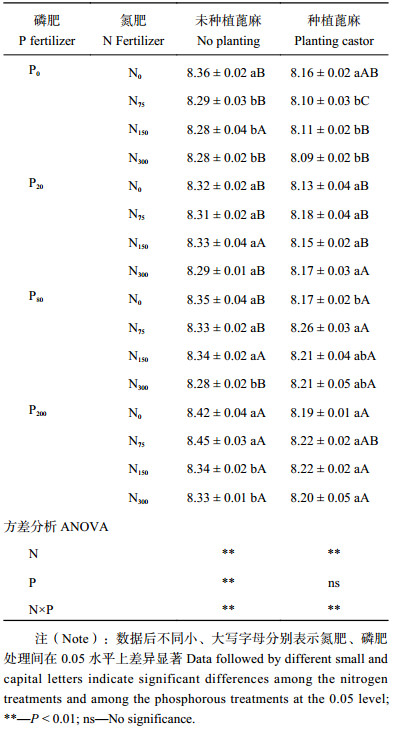
2 结果与分析 2.1 不同施肥处理下蓖麻生长情况表1可以看出,N150P20和N75P80处理蓖麻的SPAD值低于N0P0处理,其余处理均能增加SPAD值。氮、磷肥单施时SPAD值随施肥量增加而增加,N0P200和N300P0处理SPAD值最高,分别比N0P0处理增加13.6%和15.7%。两种肥料配施,施氮量为150和300 mg/kg时,SPAD值随施磷量增加先减少后增加,施氮量为75 mg/kg时,SPAD值随施磷量增加呈先增加后减少;施磷量为20 mg/kg时,N150P20处理SPAD值最低,与N75P20、N300P20处理差异显著,当施磷量为80和200 mg/kg时,SPAD值随施氮量增加而增加,N300P200处理最高,为N0P0处理的1.23倍。氮磷肥的交互作用显著,SPAD值在低量配施时出现波动,在高量配施时一直增长。施用高量氮、磷肥能显著提高蓖麻SPAD值。
| 表1 不同施肥处理下蓖麻叶片SPAD值 Table 1 SPAD values of castor leaves under different fertilization |
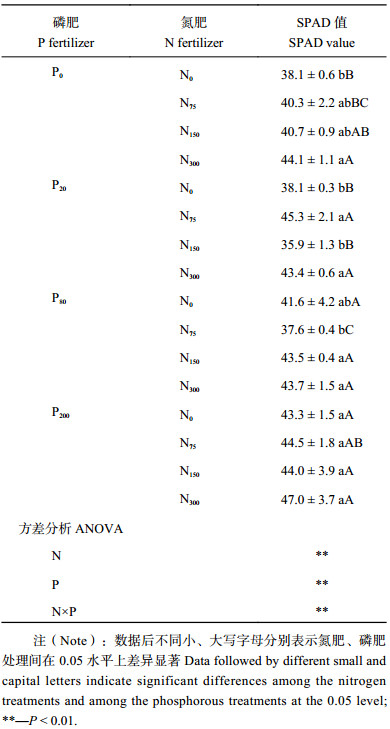 |
表2显示,氮、磷肥单施时蓖麻根重随施肥量增加先增加后减少,N75P0处理根重最大且与其他处理差异显著。氮磷肥交互作用显著,配施时高量氮肥处理根重均低于N0P0处理。N75P200处理蓖麻的根重显著高于其他组合,是N0P0处理的1.50倍。单施氮、磷肥时,蓖麻茎重、叶重的变化与根重变化一致,N0P20处理茎重、叶重最大且与其他处理差异显著。氮磷肥交互作用显著,氮、磷肥配施时,蓖麻茎重随施磷量增加缓慢增加,蓖麻叶重增加明显。N0P20、N75P200处理蓖麻茎重最大且与其他处理差异显著,分别是N0P0处理的2.01、1.91倍。N150P20、N300P20处理蓖麻茎重低于N0P0处理。但低磷与氮肥配施叶重低于单施氮、磷肥处理。N0P20、N75P200处理蓖麻叶重最大且与其他处理差异显著,分别是N0P0处理的1.82、1.94倍。单施氮、磷肥时蓖麻总重相对于不施肥处理都有增加,但蓖麻总重随着氮肥用量增加而减少,N300P0处理与N0P0处理差异不显著;N0P200处理显著增大了蓖麻干重,其他处理与N0P0处理间没有差异。
显然,氮、磷的施用对蓖麻地上部生物量的增加效果显著,但对蓖麻根系生长促进效果不明显,甚至有些处理抑制了蓖麻根系的生长。
| 表2 不同施肥处理下蓖麻干重 (g/plant) Table 2 Dry weight of castor under different fertilization |
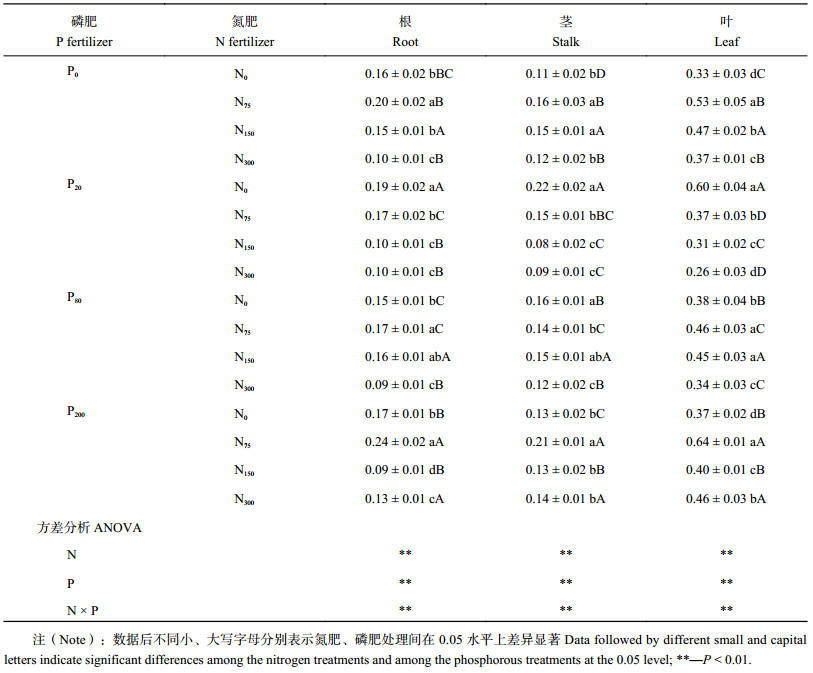 |
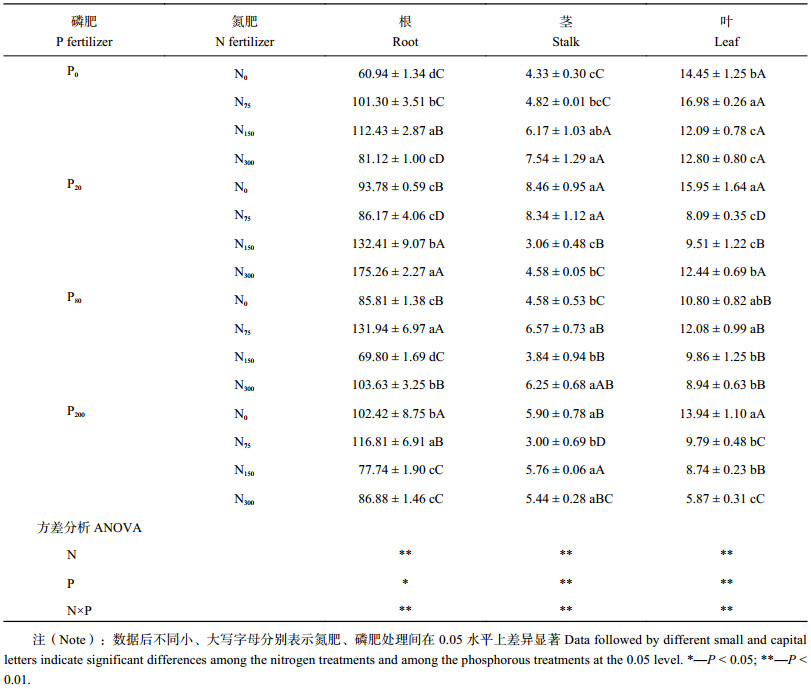
表3显示,所有施肥处理蓖麻根部Cu浓度均高于N0P0处理,其中N300P20处理根部Cu浓度最高,达175.2 mg/kg,比N0P0处理高287.6%。单施氮肥,蓖麻根部Cu浓度比N0P0处理高33.1%~84.5%,差异显著;单施磷肥,蓖麻根部Cu浓度比N0P0处理高40.8%~68.1%,差异显著。氮肥用量为75 mg/kg时,蓖麻根部Cu浓度随磷肥用量增加先升高后降低;磷肥用量为20 mg/kg时,蓖麻根部Cu浓度随施氮量增加而升高,施磷量为80和200 mg/kg时,根部Cu浓度随氮用量增加先升高后降低。单施氮肥,茎部Cu浓度随施氮量增加而增加,高量时与N0P0处理差异显著。N0P20、P20N75处理蓖麻茎部Cu浓度最高,分别比N0P0处理高95.3%、92.5%。P20N150、P80N150、P200N75处理蓖麻茎部Cu浓度仅3.00~3.84 mg/kg,低于N0P0处理。磷肥用量为20 mg/kg时,茎部Cu浓度随氮用量增加先升高后显著降低,而磷浓度为200 mg/kg时,茎部Cu浓度随氮肥用量增加先降低后升高。除N75P0、N0P20处理蓖麻叶部Cu浓度高于N0P0处理外,其他处理叶部Cu浓度均低于N0P0处理。蓖麻叶部Cu浓度随施氮量和施磷量增加而降低。氮磷配施除N300P20处理,其他处理叶部Cu浓度都显著低于单施氮肥;除P80与氮肥配施外,P20、P200与氮肥配施处理叶部Cu浓度均显著低于单施磷肥。
总体来看,氮、磷肥能提高蓖麻体内Cu浓度,低量磷肥配施氮肥能增加蓖麻根部Cu浓度,高量磷肥配施氮肥使蓖麻叶部Cu浓度减少,这可能是施肥增大蓖麻根、茎生物量引起的稀释效应。
| 表3 不同施肥处理下蓖麻体内铜含量 (mg/kg) Table 3 Cu contents of castor under different fertilization |
 |
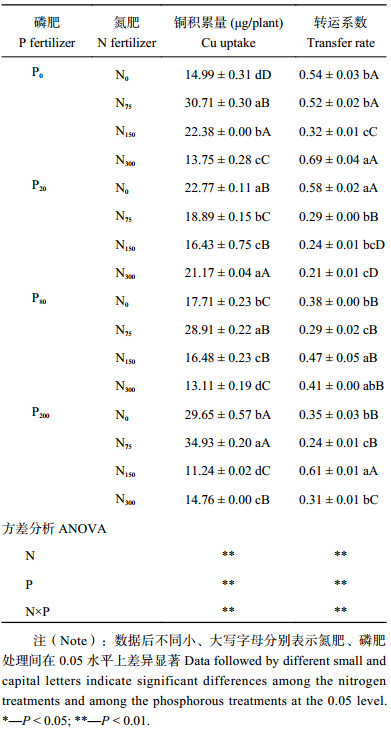
蓖麻积累Cu的变化如表4所示。单施氮肥时,铜积累量随施氮量增加而减少;单施磷肥时,铜积累量随施磷量增加先减少后增加。当施磷量为0、80、200 mg/kg时,低氮 (N75) 处理的铜积累量高于其他氮浓度处理且差异显著,其中N75P200处理蓖麻铜积累量最高,是N0P0处理的2.33倍,N75P0、N75P80处理铜积累量是N0P0处理的2.04、1.93倍,并与N75P200差异显著。铜积累量随氮肥用量增加而减少;单施磷肥和低氮 (N75) 配施磷肥处理铜积累量随施磷量增加而增加,中氮高氮 (N150、N300) 配施磷肥处理铜积累量随施磷量增加而减少。添加氮、磷肥后,铜在蓖麻各器官的分布仍为根>叶>茎,但转运系数 (地上部铜积累量/地下部积累量) 变化很大,变化规律与铜积累量变化规律相反,N300P20处理最低,N300 P0处理最高。
| 表4 氮、磷配施对蓖麻积累铜的影响 Table 4 Effects of N and P fertilizers on Cu accumulation in castor |
 |
土壤pH值的变化见表5。施肥但未种植蓖麻土壤pH值范围8.28~8.45,除N0P200、N75P200处理外,其余处理的pH值均低于N0P0处理。单施氮肥,土壤pH值与N0P0相比显著降低但不同浓度间无差异;施磷量为20 mg/kg的处理之间土壤pH值无显著差异;施氮量为0、75、300 mg/kg时,高磷处理土壤pH值都显著高于其他处理。种植蓖麻土壤pH值范围8.09~8.26,其变化规律与未种植蓖麻土壤的相似,施磷量为20、200 mg/kg的处理之间土壤pH值无显著差异,氮、磷肥配施除N150P20处理外土壤pH值都高于N0P0处理。种植蓖麻土壤pH比未种植土壤低0.2~0.4个单位。
| 表5 不同施肥处理下土壤pH值 Table 5 Soil pH under different fertilization |
 |
土壤Cu形态变化的BCR分级结果如图1所示。可以看出,所有土壤中可氧化态和残渣态Cu之和都超过总量的60%,未种植蓖麻处理之间、种植蓖麻处理之间不同形态Cu所占比例无显著变化。施肥但未种植蓖麻土壤中弱酸提取态Cu平均含量为3.54 mg/kg,占全量的0.3%,种植蓖麻土壤中弱酸提取态Cu平均含量为12.11 mg/kg,占全量的1.0%,提升了3.58倍;种植蓖麻土壤中可还原态Cu比例较未种植土壤增加约10%,残渣态Cu占比减少约10%,可氧化态Cu比例基本不变。可见,种植蓖麻可能会导致残渣态Cu向可还原态Cu转化。
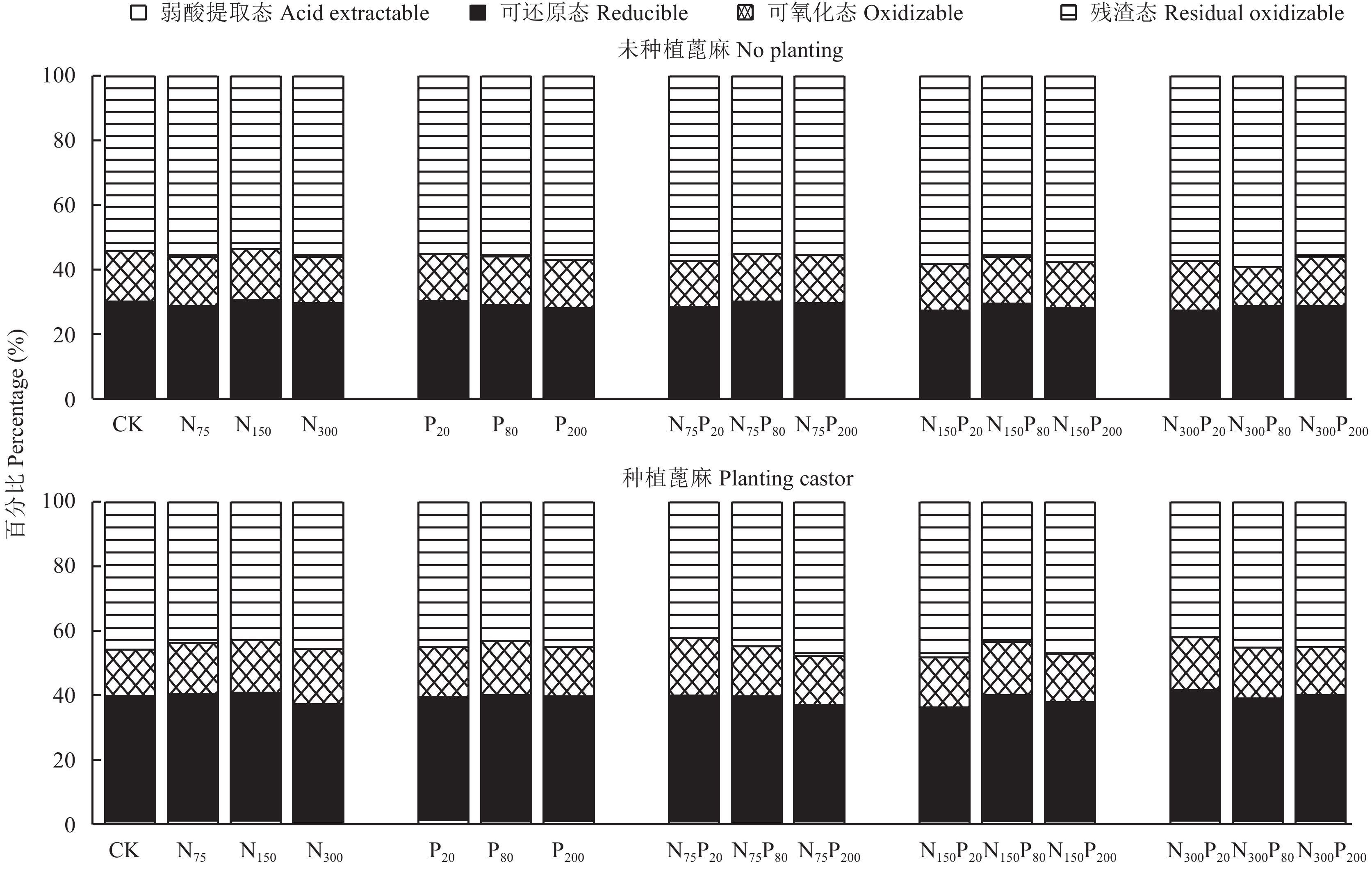 |
| 图1 土壤不同形态铜含量百分比 Fig. 1 Percentages of the different forms of Cu in soil |
植物的光合作用需要Cu参与,但Cu过量会从三方面抑制光合作用:1) 破坏类囊体的结构功能,抑制初级电子供体和受体产生;2) 增加叶绿体酶活性,加快叶绿素分解;3) 与叶绿素的巯基结合,替代Mg2+、Zn2+、Fe2+,改变叶绿素蛋白中心离子,导致叶绿素失活[2]。本试验除P20配施氮肥、N75配施磷肥处理蓖麻叶片SPAD值含量不因施肥量而变化,其余都随施肥量增加而增加,可能因为养分施用不平衡对蓖麻生理过程造成影响,养分配比会间接影响蓖麻对其他养分的吸收[22],进而影响叶绿素合成。
根干重随施肥量增加先增加后减少,可能是植物缺乏养分时,根系为获取养分而不断生长,但当土壤养分充足时,根系不需要太长就能获得足够的养分,所以在高施肥量时根干重减少,这是一种适应性的变化。蓖麻总干重随施氮量增加而减少,随施磷量增加而增加,低氮和高磷更利于蓖麻正常生长,可能是环境中Cu胁迫导致蓖麻不能正常吸收养分,施氮过多反而加重胁迫。
3.2 氮、磷肥施用对蓖麻体内铜含量的影响植物吸收重金属的量是土壤重金属种类、形态、含量,肥料种类、施用量,以及植物本身积累重金属能力的综合体现,所以施肥对植物吸收积累重金属的作用效果不一致[23]。施用硝态氮肥可增加水稻对镉的吸收[24],施用尿素和磷酸二氢铵可增加印度芥菜和蓖麻对Cd的积累[25];相反地,Fang等[26] 发现,施用重过磷酸钙可降低大白菜对Cu的吸收,高超等[27]施用磷酸钙和磷酸二铵抑制了水稻对Pb的吸收,因为营养状况改善可提高水稻抵抗重金属的能力,减少其对重金属的吸收。
本试验结果表明,施用磷肥既可增加蓖麻的生物量,又可增加蓖麻根、茎中铜的浓度。可能原因有:1) 供试蓖麻属于耐性植物,对铜富集能力强[28];2) 磷酸盐可与铁生成磷酸铁沉淀,降低土壤中铁的生物有效性[29],缺铁情况下铁通道蛋白 (IRT1) 及相关诱导转运蛋白大量表达,这些蛋白也可运输铜离子,提高植物对铜的吸收[30];3) 铜过量时,植物体内大量活性氧自由基产生,造成脂质过氧化,施用磷肥可促进非酶抗氧化保护剂产生,增大抗氧化酶类的活性[31],减少植物体内活性氧自由基,增加植物的抗性。
蓖麻体内铜积累量与转运系数变化相反,铜积累量越高转运系数越低,意味着根系是蓖麻主要的解毒场所。种植蓖麻两个月积累铜的平均值达到19.87 μg/株,最高达到34.93 μg/株,与典型的铜超积累植物海州香薷相比具有修复潜力[32–33]。
3.3 氮、磷肥施用对土壤pH值和铜形态的影响施用化肥会对土壤pH值和其它性质带来明显影响。土壤pH是影响土壤重金属形态最重要的因素,对重金属在土壤中的行为变化起主要作用[34]。NH4NO3对土壤酸化贡献较大,NH4+经硝化作用可在短期内显著降低土壤pH值,NO–3通过淋溶作用带走盐基离子,可使土壤进一步酸化,长期施用硝酸铵会导致土壤pH值下降[35–36]。NaH2PO4是强碱弱酸盐,H2PO4–交换解吸土壤胶体上的OH–会引起土壤pH值升高,起到钝化铜的作用[37]。本试验中,施肥处理的土壤pH值下降。蓖麻根系在受到重金属胁迫后,会分泌更多的苹果酸、酒石酸、柠檬酸等[10],引起根系土壤pH降低,本试验中蓖麻根系发达,种植盆中的土壤被蓖麻根系完全包裹,是土壤pH下降的主要原因,也可能是残渣态铜向氧化态铜转变的原因之一。
4 结论氮、磷肥施用能有效提高蓖麻叶片SPAD值和植株生物量,增加Cu在蓖麻体内的积累,N75P200处理的蓖麻铜积累量在供试处理中最高。施用磷肥对蓖麻吸收积累Cu的促进效果高于施用氮肥。种植蓖麻可有效增加土壤Cu的有效性,对Cu形态的影响比施加氮、磷肥对土壤中Cu形态的影响大。本试验是植物修复和化学修复,这两种土壤重金属污染原位修复方法的联合,在实际应用中需要确定具体的施肥比例,进一步弄清促进蓖麻吸收积累铜的机制。
| [1] | Ghaderian S M, Ravandi A A G. Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area, Iran[J]. Journal of Geochemical Exploration, 2012, 123(12): 25–32. |
| [2] |
林义章, 徐磊. 铜污染对高等植物的生理毒害作用研究[J].
中国生态农业学报, 2007, 15(1): 201–204.
Lin Y Z, Xu L. Physiological toxicity of copper pollution to higher plant[J]. Chinese Journal of Eco-Agriculture, 2007, 15(1): 201–204. |
| [3] |
刘春生, 史衍玺, 马丽. 过量铜对苹果树生长及代谢的影响[J].
植物营养与肥料学报, 2000, 6(4): 451–456.
Liu C S, Shi Y X, Ma L. Effect of excessive copper on growth and metabolism of apple trees[J]. Plant Nutrition and Fertilizer Science, 2000, 6(4): 451–456. DOI:10.11674/zwyf.2000.0414 |
| [4] | Ali H, Khan E, Sajad M A. Phytoremediation of heavy metals-concepts and applications[J]. Chemosphere, 2013, 91(7): 869–881. DOI:10.1016/j.chemosphere.2013.01.075 |
| [5] |
陈一萍. 重金属超积累植物的研究进展[J].
环境科学与管理, 2008, 33(3): 20–24.
Chen Y P. Research trends on heavy metals hyper-accumulators[J]. Environment Science and Management, 2008, 33(3): 20–24. |
| [6] | Nakajima H, Hara K, Yamamoto Y, Itoh K. Effects of Cu on the content of chlorophylls and secondary metabolites in the Cu-hyper accumulator Lichen Stereocaulon Japonicum [J]. Ecotoxicology and Environmental Safety, 2015, 113(4): 477–482. |
| [7] | Mutlu H, Meier M A R. Castor oil as a renewable resource for the chemical industry[J]. European Journal of Lipid Science & Technology, 2010, 112(1): 10–30. |
| [8] | González-Chávez M C A, Olivares A R, Carrillo-González R, et al. Crude oil and bio-products of castor bean (Ricinus communis L.) plants established naturally on metal mine tailings [J]. International Journal of Environmental Science and Technology, 2015, 12(7): 2263–2272. DOI:10.1007/s13762-014-0622-z |
| [9] | Andreazza R, Bortolon L, Pieniz S, Camargo F. Use of high-yielding bioenergy plant castor bean (Ricinus communis L.) as a potential phytoremediation for copper-contaminated soils [J]. Pedosphere, 2013, 23(5): 651–661. DOI:10.1016/S1002-0160(13)60057-0 |
| [10] | Huang G, Guo G, Yao S, et al. Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress [J]. International Journal of Phytoremediation, 2016, 18(1): 33–40. DOI:10.1080/15226514.2015.1058333 |
| [11] | Olivares A R, Carrillo-González R, Hernández R M S. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production [J]. Journal of Environmental Management, 2013, 114(2): 316. |
| [12] | Pigna M, Cozzolino V, Violante A, Meharg A A. Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations [J]. Water, Air, & Soil Pollution, 2009, 197(1): 371–380. |
| [13] | Alpha J M, Chen J, Zhang G P. Effect of nitrogen fertilizer forms on growth, photosynthesis, and yield of rice under cadmium stress[J]. Journal of Plant Nutrition, 2009, 32(2): 306–317. DOI:10.1080/01904160802608635 |
| [14] |
杨刚, 伍钧, 唐亚, 李昆. 不同形态氮肥施用对鱼腥草吸收转运Pb的影响[J].
农业环境科学学报, 2007, 26(4): 1380–1385.
Yang G, Wu J, Tang Y, Li K. Effects of nitrogenous fertilizers on accumulation and transfer of lead by Houttuynia cordata Thunb [J]. Journal of Agro-environment Science, 2007, 26(4): 1380–1385. |
| [15] |
冯子龙, 卢信, 张娜, 等. 农艺强化措施用于植物修复重金属污染土壤的研究进展[J].
江苏农业科学, 2017, 45(2): 14–20.
Feng Z L, Lu X, Zhang N, et al. Advances in research on plant restoration of heavy metal contaminated soil by agronomic strengthening measures[J]. Jiangsu Agricultural Sciences, 2017, 45(2): 14–20. |
| [16] | Nguyen X C. The effect of Cu concentration in soil and phosphorous fertilizer on plant growth and Cu uptake by Brassia juncea L. grown on contaminated soils [J]. VNU Journal of Science, Earth Sciences, 2008, 24: 113–117. |
| [17] | Yang Y, Chen R, Fu G, et al. Phosphate deprivation decreases cadmium (Cd) uptake but enhances sensitivity to Cd by increasing iron (Fe) uptake and inhibiting phytochelatins synthesis in rice (Oryza sativa) [J]. Acta Physiologiae Plantarum, 2016, 38(1): 28. DOI:I10.1007/s11738-015-2055-9 |
| [18] |
黄化刚, 李廷强, 朱治强, 等. 可溶性磷肥对重金属复合污染土壤东南景天提取锌/镉及其养分积累的影响[J].
植物营养与肥料学报, 2012, 18(2): 382–389.
Huang H G, Li T Q, Zhu Z Q, et al. Effects of soluble phosphate fertilizer on Zn/Cd phytoextraction and nutrient accumulation of Sedum alfredii H. in co-contaminated soil [J]. Plant Nutrition and Fertilizer Science, 2012, 18(2): 382–389. DOI:10.11674/zwyf.2012.11294 |
| [19] | Liang S X, Sun H W. Determination of six nutritional elements in Chinese herbal medicines by graphite furnace atomic absorption spectrometry[J]. Spectroscopy & Spectral Analysis, 2002, 22(5): 847. |
| [20] |
鲍士旦. 土壤农化分析(第3版)[M]. 北京: 中国农业出版社, 2000.
Bao S D. Soil and agricultural chemistry analysis (3rd Edn)[M]. Beijing: China Agriculture Press, 2000. |
| [21] | Sutherland R A, Tack F M G. Fractionation of Cu, Pb and Zn in certified reference soils SRM 2710 and SRM 2711 using the optimized BCR sequential extraction procedure[J]. Advances in Environmental Research, 2004, 8(1): 37–50. |
| [22] | Wu L H, Li H, Luo Y M, Christie P. Nutrients can enhance phytoremediation of copper-polluted soil by Indian Mustard[J]. Environmental Geochemistry and Health, 2004, 26(2): 331–335. DOI:10.1023/B:EGAH.0000039598.24033.4e |
| [23] | Liu M, Sun J, Li Y, Xiao Y. Nitrogen fertilizer enhances growth and nutrient uptake of Medicago sativa inoculated with Glomus tortuosum grown in Cd-contaminated acidic soil [J]. Chemosphere, 2017, 167: 204–211. DOI:10.1016/j.chemosphere.2016.09.145 |
| [24] | Yang Y, Xiong J, Chen R, et al. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa) [J]. Environmental and Experimental Botany, 2016, 122: 141–149. DOI:10.1016/j.envexpbot.2015.10.001 |
| [25] | Bauddh K, Singh R P. Effects of organic and inorganic amendments on bio-accumulation and partitioning of Cd in Brassica juncea and Ricinus communis [J]. Ecological Engineering, 2015, 74: 93–100. DOI:10.1016/j.ecoleng.2014.10.022 |
| [26] | Fang Y, Cao X, Zhao L. Effects of phosphorus amendments and plant growth on the mobility of Pb, Cu, and Zn in a multi-metal-contaminated soil[J]. Environmental Science and Pollution Research International, 2012, 19(5): 1659–1667. DOI:10.1007/s11356-011-0674-2 |
| [27] |
高超, 李军, 韩颖, 等. 不同磷肥对水稻铅积累的影响及其机理[J].
环境科学学报, 2015, 35(1): 288–293.
Gao C, Li J, Han Y, et al. Effects of different types of phosphate fertilizers on lead accumulation in rice[J]. Acta Scientiae Circumstantiae, 2015, 35(1): 288–293. |
| [28] | Boda R K, Majeti N V P, Suthari S. Ricinus communis L. (castor bean) as a potential candidate for revegetating industrial waste contaminated sites in peri-urban Greater Hyderabad: remarks on seed oil [J]. Environmental Science and Pollution Research International, 2017, 24: 19955–19964. DOI:10.1007/s11356-017-9654-5 |
| [29] |
洪顺山, 朱祖祥. 从磷酸盐位探讨土壤中磷的固定机制及其有效度问题[J].
土壤学报, 1979, (2): 94–109.
Hong S S, Zhu Z X. The mechanism of phosphate fixation and availability of phosphorus as interpreted by phosphate potentials of soils[J]. Acta Pedologica Sinica, 1979, (2): 94–109. |
| [30] |
施积炎, 陈英旭, 田光明, 林琦. 海州香薷和鸭跖草铜吸收机理[J].
植物营养与肥料学报, 2004, 10(6): 642–646.
Shi J Y, Chen Y X, Tian G M, Lin Q. Copper uptake mechanism of Elsholtzia splendens and Commelina communis [J]. Plant Nutrition and Fertilizer Science, 2004, 10(6): 642–646. DOI:10.11674/zwyf.2004.0617 |
| [31] | Huang G, Rizwan M S, Ren C, et al. Influence of phosphorous fertilization on copper phytoextraction and antioxidant defenses in castor bean (Ricinus communis L.) [J]. Environmental Science and Pollution Research, 2016, 25(1): 1–9. |
| [32] | Jiang L Y, Yang X E, Chen J M. Copper tolerance and accumulation of Elsholtzia splendens Nakai in a pot environment [J]. Journal of Plant Nutrition, 2008, 31(8): 1382–1392. DOI:10.1080/01904160802206257 |
| [33] | Song J, Zhao F J, Luo Y M, et al. Copper uptake by Elsholtzia splendens and Silene vulgaris, and assessment of copper phytoavailability in contaminated soils [J]. Environmental Pollution, 2004, 128(3): 307–315. DOI:10.1016/j.envpol.2003.09.019 |
| [34] | Adamczyk-Szabela D, Markiewicz J, Wolf W M. Heavy metal uptake by herbs. IV. Influence of soil pH on the content of heavy metals in Valeriana officinalis L. [J]. Water Air & Soil Pollution, 2015, 226(4): 106. |
| [35] |
楼玉兰, 章永松, 林咸永. 氮肥形态对污泥农用土壤中重金属活性及玉米对其吸收的影响[J].
浙江大学学报(农业与生命科学版), 2005, 31(4): 392–398.
Lou Y L, Zhang Y S, Lin X Y. Effects of forms of nitrogen fertilizer on the bioavailability of heavy metals in the soils amended with biosolids and their uptake by corn plant[J]. Journal of Zhejiang University (Agriculture & Life Sciences Edition), 2005, 31(4): 392–398. |
| [36] |
王林, 周启星, 孙约兵. 氮肥和钾肥强化龙葵修复镉污染土壤[J].
中国环境科学, 2008, 28(10): 915–920.
Wang L, Zhou Q X, Sun Y B. Intensification of Solarium nigrum L. remedying cadmium contaminated soils by nitrogen and potassium fertilizers [J]. China Environmental Science, 2008, 28(10): 915–920. DOI:10.3321/j.issn:1000-6923.2008.10.011 |
| [37] |
刘昭兵, 纪雄辉, 彭华, 等. 磷肥对土壤中镉的植物有效性影响及其机理[J].
应用生态学报, 2012, 23(6): 1585–1590.
Liu Z B, Ji X H, Peng H, et al. Effects of phosphorous fertilizers on phytoavailability of cadmium in its contaminated soil and related mechanisms[J]. Chinese Journal of Applied Ecology, 2012, 23(6): 1585–1590. |
 2018, Vol. 24
2018, Vol. 24  doi:
doi: 

