Seed priming is a useful strategy to facilitate the rapid and uniform field emergence of crop seeds, which is essential to achieve high yield in annual crops. The germination of seed requires a set of food stores including starch, protein, lipid and micronutrients to initiate metabolism processes manipulated by specific enzymes. Seed priming is able to enhance the seed vigor through mobilizing food reserve for seedling tissues or activating and synthesizing the related enzymes, thus resulting in faster and more uniform seedling emergence and development[1].
Over the past few years, various seed priming approaches have been developed[2–3], among which the pretreatments of seeds with natural or synthetic chemicals are predominantly researched in many horticultural and field crops[4–6]. Specifically, some growth regulating substances are found to promote the biosynthesis of certain chemical constituents in plants and induce faster and more vigorous seedling establishment. In this respect the amino acid family, which has a high integrity with different metabolic pools of plants, is a group of representative chemicals used to prime plant seeds with improved nutrient supply[7–8]. For example, pre-treating the fennel (Foeniculum vulgare Mill) seed with methionine and tryptophan was reported to bring about significantly accelerated plant growth because of boosted synthesis of important plant constituents[9]. Salicylic acid[10–13], humic acid[14] and ascorbic acid[15] have also been used for seed priming. These chemicals not only significantly increase seed germination and seedling vigor under greenhouse conditions, but also contribute to the stress tolerance of plants. For example, salicylic acid-treated seeds are more resistant to cold, and humic acid-treated seeds can resist to salt-alkali stress[12–14]. However, the application of these traditional seed priming chemicals is limited by their high costs. As a fact of matter, some low-cost organic carbons have also demonstrated satisfactory performance on regulating the plant growth to achieve a high yield. The seed priming in ethanol solution is proved effective to stimulate germination and seedling development of rice and tomato by breaking down seed dormancy[16–17]. Such a result enlightens us to seek for economical seed priming chemicals from organic carbons into practice.
So far a variety of parameters including germination percentage, seedling length, seedling weight, and activities of specific enzymes are available for evaluating the effect of seed priming on plant growth. Ideally, an effective seed priming method should induce the maximum seed germination and the fastest and most uniform seedling development. Unfortunately, it is difficult to simultaneously satisfy all these requirements by any individual seed priming method. Usually the seed priming condition which obtains the maximum seed germination is not the one to get the fastest seedling growth. In order to quantitatively compare the different seed priming methods, it is necessary to establish an integrated evaluation index that takes the multi- criteria into consideration together.
The grey relational analysis might provide a possible way to develop such an evaluation index. Grey relational analysis is developed to handle the uncertain systematic problem with only partially known information, by applying grey relational coefficient to solve the complicated interrelationships between multiple variables[18–19]. In the grey relational analysis the global comparison among several sets of data is undertaken through measuring the degree of similarity between sequences based on the grade of relation[20]. Till date the grey relation analysis has been applied in many different systems to evaluate system performance with multiple responses[21–24].
This work aims to evaluate the feasibility of C2-C4 organic acids and alcohols as alternatives to amino acids in the field of seed priming. Six compounds including acetate, propionate, butyrate, ethanol, propanol and butanol were used to treat the seeds of corn, green pea and wheat, and the effects of seed priming on seed germination and seedling development were investigated. The germination percentage, shoot length, root length and seedling weight were employed as the evaluation indices, and the grey relational analysis was used to integrate these indices in order to obtain a comprehensive assessment on the priming chemicals.
1 Materials and Methods 1.1 Seed priming treatmentsThe experiments were laid out in completely randomized design (CRD) on the seeds of corn (Zea mays L.), green pea (Leguminosae pisum L.) and wheat (Triticum aestivum L.). Seed priming treatments in acetate, propionate, butyrate, ethanol, propanol, butanol and glycine solutions were performed with the hydro-priming treatment in distilled water as a control. The concentrations of organic acids and glycine varied in the range from 10 to 50 mg/L, and alcohols from 3 to 13 mg/L. The glycine is presented a representative amino acid for comparison. The seeds were soaked in chemical solutions at 25℃ for 24 h, and then washed with distilled water and dried at room temperature for further studies.
1.2 Assay of hydrolytic enzymesThe primed seeds were firstly ground in 0.1 mol/L buffer in ice bath. The pH 7.2 phosphate buffer was used for the protease activity determination and pH 5.6 citrate buffer for the β-amylase measurement. The mixture was centrifuged at 12, 000 × g for 30 min at 4℃, and the supernatant was used for the enzyme assay. The protease activity was determined by casein digestion assay following Drapeau[25], and the β-amylase activity was determined as described by Benfield[26]. The enzyme activity was standardized by the fresh weight of seed.
1.3 Germination test and seedling growthFour replicates of 30 seeds were imbedded on three layers of moist filter paper in 9 cm diameter Petri dishes at constant temperature of 25℃. Day and night lengths were kept at 12 h and 12 h, respectively, and relative humidity was maintained at 80%. The seeds were evaluated for normal, abnormal seedling, un-geminated and dead seeds 5 days after sowing. Germination percentage was calculated on the basis of normal seedling. After the species were cultivated for 14 days, five normal seedlings from each replicate were taken at random and the shoot and root lengths were measured The five seedlings were dried overnight at 80℃ and weighted together to obtain the dry weight.
1.4 Grey relational analysisThe experimental data of germination percentage, shoot length, root length and seedling weight were first normalized within a range between 0 and 1 to facilitate calculation and comparison[27]. Since the expectancy was “larger-the-better” for above four responses, the normalized values were expressed as:
| ${x_i}(k) = \frac{{{\eta _i}(k) - \mathop {\min {\eta _i}(k)}\limits_{\forall k} }}{{\mathop {\max {\eta _i}(k)}\limits_{\forall k} - \mathop {\min {\eta _i}(k)}\limits_{\forall k} }}$ | (1) |
where xi (k) is the normalized value, ηi (k) is the original experimental value of the kth response in the ith experiment, min ηi (k) and max ηi (k) refer to the smallest and largest values of ηi (k), respectively.
Next, the grey relational coefficient ξi (k) was calculated from the normalized experimental data according to the following equation[19]:
| ${\xi _i}(k) = \frac{{\Delta \min + \zeta \Delta \max }}{{{\Delta _{0i}}(k) + \zeta \Delta \max }}$ | (2) |
where x0 (k) is the optimal normalized value of the kth response;
The grey relational grade, also designated as priming grade, was defined as the weighted average of all grey relational coefficients as:
| ${y_{0i}} = \sum\limits_{k = 1}^n {{w_k}} {\xi _i}(k)$ | (3) |
where wk is the weight of the kth response and ∑wk = 1. Considering the objective “germination percentage” is usually more emphasized in relevant studies, the wk is specified as 0.4 for “germination percentage”, and as 0.2 for the other three responses.
2 Results and Analysis 2.1 Effects of chemical priming treatments on the hydrolytic enzyme activities of seedsAs shown in Fig. 1, chemical priming treatments induce universal increments in the protease activities of corn, green pea and wheat seeds, compared to the activity values of 20.5, 23.7 and 13.2 units/g obtained in the hydro-primed controls. Notably, the organic carbons even demonstrate more significant enhancement on the protease activity than the glycine. The glycine at a concentration of 30 mg/L increases protease activities by 4.3 units/g for corn seed and 0.9 units/g for green pea seed, respectively. In comparison, a 9.7 units/g increment of protease activity is observed after the corn seed is primed in 8 mg/L propanol, and the protease activity in green pea seed is improved by 1.2 units/g in 20 mg/L acetate solution.
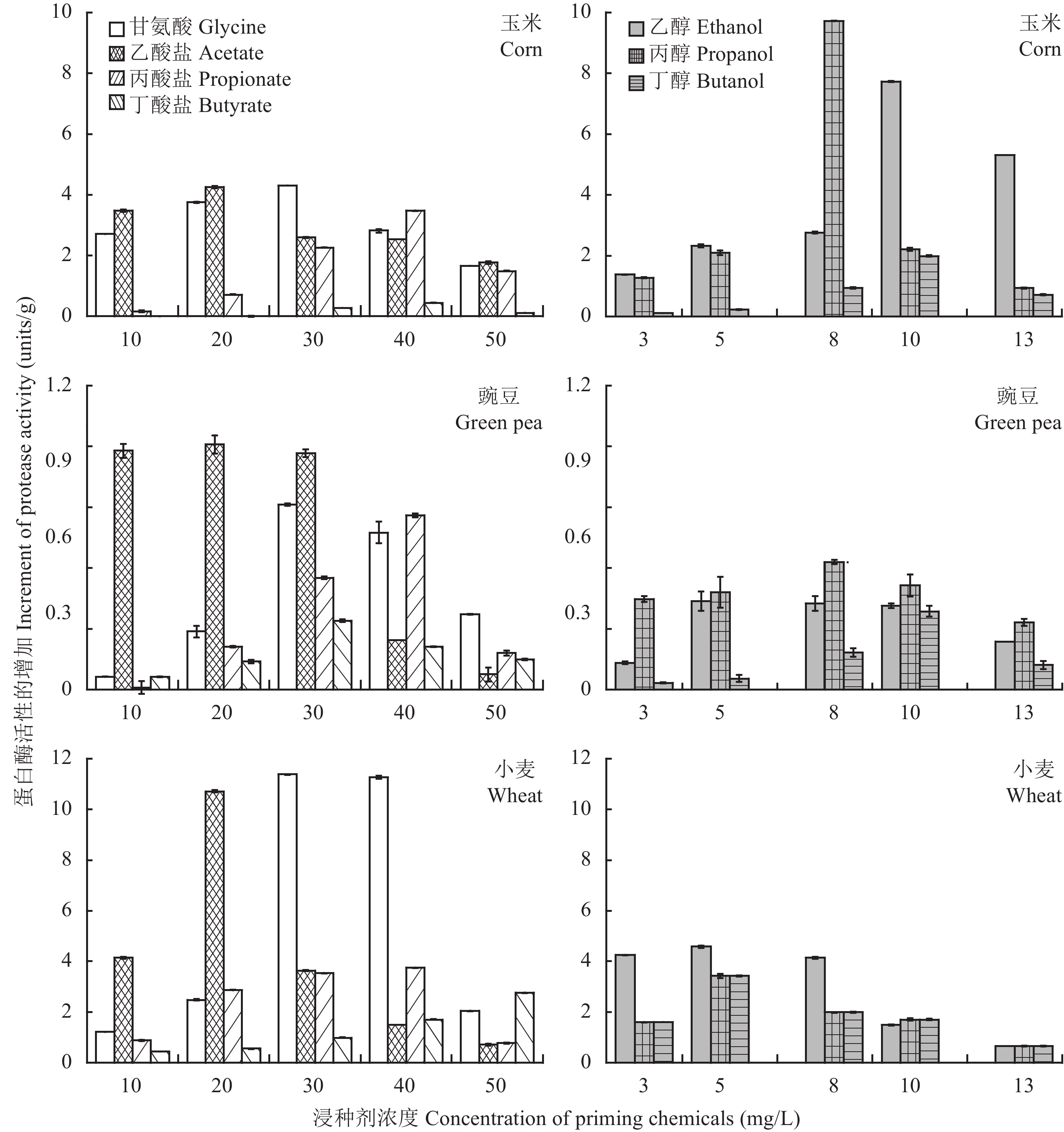 |
| 图1 不同溶液浸种后玉米、豌豆和小麦种子中蛋白酶活性的增加 Fig. 1 Increment of protease activities in the seeds of corn, green pea and wheat after primed in different chemical solutions |
In analogy to the case of protease activity, the amylase activity is also improved by chemical priming treatments (Fig. 2). The hydro-primed corn, green pea and wheat seeds show amylase activities of 30.4, 18.0 and 20.3 units/g, respectively. Amylase activity in corn seed is increased by 12.0 units/g at glycine concentration of 40 mg/L. More noticeable increment of amylase activity as high as 25.7 mg/L is observed in the treatment with 20 mg/L acetate. Such a concentration of acetate also exhibits favorable effect on the wheat seed, whose amylase activity is enhanced by more than one time. It seems the chemical priming treatments only slightly raise the amylase activity in green pea seed, while the highest value is still found in the treatment with 20 mg/L acetate.
 |
| 图2 不同溶液浸种后玉米、豌豆和小麦种子中淀粉酶活性的增加 Fig. 2 Increment of amylase activities in the seeds of corn, green pea and wheat after primed in different chemical solutions |
The germination percentages of hydro-priming treated corn, green pea and wheat seeds are calculated to be 82.5%, 75% and 76%, respectively. Chemical priming treatments result in an increase from 2% to 15% in the seedling emergence of the three plant species (Fig. 3). Among the several tested chemicals, the acetate at a concentration of 20 mg/L presents the strongest enhancement on the germination percentages of the species, giving the values of 90%, 95% and 92% for them.
The response of seedling development to chemical priming is evaluated in terms shoot length, root length and weight of seedling. The hydro-primed corn, green pea and wheat achieve shoot lengths of 16.2, 13.0 and 13.5 cm and root lengths of 16.0, 9.8 and 11.4 cm after 14 days of cultivation. As shown in Fig. 4 and 5, both the shoot and root lengths of the three plant species display various degrees of increase after the seeds are primed in chemical solutions. The shoot length of corn is remarkably lifted by 70% when primed with 8 mg/L ethanol, whereas the 30 mg/L acetate is more effective on the green pea whose shoot length is promoted by 51%. As for the wheat the highest shoot length increment of 25.2% is observed in the propionate treatment at a concentration of 40 mg/L. Priming the corn seeds in 20 mg/L acetate solution yields a root length of 23.0 cm, which is only 1.7% lower than the maximum value of 23.0 cm obtained in 20 mg/L glycine treatment. The longest root of green pea is found in the treatment with 20 mg/L propionate, accounting for 32% increment compared to the hydro-primed one. The root length of wheat is increased to the highest of 17.8 cm by seed priming with 30 mg/L acetate, which is 56% higher than the control. As shown in Fig. 6, the propanol shows favorable effect to enhance the weight of seedling. The 5 mg/L propanol treatment leads to 73% and 18% increment on the seedling weight of corn and wheat, while for green pea the maximum weight increment of 30% is observed at propanol concentration of 10 mg/L. In summary, the C2-C4 organic acids and alcohols demonstrate noticeably accelerating effects on both the seed germination and seedling development, making them in direct competition to the glycine as seed priming chemicals.
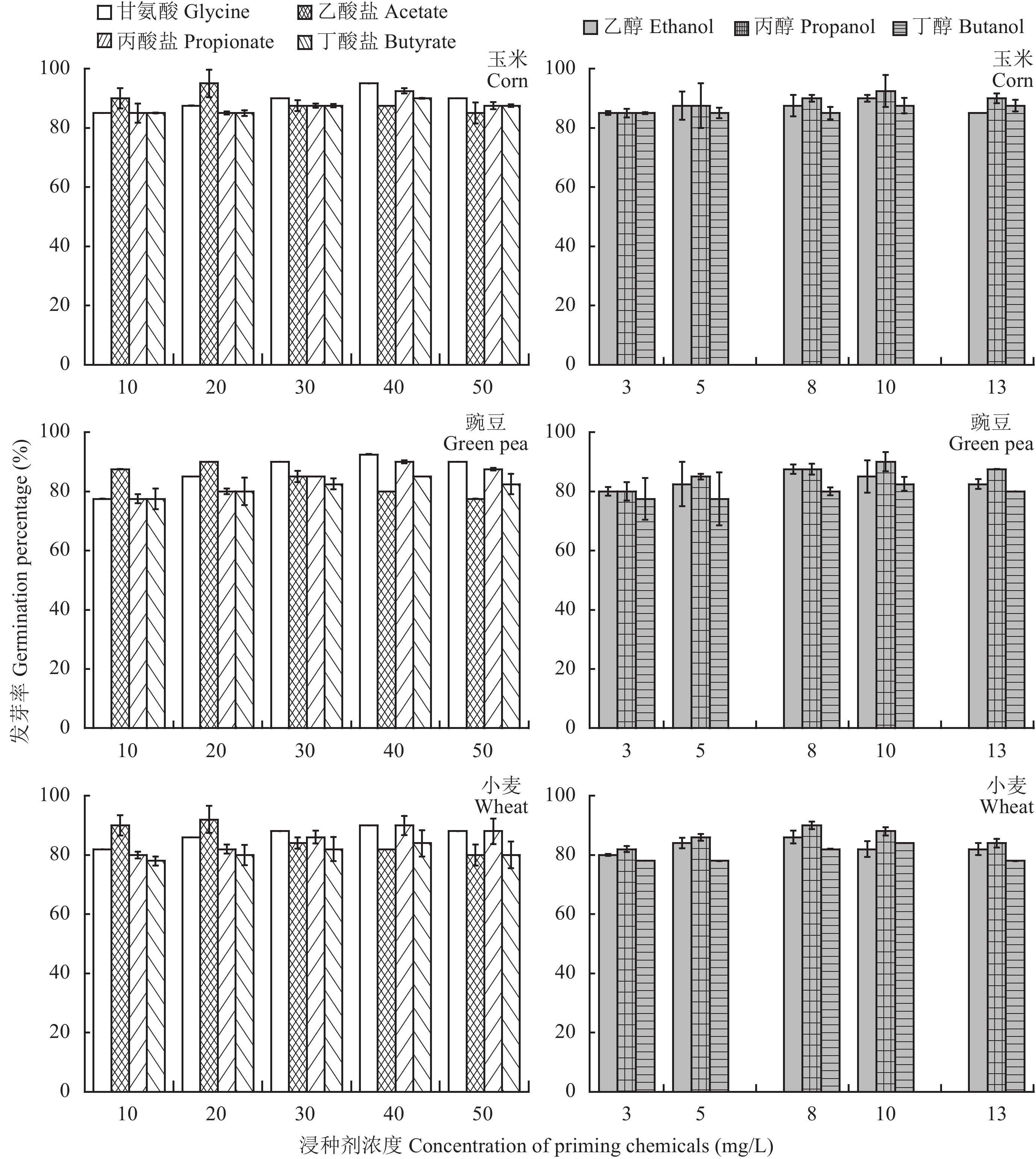 |
| 图3 不同溶液浸种后玉米、豌豆和小麦种子的发芽率 Fig. 3 Germination percentages of corn, green pea and wheat after the seeds are primed in different chemical solutions |
 |
| 图4 不同溶液浸种后玉米、豌豆和小麦的枝条长度 Fig. 4 Shoot lengths of corn, green pea and wheat after the seeds are primed in different chemical solutions |
 |
| 图5 不同溶液浸种后玉米、豌豆和小麦的根长度 Fig. 5 Root lengths of corn, green pea and wheat after the seeds are primed in different chemical solutions |
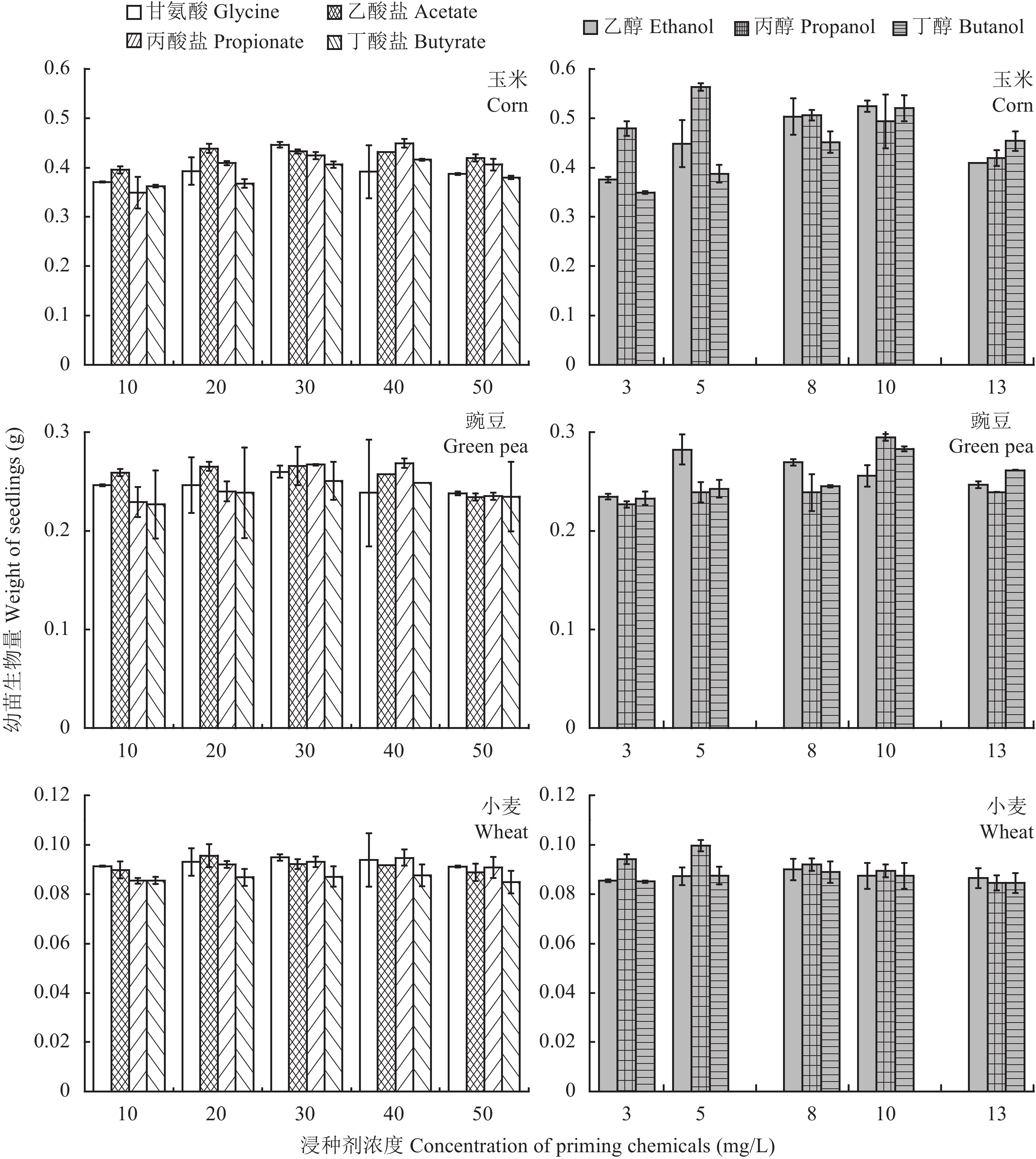 |
| 图6 不同溶液浸种后玉米、豌豆和小麦的幼苗生物量 Fig. 6 Seedling weights of corn, green pea and wheat after the seeds are primed in different chemical solutions |
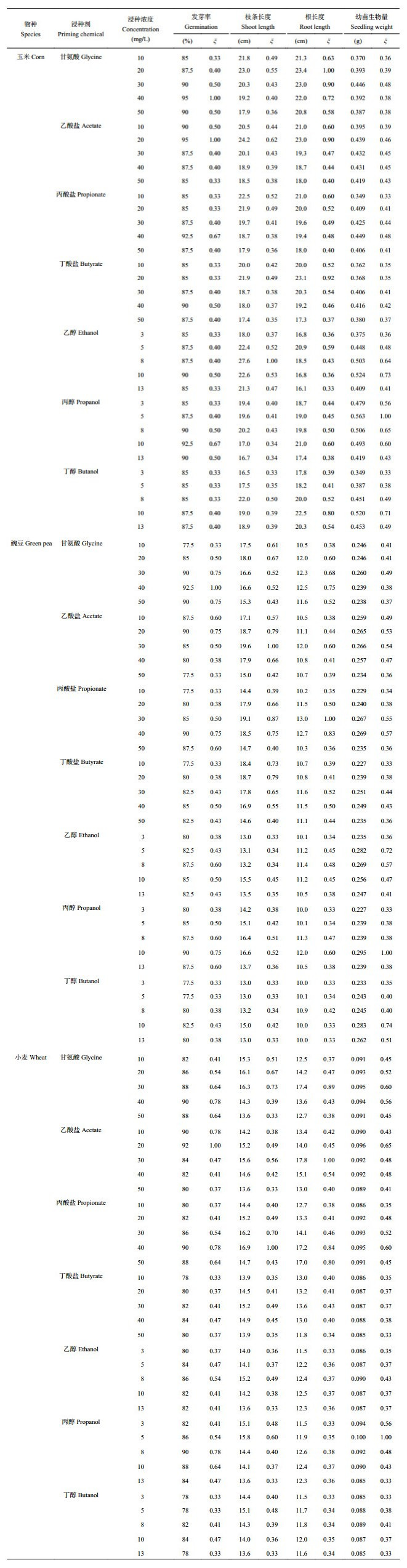
The grey relational analysis is employed to get a comprehensive assessment on the priming chemicals in terms of germination percentage, shoot length, root length and seedling weight. The grey relational coefficients ξi (k) for the four responses are listed in Table 1, and the calculated priming grades are graphically compared in Fig. 7. Totally speaking, acetate, propionate and propanol exhibit relatively better performance than butyrate, ethanol and butanol as seed priming chemicals. The optimal concentration of glycine to prime the seeds of corn and green pea is 40 mg/L, presenting priming grades of 0.701 for corn and 0.730 for green pea. However, the 20 mg/L acetate reveals a priming grade of 0.797 on the corn seed, which is even higher than that of glycine treatment. Besides, the 40 mg/L propionate and 10 mg/L propanol demonstrate enhanced effect on the green pea seed competitive to the glycine, giving their grade vales of 0.730 and 0.725, respectively. In the case of wheat seed the highest priming grade of 0.799 is obtained at 40 mg/L propionate, and the 20 mg/L acetate receives the second highest priming grade of 0.720. Such values are higher than the priming grade of 0.699 obtained in the glycine treatment at the optimum concentration of 30 mg/L.
| 表1 种子萌发和幼苗发育基于灰色关联分析的实验指标 Table 1 Grey relational coefficients of the experimental results for the indices related to seed germination and seedling development |
 |
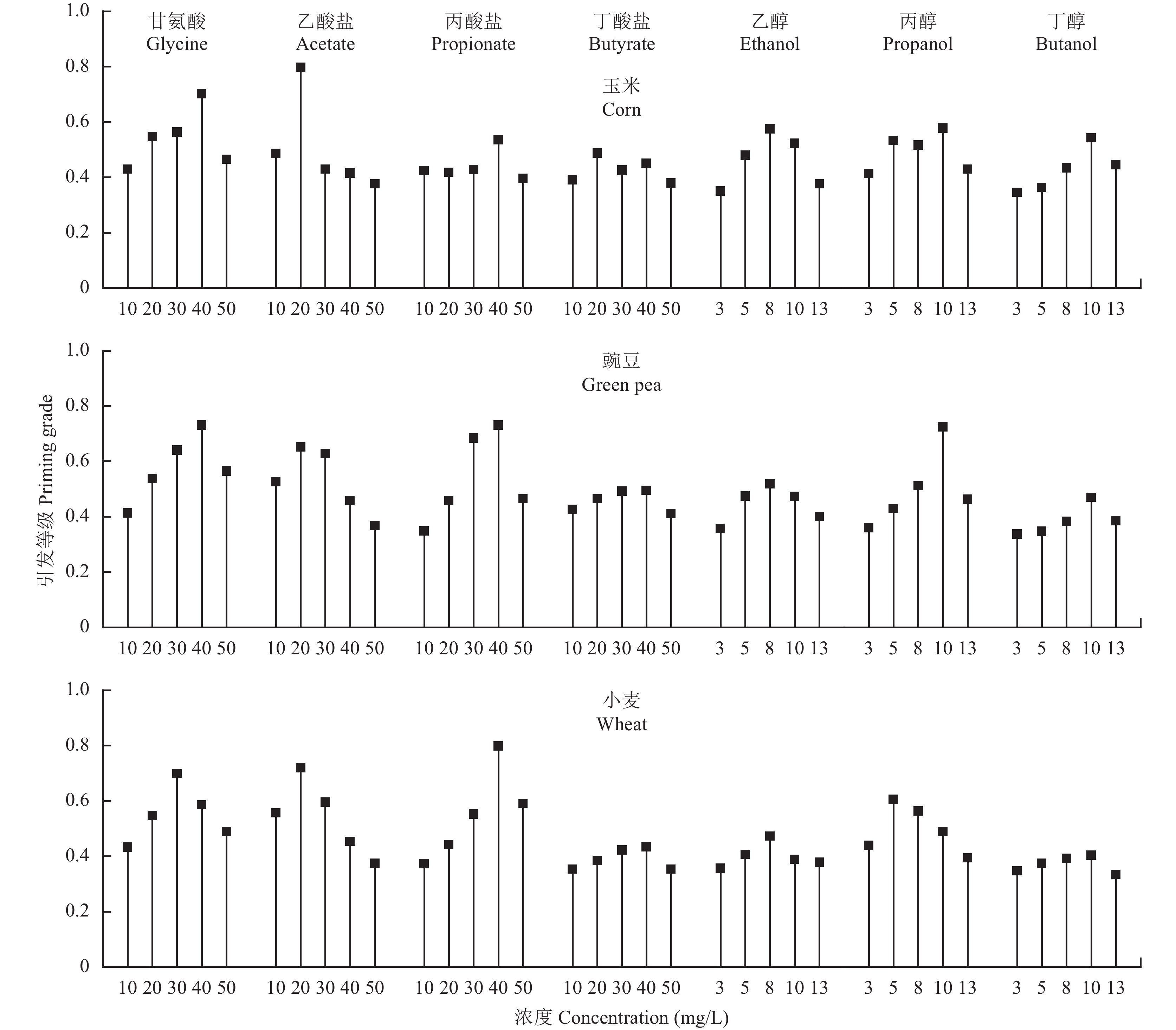 |
| 图7 不同浓度的浸种剂对玉米、豌豆和小麦种子引发等级的影响 Fig. 7 Priming grades of the evaluated chemicals at varied concentrations in the seed priming treatments of corn , green pea and wheat |
Both the protease and amylase activities display various degrees of enhancement after the seeds of corn, green pea and wheat are primed by C2-C4 organic acids and alcohols. Generally speaking, the effect of chemical priming on the protease and amylase activities is strongly depended upon the concentration of chemical, and individual chemical has its own optimal concentration to enhance the enzyme activity of plant species. Protease and amylase are important enzymes involved in the metabolism of germinating seed. The function of protease is to hydrolyze proteins into soluble peptides which are further dissociated to amino acids by peptidase[28]. The amylase is able to break down stored carbohydrate reserves in the seed with monosaccharides as products. Since the amino acids and monosaccharides are essential nutrients for the tissue development of seedling, seed priming with organic carbons is expected to facilitate the protein and carbohydrate metabolism that leads to better assimilate translocation and more vigorous seedling establishment. Particularly, the acetate and propanol show more significant enhancement on both the protease and amylase activities than the glycine, thus being promising alternatives to amino acids as seed priming chemicals.
As anticipated, enhanced germination percentage, shoot length, root length and seedling weight are observed for the chemical primed plants compared to the hydro-primed controls. However, it can be found from Fig. 3–6 that the seed germination and seedling development show asynchronous responses to individual priming chemical. In another word, the highest seed germination percentage, longest seedling shoot and root, and largest seedling weight do not always simultaneously occur in the same seed priming treatment. Thus, it is difficult to give a judgment regarding the optimal priming chemical based on single evaluation response alone. In order to get a comprehensive assessment on the priming chemicals, the grey relational analysis is employed to establish a collaborative relationship between the germination percentage, shoot length, root length and seedling weight. The grey relational analysis procedure consists of experimental data normalization and grey relational coefficient generation. The normalized data of evaluation responses have the same order, thus the interruptions caused by different units and scales of original data are avoided. The grey relational coefficient calculated from normalized data offers information about the relationship between the optimal and actual normalized results. A high value of grey relational coefficient indicates that the experimental result is close to the optimal value for individual evaluation response. The obtained grey relational coefficients are within the same scale among different evaluation responses, thus able to be integrated to obtaining a final evaluation grade. By comparing the priming grade, the 20 mg/L acetate is selected as the optimal chemical to prime the seed of corn, while the 40 mg/L propionate obtains the highest priming grades on the seeds of green pea and wheat. Notably, these treatments receive better effects on seed germination and seedling development than glycine, presenting great potential in practical applications.
Chemical seed-priming technique has been found effective for better germination and seedling establishment of many plant species under controlled conditions[29]. So far various compounds including inorganic salts, synthetic hormones, high-molecular polymers and amino acids have been tried as seed priming chemicals[2–3]. However, the environmental influence and economic feasibility of these chemicals remain to be evaluated for their large-scale application. This work for the first time, to the best of our knowledge, demonstrates the low molecular organic acids and alcohols are suitable growth regulating substances to liberate plant seeds from dormancy and to stimulate seedling establishment. Notably, these organic carbons are anticipated to be effective on a wide range of plant species, according to their applications on the corn, green pea and wheat. Besides, the C2-C4 organic acids and alcohols tested here are common compositions in anaerobic digestion effluents[30–31], which may provide an environmental friendly and economical favorable resource for the production of seed-priming agents to adapt to a sustainable agriculture pattern.
4 ConclusionSix compounds, including acetate, propionate, butyrate, ethanol, propanol and butanol, are used to treat the seeds of corn, green pea and wheat. The six tested chemicals show various degrees of enhancement on the protease and amylase activities of seeds, thus advancing the metabolism of plants. Grey relational analysis is employed to integrate the germination percentage, shoot length, root length and seedling weight for a comprehensive assessment of the priming chemicals. The 20 mg/L acetate is selected as the optimal chemical to prime the seed of corn, while the 40 mg/L propionate obtains the highest priming grades on the seeds of green pea and wheat. Notably, the acetate, propionate and propanol demonstrate promising potential as alternatives to amino acids in the field of seed priming for a wide range of plant species.
| [1] | Nascimento W M, West S H. Priming and seed orientation affect emergence and seed coat adherence and seedling development of muskmelon transplants[J]. Horticultural Science, 1998, 33(5): 847–848. |
| [2] | Jisha K C, Vijayakumari K, Puthur J T. Seed priming for abiotic stress tolerance: an overview[J]. Acta Physiologiae Plantarum, 2013, 35(5): 1381–1396. DOI:10.1007/s11738-012-1186-5 |
| [3] | Paparella S, Araujo S S, Rossi G, et al. Seed priming: state of the art and new perspectives[J]. Plant Cell Reports, 2015, 34(8): 1281–1293. DOI:10.1007/s00299-015-1784-y |
| [4] | Ashraf M, Foolad R M. Pre-sowing seed treatment—a shotgun approach to improve germination, plant growth and crop yield under saline and non-saline conditions[J]. Advances in Agronomy, 2015, 88: 223–271. |
| [5] | Barassi C A, Ayrault G, Creus C M, et al. Seed inoculation with Azospirillum mitigates NaCl effects on lettuce[J]. Scientia Horticulturae, 2006, 109(1): 8–14. DOI:10.1016/j.scienta.2006.02.025 |
| [6] | Kaur S, Gupta A K, Kaur N. Seed priming increases crop yield possibly by modulating enzymes of sucrose metabolism in chickpea[J]. Journal of Agronomy and Crop Science, 2005, 191(2): 81–87. DOI:10.1111/jac.2005.191.issue-2 |
| [7] | Coruzzi G, Last R. Amino acids [A]. Biochemistry and molecular biology of plants[M]. Rockville: American Society of Plant Physiologists, 2000. |
| [8] | Arif M, Ali S, Shah A, et al. Seed priming maize for improving emergence and seedling growth[J]. Sarhad Journal of Agriculture, 2005, 21: 239–243. |
| [9] | El–Awadi M E, Hassan E A. Physiological responses of fennel (Foeniculum vulgare Mill) plants to some growth substances [J]. Journal of American Science, 2010, 6(7): 120–125. |
| [10] | Mahesh H M, Murali M, Pal M A C, et al. Salicylic acid seed priming instigates defense mechanism by inducing PR-proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb [J]. Plant Physiology and Biochemistry, 2017, 117: 12. DOI:10.1016/j.plaphy.2017.05.012 |
| [11] |
朱霞, 王晓丽, 陈诗. 水杨酸对云南松种子萌发及幼苗生长的影响[J].
山东林业科技, 2009, 39(4): 15–17.
Zhu X, Wang X L, Chen S. Effects of soaking with salicylic acid on seeds germination and seedlings growth of Pinus yunnanensis [J]. Journal of Shandong Forestry Science and Technology, 2009, 39(4): 15–17. |
| [12] | Szalai G, Pál M, Árendás T, et al. Priming seed with salicylic acid increases grain yield and modifies polyamine levels in maize[J]. Cereal Research Communications, 2016, 44(4): 537–548. DOI:10.1556/0806.44.2016.038 |
| [13] | Pouramir–Dashtmian F, Khajeh–Hosseini M, Esfahani M. Improving chilling tolerance of rice seedling by seed priming with salicylic acid[J]. Archives of Agronomy and Soil Science, 2014, 60(9): 1291–1302. DOI:10.1080/03650340.2014.892584 |
| [14] |
郭伟, 王庆祥. 腐植酸浸种对盐碱胁迫下小麦幼苗抗氧化系统的影响[J].
应用生态学报, 2011, 22(10): 2539–2545.
Guo W, Wang Q X. Effects of seed soaking with humic acid on wheat seedlings antioxidant system under salt alkali stress[J]. Chinese Journal of Applied Ecology, 2011, 22(10): 2539–2545. |
| [15] | Alcântara B K, Rizzi V, Gaziola S A, et al. Soluble amino acid profile, mineral nutrient and carbohydrate content of maize kernels harvested from plants submitted to ascorbic acid seed priming[J]. Anais da Academia Brasileira de Ciências, 2017, 89(1): 695–704. |
| [16] | Farooq M, Basra S M A, Rehman H, et al. Germination and early seedling growth as affected by pre-sowing ethanol seed treatments in fine rice[J]. International Journal of Agriculture and Biology, 2006, 8(1): 19–22. |
| [17] | Afzal I, Munir F, Ayub C M, et al. Ethanol priming, an effective approach to enhance germination and seedling development by improving antioxidant system in tomato seeds[J]. Acta Scientiarum Polonorum Hortorum Cultus, 2013, 12(4): 129–137. |
| [18] | Fang F, Zeng R J, Sheng G P, et al. An integrated approach to identify the influential priority of the factors governing anaerobic H2 production by mixed cultures [J]. Water Research, 2010, 44(10): 3234–3242. DOI:10.1016/j.watres.2010.03.001 |
| [19] | Siddhi J H, Rajadurai A, Mohan B, et al. Multi response optimisation of sintering parameters of Al-Si alloy/fly ash composite using taguchi method and grey relational analysis[J]. International Journal of Advanced Manufacturing Technology, 2009, 45(3): 362–369. |
| [20] | Julong D. Introduction to grey system theory[J]. The Journal of Grey System, 1989, 1(1): 1–24. |
| [21] | Chiang K T, Chang F P, Tsai T C. Optimum design parameters of Pin-Fin heat sink using the grey-fuzzy logic based on the orthogonal arrays[J]. International Communications in Heat and Mass Transfer, 2006, 33(6): 744–752. DOI:10.1016/j.icheatmasstransfer.2006.02.011 |
| [22] | Liu N M, Horng J T, Chiang K T. The method of grey-fuzzy logic for optimizing multi-response problems during the manufacturing process: a case study of the light guide plate printing process[J]. The International Journal of Advanced Manufacturing Technology, 2009, 41(1): 200–210. |
| [23] | Sun M, Li W W, Yu H Q, et al. A novel integrated approach to quantitatively evaluate the efficiency of extracellular polymeric substances (EPS) extraction process[J]. Applied Microbiology and Biotechnology, 2012, 96(6): 1577–1585. DOI:10.1007/s00253-012-4478-1 |
| [24] | Zhai L F, Sun M, Song W, et al. An integrated approach to optimize the conditioning chemicals for enhanced sludge conditioning in a pilot-scale sludge dewatering process[J]. Bioresource Technology, 2012, 121: 161–168. DOI:10.1016/j.biortech.2012.06.093 |
| [25] | Drapeau G. Protease from Staphylococcus aureus [A]. Lorand L. Methods in enzymology[M]. New York: Academic Press, 1974. |
| [26] | Bernfeld P. Amylase α and β[A]. Colowick S P, Kaplan N O. Methods in enzymology [M]. New York: Academic Press, 1955. |
| [27] | Haq A N, Marimuthu P, Jeyapaul R. Multi response optimization of machining parameters of drilling Al/SiC metal matrix composite using grey relational analysis in the Taguchi method[J]. The International Journal of Advanced Manufacturing Technology, 2008, 37(3): 250–255. |
| [28] | Callis J. Regulation of protein degradation[J]. Plant Cell, 1995, 7: 845–857. DOI:10.1105/tpc.7.7.845 |
| [29] | Basra S M A, Farooq M, Tabassam R, et al. Physiological and biochemical aspects of pre-sowing seed treatments in fine rice (Oryza sativa L.) [J]. Seed Science and Technology, 2005, 33(3): 623–628. DOI:10.15258/sst |
| [30] | Jain S, Jain S, Wolf I T, et al. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste[J]. Renewable and Sustainable Energy Reviews, 2015, 52: 142–154. DOI:10.1016/j.rser.2015.07.091 |
| [31] | Batstone D J, Virdis B. The role of anaerobic digestion in the emerging energy economy[J]. Current Opinion in Biotechnology, 2014, 27: 142–149. DOI:10.1016/j.copbio.2014.01.013 |
 2018, Vol. 24
2018, Vol. 24  doi:
doi: 

