土壤缺磷是限制作物产量的一个重要因素,据统计,全世界约有57万公顷的耕地缺磷[1],而我国约有2/3的耕地磷素缺乏尤为突出[2]。长期以来,施用磷肥是粮食增产的重要举措。大量研究显示,施入土壤的磷肥当季利用率不超过20%[3],其中绝大部分磷与土壤中Ca2+、Fe3+、Fe2+、Al3+ 等离子结合转化为难溶磷,不易被植物吸收利用[1]。迄今,世界各国依靠大量消费磷肥确保作物增产,保障粮食安全,从而导致磷矿资源的消费量逐年增加。我国90%以上磷矿为中低品位,按现有的年消费量,我国磷矿使用年限将不足20年[3]。因此,提高磷肥及磷矿资源的利用效率和土壤磷库的有效性,延长磷矿资源的使用期限,对世界各国农业的可持续发展具有重要意义[4–5]。
溶磷微生物能活化土壤难溶磷、提高磷肥利用率、增加作物吸磷量、促进作物增产[4–6],其生物肥料制剂已经成为一种高效、经济、环境友好的转化土壤难溶磷的生物措施。溶磷微生物依靠自身产生有机酸[7–8]、分泌H+ 离子[9],将难溶磷转化为有效磷,同时通过自身产生植物促生物质[10],在磷素的生物有效性和作物增产中发挥重要作用。自1903年Staltrom[10]从土壤中成功分离到溶磷细菌以来,溶磷微生物一直是研究热点,国内外相继开展了溶磷微生物的大量筛选工作[12–13],以期通过接种溶磷微生物制剂提高土壤难溶磷的有效性和磷肥利用率,从而减少磷矿资源的消费量[7, 14]。已有的报道表明,真菌的溶磷能力大于细菌,且溶磷性能稳定[11]。
世界各国科学家分离了大量溶磷真菌,其种类主要是青霉菌[6]、曲霉菌[8]、木霉菌[15]、酵母菌[16]、篮状菌[17]、正青霉菌[18]、根霉菌[19]、镰刀菌[20]、小菌核菌[21]和轮枝菌[22]等,其中绝大部分为青霉菌和曲霉菌。溶磷青霉菌是一类重要的溶磷微生物资源,现已报道的溶磷青霉菌有微白青霉[23]、产黄青霉[24]、桔青霉[25]、常现青霉[23]、微紫青霉[26]、梅利尼青霉[25]、黑青霉[27]、特异青霉[28]、橄榄色青霉[23]、岛青霉[23]、草酸青霉[13]、产紫青霉[26]、局限青霉[23]、红色青霉[26]、皱褶青霉[27]、托姆青霉[28]、斜卧青霉[29]、指状青霉[30]、变换青霉[30]、绳状青霉[32]、拜莱青霉[32]、紫棕青霉 (P. cf. fuscum)[33]、嗜松青霉[34]、淡紫青霉[35]、黄灰青霉[9]和棘孢青霉[36],达26种之多。
绝大多数溶磷菌株是从作物根际土壤中分离,而一些溶磷菌株则是从特殊环境中获得,以期更好地利用该类菌株具有溶磷功能和兼具适应土著环境的特性。Chatli等[36]从低温沙漠地区分离了溶磷青霉菌株FC28、FC39,对磷酸三钙的溶解量分别为136.9 mg/L和103.3 mg/L;Bhattacharya等[37]从蚯蚓粪便中分离到溶磷菌株V1,对磷矿粉最大溶解率为36%,土壤有效磷含量提高13.65 mg/kg;Naveenkumar等[38]从槟榔壳废弃物中获得了溶磷菌Strain-1,对磷酸三钙的溶解量为200 mg/L;Rinu等[39]从海拔1800~3610 m的喜马拉雅山脉土壤中分离到溶磷菌株ITCC2546、ITCC4210和ARIFCC771,在盆栽实验中使玉米种子干重分别增加10.71%、33.33%和21.43%,小麦种子干重分别增加33.33%、37.04%和25.92%;李海云等[40]从猪粪堆肥中筛选到溶磷产黄青霉菌株PSM-1,对磷酸三钙的溶解量为138.36 mg/L;Nath等[41]从茶树叶中分离出溶磷菌Penicillium sp. 1和Penicillium sp. 2,对磷酸三钙的溶解量分别为86.10 mg/L和84.25 mg/L;Chai等[42]从明矾矿石中筛选到溶磷青霉菌株PSM11-5,对磷矿粉的溶解率达74.5%,且耐受重金属Cd2+、Co2+ 和Cr6+ 的能力较好。
我国盐碱地耕地面积约有1300万公顷,是我国最具增产潜力的低产农田。盐碱土壤主要面对盐碱胁迫和养分吸收两大障碍[16],鉴于目前盐碱土壤治理采用的管道排盐[44]、抗 (耐) 盐育种[17]收效甚微,功能微生物及其制剂的应用正在展现巨大潜能[44]。无论盐分胁迫土壤,还是非盐分胁迫的土壤,有效磷含量低是制约作物产量的主要因素之一[13]。因此,溶磷微生物,尤其耐盐溶磷微生物受到农业生产的高度重视[17]。国内外已有少量耐盐溶磷菌株的研究报道,其中,耐盐溶磷青霉菌主要有菌株PSF2[44]、菌株PSFWRB-2[45]和菌株QL1501[46]。此外,红酵母菌 (Rhodotorulasp.) PS4[16]、木霉菌TRC3[15]和疣孢蓝状菌 (Talaromyces verruculosus) P10[17]等溶磷菌株的耐盐能力较突出。本研究旨在筛选具有耐盐能力的高效溶磷真菌,并对菌株的溶磷能力、代谢产物及促生增产效果进行研究,为开发适用于盐碱农田土壤的溶磷生物肥料提供菌种资源。
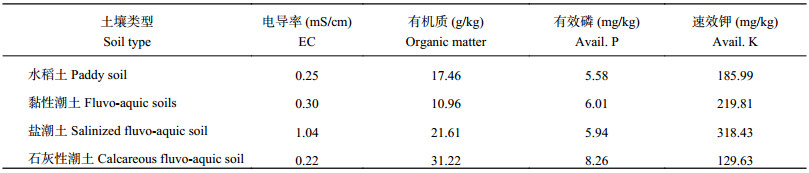
1 材料与方法 1.1 供试材料 1.1.1 土壤来源从内蒙古五原县 (东经107°35′70″~108°37′50″,北纬40°46′30″~41°16′45″) 轻中度盐碱农田采集向日葵根围土壤样品150个,土壤pH值为8.67,电导率EC为0.96 ms/cm。盆栽土壤来自北京市石灰性潮土、安徽省阜阳黏性潮土、安徽水稻土和山东沿海盐潮土。土壤理化性状见表1。
| 表1 供试土壤理化性状 Table 1 The physico-chemical properties of test soils |
 |
本研究菌剂使用的载体为草炭,该草炭产自我国东北地区,有效磷97.14 mg/kg、速效钾48.36 mg/kg、有机质219.74 g/kg。
1.1.3 培养基1) 菌株筛选与培养:无机磷培养基、PDA培养基、牛肉膏蛋白胨培养基等[4]。
2) Pikovskaya改良培养基 (g/L):葡萄糖 10.0 g、(NH4)2SO4 0.5 g、酵母抽提物 0.5 g、KCl 0.2 g、FeSO4·7H2O 0.001 g、MnSO4·4H2O 0.001 g、Ca3(PO4)2 5.0 g、MgSO4·7H2O 0.1 g、pH 7.0[47]。
3) PDY培养基:土豆200 g与1 L蒸馏水混合煮沸20 min过滤,滤液补水至1 L,加入蔗糖10 g、酵母粉1 g。
4) PDA培养基:PDY加琼脂18 g/L。
5) 耐盐培养基:PDA加NaCl。分别配制NaCl含量为0%、5%、7.5%、10%和12.5%的培养基。
以上培养基灭菌条件均为121℃,20 min。
1.1.4 供试磷源1) 分析纯磷酸三钙[Ca3(PO4)2 (P 20%)]、分析纯磷酸铝[AlPO4 (P 25.41%)],购于天津科密欧试剂公司。
2) 磷矿粉:贵州开阳磷矿粉 (Kaiyang rock phosphate,Kaiyang RP,P 14.79%);江苏锦屏磷矿粉 (Jinping rock phosphate,JPRP,P 15.01%);云南晋宁磷矿粉 (Jinning rock phosphate,JNRP,P 14.75%);河北钒山磷矿粉 (Fanshan rock phosphate,FSRP,P 14.80%) ;云南昆阳磷矿粉 (Kunyang rock phosphate,Kunyang RP,P 14.90%)。磷矿石均粉碎过100目 (149 μm) 筛,钼锑抗比色法测定有效磷含量。
1.1.5 液质联用 (Liquid chromatograph-mass spectrometer,LC-MS) 试剂1) 有机酸标准品 酒石酸 (DL-Tartaric acid)、草酸 (Oxalic acid) 、柠檬酸 (Citric acid)、苹果酸 (DL-Malic acid)、乳酸 (L(+)-Lactic acid)、琥珀酸 (Succinic acid) 和延胡索酸 (Fumaric acid),购自德国Dr. Ehrenstorfer公司。
2) 植物激素标准品 生长素 (IAA)、玉米素 (Zeatin),购于德国Dr. Ehrenstorfer公司。磷酸二氢钾和磷酸为国产优级纯,甲酸铵、丙酸和甲醇 (Methyl alcohol) 为色谱纯 (美国Tedia公司)。试验用水为超纯水 (电阻率为18.20 MΩ.cm)。
1.1.6 Hoagland培养液配方 (mol/L)K2SO4 8 × 10–4、MgSO4 6.5 × 10–4、KCl 1 × 10–4、Ca(NO3)2 2 × 10–3、CuSO4 1 × 10–7、MnSO4 1 × 10–6、EDTA-Fe 1 × 10–7、H3BO3 1 × 10–5、ZnSO4 1 × 10–6、(NH4)6Mo7O24 5 × 10–9。用0.01 mol/L NaOH溶液和0.01 mol/L HNO3溶液调节pH至5.8[48]。
1.1.7 供试菌株草酸青霉 (Penicillium oxalicum) 菌株M2为本试验筛选菌株;斜卧青霉 (Penicillium decumbens) 菌株P83为本实验室保存菌株。斜卧青霉P83和拜莱青霉ATCC20851作为对照菌株。
1.1.8 主要的试剂和仪器真菌总DNA提取试剂盒、Taq酶和dNTP购自天根生化科技有限公司,其他试剂为国产分析纯,购自国药集团化学试剂有限公司。引物由上海生工生物技术有限公司合成,ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') 和ITS4 (5'-GCTGCGTTCTTCATCGATGC-3')[49];pH计为Mettler Toledo S40 seven multi;电导仪为Mettler Toledo FEP30;培养箱为DHP-9162型,购自上海一恒科技有限公司;PCR仪为GeneAmp PCR System 9700;高速离心机为Heraeus公司的Sorvall Biofuge Stratos;光学显微镜为OLYMPUS BH-2;扫描电子显微镜为FEI-QUANTA200;紫外分光光度仪为天美科技有限公司UV-7501。三重串联四极杆液质联用仪器 (美国Agilent):主机Agilent LC 1290 Infinity2,Agilent QQQ 6470;工作站Agilent Masshunter。
1.2 试验方法 1.2.1 溶磷菌株的分离鉴定采用梯度稀释法获得系列稀释倍数的土壤菌悬液。称取1g保藏于4℃冰箱的新鲜土壤,用0.85%灭菌的生理盐水将土壤样品稀释至10–2~10–5倍,分别吸取0.1 mL土壤悬液涂布于无机磷培养基平板上,每个浓度土壤悬液重复涂布3个平板。置于培养箱中28℃倒置培养,选择溶磷圈较大的菌株,转接到PDA固体培养基平板上,纯化后接入PDA斜面保藏。
1.2.2 悬浮培养试验将100 mL含5 g/L难溶磷的Pikovskaya培养液盛入三角瓶中,难溶磷磷源分别为磷酸三钙、磷酸铝、开阳磷矿粉 (Kaiyang RP)、锦屏磷矿粉 (JPRP)、晋宁磷矿粉 (JNRP)、钒山磷矿粉 (FSRP) 和昆阳磷矿粉 (Kunyang RP)。于121℃灭菌30 min,接种1 mL溶磷菌悬液于无菌培养基中。试验处理包括:1) 对照 (CK),接入1 mL灭菌培养液;2) 黑曲霉菌P83;3) 草酸青霉菌M2。每个处理3次重复。置于28℃、160 r/min摇床培养,分别于3、6和9 d,取菌液5 mL,12000 r/min、4℃离心5 min,取上清液测定有效磷含量。
1.2.3 供试菌株在磷酸三钙、磷酸铝和磷矿粉溶磷过程中的pH值变化试验设置同1.2.2的方法,分别测定在磷酸三钙、磷酸铝和5种磷矿粉中接种菌株M2、P83时,溶液pH值的变化。
1.2.4 供试菌株耐盐性测定用无菌接种环从长满菌丝体的PDA培养液中,挑取一个较大的小球体接入固体耐盐培养基中央,置于28℃恒温培养箱中,从第3 d开始每日观察并记录菌丝在NaCl含量分别为0%、5%、7.5%、10%和12.5%的PDA平板中的生长状况。
1.2.5 溶磷菌剂的制备将50 g草炭置于500 mL三角瓶中,121℃间歇式灭菌3次,每次30 min。供试溶磷菌株M2、P83分别接种于PDA固体平板上,28℃倒置培养5 d,用接种环轻轻刮取孢子到50 mL灭菌PDB培养液中。在显微计数板上计数,用培养液平衡至孢子数量约为1 × 108 cfu/mL时,供试菌株的菌液与灭菌草炭以1∶1 (v/w) 混合均匀,28℃恒温箱中培养1周备用。
1.2.6 液质联用 (LC-MS) 测定草酸青霉菌M2溶磷过程中产生的有机酸在200 mL三角瓶中装入100 mL的Pikovskaya液体培养基,磷源分别为磷酸三钙、磷酸铝和昆阳磷矿粉三种。灭菌后接种1 mL 106 cfu溶磷真菌M2的孢子悬液,在28℃,170 r/min摇床振荡培养6 d。将菌液12000 r/min离心2 min取上清,加入2滴0.05%百里酚以抑制微生物活动并防止有机酸分解。将菌液过水相0.22 μm滤膜后上机测试。
有机酸标准液配制:分别精确称取酒石酸 (Tartaric acid)、草酸 (Oxalic acid)、苹果酸 (Malic acid)、柠檬酸 (Citric acid)、乳酸 (Lactic acid)、延胡索酸 (Fumaric acid) 和琥珀酸 (Succinic acid)7种有机酸的标准品 (精确至0.0001 g),用流动相A溶解稀释,用孔径0.22 μm的微孔滤膜过滤,定容于50 mL容量瓶中,配制质量浓度为100 mg/L的储备液,4℃冷藏。
标准曲线绘制:根据文献[12]及实际操作中紫外吸收灵敏度,将草酸、酒石酸、苹果酸、乳酸、柠檬酸、延胡索酸、琥珀酸按照2.5∶5∶5∶5∶5∶0.5∶15的比例配制混标,逐级稀释成5、10、20倍分别为a、b、c和d级别的标准液,现配现用。其中a级别标准溶液的质量浓度为56、120、140、103、150、10.8和300 mg/L。
1) 高效液相条件 流动相A配制:精准称量KH2PO4 (精确至0.000 1 g),用超纯水溶解配制浓度为0.01 mol/L,加入适量H3PO4调节pH值至2.73,经水系孔径为0.22 μm的微孔滤膜过滤,超声脱气后备用。流动相B为0.1%丙酸水∶甲醇 = 80∶20,经超声脱气后备用。色谱柱为ZORBAX SB C18 (5 μm,250 mm × 4.6 mm),流速为0.5 mL/min,柱温为25℃,进样量10 μL,运行时间10 min。
2) 质谱条件 负离子扫描,SIM模式,干燥气温度300℃,鞘气温度250℃,毛细管电压3500 V,喷嘴电压500 V。
1.2.7 液质联用 (LC-MS) 测定草酸青霉菌M2溶磷过程中分泌的植物激素试验设计同1.2.6的方法。
植物激素标准液配制:分别精确称取生长素 (IAA) 和玉米素 (Zeatin) 标准品 (精确至0.0001 g),以甲醇溶解稀释,用孔径0.22 μm的微孔滤膜过滤,定容于50 mL容量瓶中,配制质量浓度为100 mg/L的储备液,4℃冷藏。
1) 高效液相条件 流动相为5 mmol/L甲酸铵水∶甲醇 = 70∶30。色谱柱为Eclipse XDB C18 (3.5 μm,2.1 mm × 150 mm),流速为0.3 mL/min,柱温为25℃,进样量10 μL,运行时间6 min。
2) 质谱条件 正离子扫描,MRM模式,干燥气温度300℃,鞘气温度250℃,毛细管电压3500 V,喷嘴电压500 V。
1.2.8 盆栽试验玉米种子用0.4%的次氯酸钠进行表面消毒,用无菌蒸馏水冲洗4次,除去次氯酸钠,再用55℃无菌蒸馏水浸种,30℃温箱培养过夜后将种子转入灭菌纱布上催芽。试验使用塑料盆 (18 cm × 15 cm × 15 cm),每盆装土壤750 g。供试土壤有四种,水稻土、黏性潮土、盐潮土和石灰性潮土。供试磷源为三种,分别是Ca3(PO4)2、AlPO4 和昆阳磷矿粉 (RP),每种磷源用量为1.0 g/kg土壤。菌剂处理为:1) 对照 (CK),加入灭菌草炭和Pikovskaya培养液;2) 接种溶磷菌P83;3) 接种溶磷菌M2。称取菌剂1.50 g/盆,与2.25 g灭菌草炭混匀后,与土壤混合均匀。试验采用完全随机设计方案。每个处理重复4次,共计144盆。温室种植 (2014年3月28日种植,5月4日收获,北京白天平均28℃,夜间平均18℃),每盆播种2粒发芽的玉米种子。生长期间保持土壤湿度在65%~75%。采集玉米根际土壤样品和收获玉米植株,测定植株鲜重、干重和土壤有效磷含量。玉米品种为郑单958。
1.2.9 小区试验2014年5月在北京市唐家岭布置了田间小区试验。供试菌株为M2、P83和ATCC20851为对照菌株,试验地面积为40 m2 (10 m × 4 m),将其划分为1.8 m × 0.8 m的小区,每个小区接种菌剂300 g。菌剂处理为:1) 对照 (CK),只加入灭菌草炭和Pikovskaya培养液;2) 接种ATCC20851溶磷菌剂;3) 接种P83溶磷菌剂;4) 接种M2溶磷菌剂。每个处理重复4次,溶磷菌剂与表层15 cm土壤混合均匀后培垄 (200 cm × 20 cm × 20 cm),每个垄种植花生16棵,155 d收获。测定花生植株鲜重和干重、花生果实鲜重和干重。将小区产量折合为每公顷的产量,收获时采集花生根部土壤测定土壤有效磷含量。
1.3 数据统计分析采用SAS 9.2统计软件对数据进行统计分析 (SAS Institute Inc., Cary, NC, USA)。利用SPSS 19.0软件中的一般线性模型 (General Lineral Model for Univariate,GLM–Univariate) 进行多因素方差分析。
2 结果和分析 2.1 溶磷菌的筛选、鉴定从向日葵根围土壤样品中共分离到5株溶磷真菌,其中真菌M2在无机磷培养基上生长良好,7天能够将9 cm培养皿内的无机磷全部溶解,说明该菌溶磷能力较强,故选择菌株M2作为本试验的供试菌株。
菌株M2在PDA培养基中28℃培养,菌落生长迅速,菌丝体为白色,菌落中央有脐凸。3天后出现灰绿色分生孢子,5天时分生孢子多且极易脱落,无渗出液背面为浅黄色。显微镜下观察到菌丝成束且无隔断,呈帚状枝单轮生,壁光滑。分生孢子为圆形,直径约3 μm。
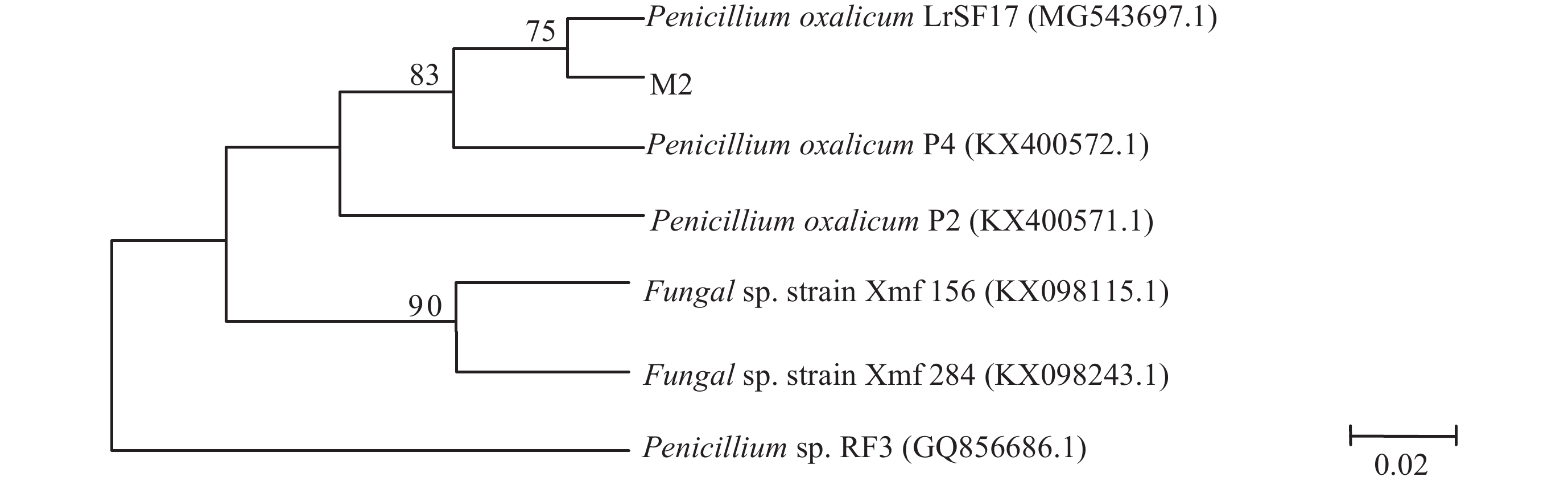 |
| 图1 菌株M2基于ITS rDNA序列同源性构建的系统发育树 Fig. 1 Molecular phylogenetic analysis of fungal strain M2 from ITS region |
利用ITS rDNA特异引物对菌株进行PCR扩增得到562 bp的目的DNA片段,利用Blast软件与GenBank的序列进行同源性比较,结果显示,菌株M2与草酸青霉 (Penicillium oxalicum) 的同源性达到99%。利用MEGA6的Neighbor-Joining软件进行系统发育树的构建 (图1),菌株M2与Penicillium oxalicum处于相同的发育地位,说明菌株M2与Penicillium oxalicum在ITS rDNA序列上具有高度的相似性,因此形态学和ITS序列鉴定结果一致,菌株M2为草酸青霉。
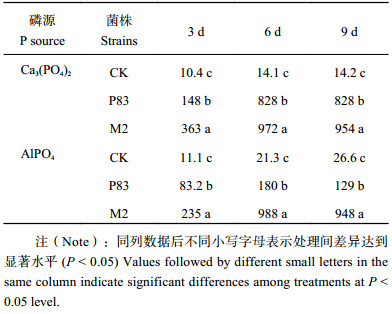
2.2 溶磷菌株M2的溶磷效果 2.2.1 溶磷菌株对不同难溶磷酸盐的作用效果接种溶磷菌株的溶磷量明显高于对照 (表2)。以磷酸三钙和磷酸铝为磷源的处理中,接种溶磷菌株摇瓶中培养6 d,菌株溶磷量最高,菌株M2对磷酸三钙和磷酸铝的溶解率分别为59.2%和46.3%;菌株P83的溶解率分别为51.4%和6.29%。可见菌株M2对磷酸三钙和磷酸铝的溶解能力大于菌株P83,更显著高于对照。
| 表2 接种草酸青霉菌M2和斜卧青霉菌 P83的不同磷源培养液中有效磷含量 (mg/L) Table 2 Soluble P content in cultures of isolates M2 and P83 grown in PVK broth plus Ca3 (PO4)2 and AlPO4 |
 |
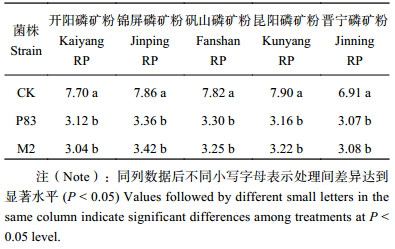
液体摇床培养条件下,以我国5种磷矿粉为磷源,接种菌株M2与菌株P83,结果显示 (表3),2株溶磷菌株的溶磷能力显著高于对照,菌株M2的溶磷量显著大于菌株P83。
综上所述,菌株M2在磷酸三钙、磷酸铝和5种磷矿粉中的溶磷能力大于菌株P83;对磷酸三钙的溶解能力大于磷酸铝和磷矿粉。
| 表3 5种磷矿粉接种M2和P83菌株6天后培养液中有效磷含量 (mg/L) Table 3 Soluble P content in cultures of isolates M2 and P83 plus 5 phosphorous rocks for 6 days incubation |
 |
在起始pH值为7.0的磷酸三钙和磷酸铝培养液中 (图2),菌株M2、P83与CK相比,溶液pH值明显下降且差异显著,但两菌株对溶液pH值的影响差异不显著。
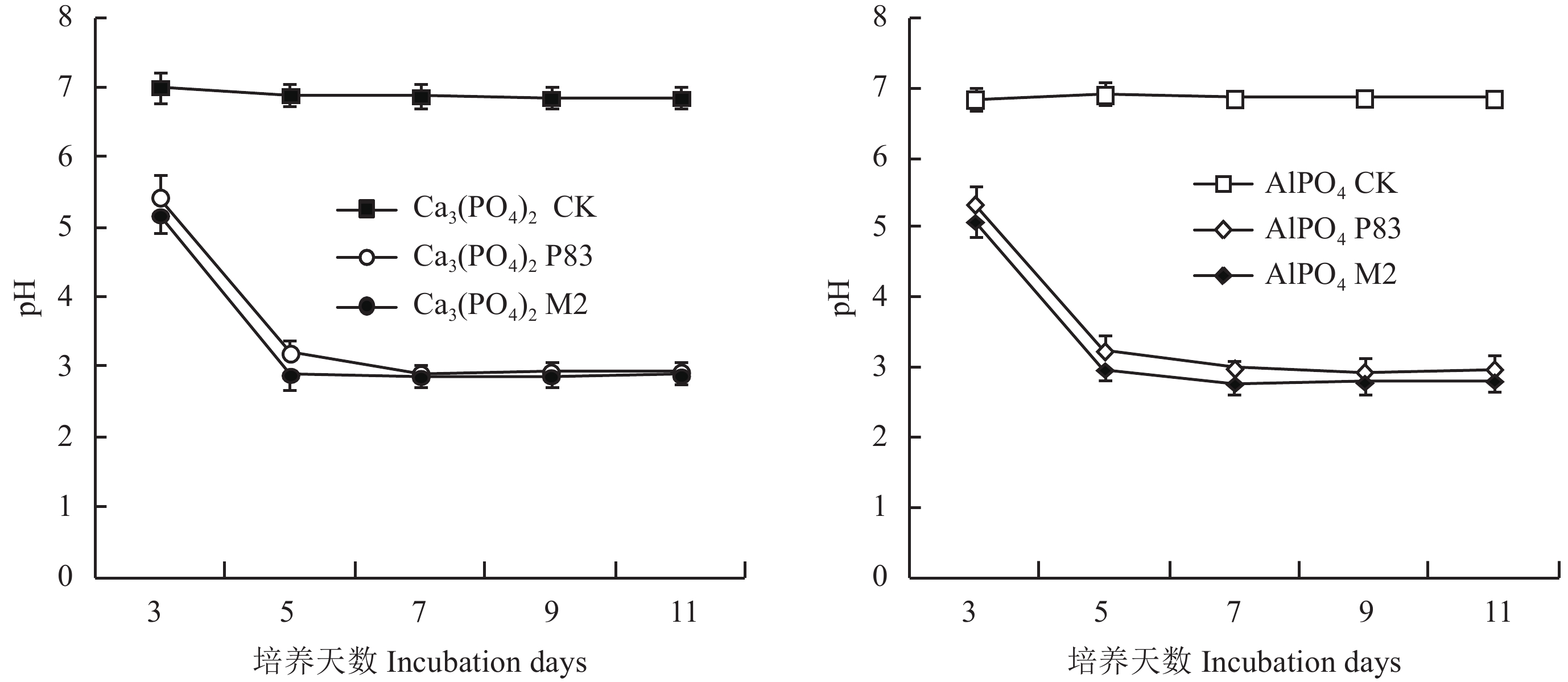 |
| 图2 PVK培养液接种M2和P83菌株后不同时间pH值变化 Fig. 2 Change in pH over time with incubation of isolates M2 and P83 in PVK broth culture with Ca3(PO4)2 and AlPO4 |
溶磷菌M2和P83接种于分别使用5种磷矿粉的培养液体中,结果显示,所有接种处理的溶液pH值皆比CK显著下降 (表4),但两菌株对溶液pH值的影响差异不显著。
| 表4 5种磷矿粉培养液中接种M2 和P83菌株 6 d 后pH值变化 Table 4 Change in pH with incubation of isolates M2 and P83 in PVK broth with rock phosphates for 6 days |
 |
溶磷菌株M2、P83接入含NaCl 0、5%、10%和12.5%的培养基中,置于28℃恒温培养箱中,从第3 d开始每日观察并记录菌落的生长状况。与NaCl含量为0相比,在盐含量为5%的培养基中,菌株P83的生长严重被抑制,而菌株M2的生长状况与CK中相似;在盐含量为7.5%时,菌株P83无生长,菌株M2的生长被轻度抑制;在盐含量为10%时,菌株M2被严重抑制。可见,溶磷真菌M2最高可耐受7.5%的盐浓度(表5)。
| 表5 溶磷菌P83 和M2 在不同氯化钠浓度培养基中的生长状况 (5 d) Table 5 Impact of various NaCl concentrations on isolates P83 and M2 growth on PDA plates for 5 days incubation |
 |
在3种难溶磷培养液中,菌株M2以分泌草酸和柠檬酸为主,其次为苹果酸、乳酸和琥珀酸,微量的酒石酸和延胡索酸 (图3)。菌株M2总有机酸分泌量Ca3(PO4)2 > 磷矿粉 > AlPO 4,分别为999.95、911.55和778.27 mg/L。
 |
| 图3 接种菌株M2的Ca3(PO4)2、AlPO4 和磷矿粉培养液中的有机酸含量 (培养6 d) Fig. 3 Organic acids produced by isolate M2 in PVK broth culture with 3 phosphates at 6 days incubation |
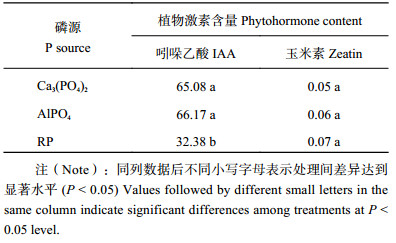
菌株M2接入分别含3种难溶磷培养液中,菌株M2生长素分泌量AlPO4 > Ca 3(PO4)2 > RP,分别为66.17 mg/L、65.08 mg/L和32.38 mg/L;分泌玉米素含量在三种难溶磷中无明显差异,含量为0.05~0.07 mg/L ( 表6)。
| 表6 不同磷源培养液接种M2菌株6天后吲哚乙酸和玉米素含量 (mg/L) Table 6 IAA and Zeatin produced by isolate M2 with 3 insoluble phosphates for 6 days incubation |
 |
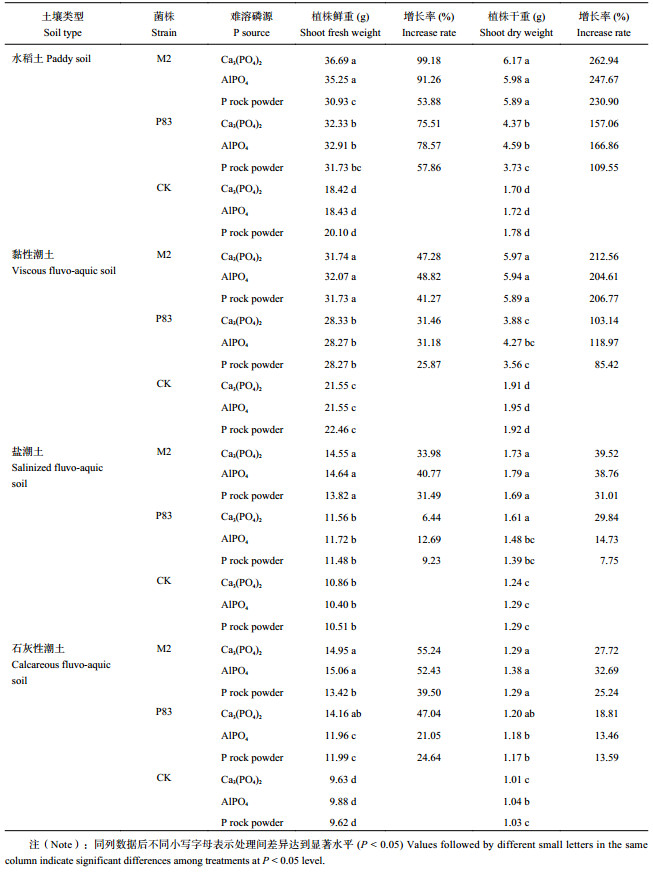
接种菌剂M2和P83明显提高土壤中有效磷含量 (表7)。菌剂M2分别接种于4种土壤,有效磷含量皆高于对照和P83。试验结果表明,同一土壤同一菌剂不同磷源间,土壤有效磷含量差异不显著;同一土壤同一磷源不同菌剂间,所有处理中接种菌剂M2和P83的土壤有效磷含量均显著高于CK;菌剂M2对磷酸铝和磷矿粉的溶解能力明显优于菌剂P83,但对磷酸三钙的溶解能力差异不显著;同一磷源同一菌剂不同土壤间土壤有效磷含量差异不显著。以土壤种类为主要因素,对所有接种菌剂和不同磷源处理下的数据进行平均,黏性潮土有效磷含量最高为32.93 mg/kg,表明溶磷菌剂M2和P83在黏性潮土中活化难溶磷的能力最强。接种菌剂和不接菌 (CK) 处理下,接种菌剂M2土壤有效磷含量最高 (33.06 mg/kg)。不同磷源处理下,以磷酸钙为磷源的土壤有效磷含量最高,为33.09 mg/kg,表明菌剂M2和P83在土壤中活化磷酸钙的能力最强。4种土壤、2种菌剂和3种难溶磷源共36个处理中,以磷酸三钙为磷源的黏性潮土接种菌剂M2,土壤有效磷含量最高,表明菌剂M2在黏性潮土中对磷酸三钙的溶解效果最好。综上说明,菌剂M2比菌剂P83对提高土壤有效磷具有显著优势,菌剂M2与4种土壤的适配性均高于菌剂P83,具有较强应用潜力。
| 表7 盆栽玉米接种M2和P83菌株和供应不同难溶磷源对4种土壤有效磷的影响 Table 7 Impact of isolates M2 and P83 on soil available P influenced by soil types and insoluble P sources on corn plants in greenhouse pot studies |
 |
对玉米生物量的影响与土壤有效磷含量相同,接种菌剂M2和P83能提高玉米生物量 (表8)。同种土壤中,接种菌剂M2的玉米生物量,均高于菌剂P83。4种土壤中接种菌剂和不同磷源处理下,水稻土中玉米生物量最高,鲜重、干重分别为33.31 g、5.12 g,表明菌剂M2和P83在水稻土中对玉米的促生效果最好。接种菌剂和不接菌 (CK) 处理下,接种菌剂M2的玉米生物量最高,鲜重、干重分别为23.73 g和3.75 g。水稻土中使用磷酸三钙的同时接种菌剂M2,植株干重增长率最大 (262.94%)。接入菌剂M2与P83相比,M2的植株干重和鲜重都有增加。表明菌剂M2在4种土壤中对玉米生物量的促生效果好于菌剂P83。
综上表明,菌剂M2对玉米生物量的作用效果优于菌剂P83,菌剂M2与4种土壤的适配性均高于菌剂P83。
| 表8 接种M2和P83菌株和供应不同难溶磷源对4种土壤盆栽玉米生物量的影响 Table 8 8 Impact of isolates M2 and P83 on corn plant fresh biomass influenced by soil types and insoluble P sources in greenhouse pot studies |
 |
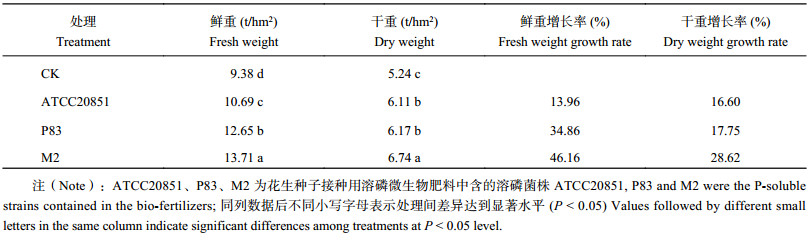
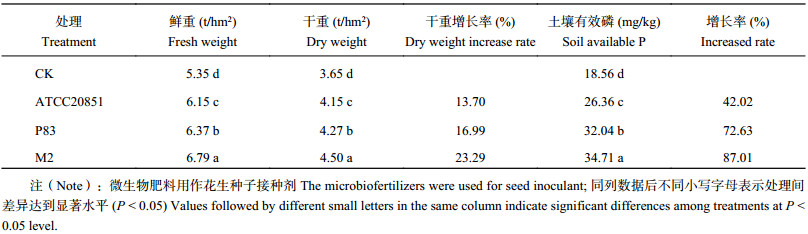
接种菌剂对花生的生长有明显促生效果,对花生产量的促生效果为菌剂M2 > P83 > ATCC20851 > CK ( 表9)。菌剂M2与ATCC20851相比,植株鲜重和干重分别提高3.02 t/hm2和0.63 t/hm2;与菌剂P83相比,分别增加1.06 t/hm2和0.57 t/hm2。综上表明,添加菌剂M2对小区试验花生生物量的促生效果最好。
| 表9 田间花生接种M2,P83和ATCC20851菌剂对其秸秆产量的影响 Table 9 Effect of M2, P83 and ATCC20851 biofertilizers on peanut straw yields in field study |
 |
对花生果实的促生效果为菌剂M2 > P83 > ATCC20851 > CK。对花生果实产量影响最大的为菌剂M2,比CK花生果实鲜重、干重增产1.44 t/hm 2和0.85 t/hm2;菌剂M2比菌剂ATCC20851处理的花生果实鲜重、干重增加10.41%和8.43%。添加菌剂能显著提高土壤中有效磷含量 (表10)。对土壤中有效磷含量影响最大的为菌剂M2。综上可见菌剂M2在小区试验中对花生产量以及土壤磷养分促进效果最好。
| 表10 不同溶磷菌生物肥料对田间花生产量和土壤有效磷的影响 Table 10 Effect of M2, P83 and ATCC20851 biofertilizers on peanut seed yields and soil available P infield study |
 |
草酸青霉菌M2在液体摇瓶培养和土壤条件下,对多种难溶磷都表现出较强的溶解能力。现已报道的溶解磷酸三钙较强的青霉菌株有P4 (P. oxalicum)[12]、P83 (P. decumbens)[29]和FS23 (P. canescens)[50],换算成培养基中加入磷酸三钙5 g/L,培养液中最大可溶性磷含量分别为931.45、956和754 mg/L。菌株M2在加入5 g/L磷酸三钙培养液中,有效磷最大浓度为971.87 mg/L,在溶解磷酸三钙上要优于上述菌株。以往研究表明,多数溶磷菌株对磷酸三钙的溶解能力高于磷酸铝和磷矿粉[13, 41]。关于溶解磷酸铝较好的溶磷真菌菌株已有较多报道,其中,菌株B1-A[51]、菌株FS1[50]和菌株Z60[52]对磷酸铝溶解能力较强,水溶性磷最大浓度分别为942.3、713和624.1 mg/L,其他能溶解磷酸铝的青霉菌株可溶性磷含量大多约100 mg/L[51–52],草酸青霉菌M2对磷酸铝的溶解量为987.74 mg/L,优于菌株B1-A、FS1和Z60。报道显示,液体培养中青霉菌溶解磷矿粉的能力为29~219 mg/L[9-10],菌株M2溶解昆阳磷矿粉释放的有效磷达555.83 mg/L。比较而言,菌株M2对磷矿粉的溶解能力高于已报道的多数菌株,仅略低于菌株An2[8]。菌株An2溶解磷酸铝 (折合5 g/L) 的有效磷为1178 mg/L,高于菌株M2,但对磷酸三钙和磷矿粉的溶解能力明显低于菌株M2,菌株An2的最大溶解量分别为861 mg/L和89.5 mg/L;同时菌株M2对NaCl的最大耐受力为10%,而菌株An2仅能耐受5%的NaCl。迄今已报道耐盐能力最强的溶磷菌株为PSF2[44],可耐受12.5%的NaCl,菌株M2的耐盐能力仅低于菌株PSF2。从溶磷能力而言,菌株PSF2仅能溶解磷酸三钙 (521 mg/L)。因此,菌株M2不仅表现出高效的溶磷能力,而且具有一定的耐盐能力。
菌株M2与4种典型土壤的适配性结果显示,M2具有很强的将土壤难溶磷转化为有效磷 (表7) 和促进作物生长能力 (表8)。菌株M2与我国黏性潮土配合时生物量和有效磷含量最高,其次是与水稻土配合。接种M2于4种土壤中,所有接种M2处理的土壤有效磷均显著增加,增加率最大值为310.95% (表7),接种菌剂M2对玉米生物量最大增加率达262.94%,凸显了菌剂M2优良的促生效果。因此,M2在上述2种土壤中都能够高效溶解土壤磷和外源加入的难溶磷,促进作物生长,为M2生物肥料的生产应用提供了重要依据。
目前,关于溶磷菌的溶磷机制一般被认为是菌株在生长过程中产生低分子量有机酸,这些有机酸通过羟基或羧基与难溶性磷酸盐上的钙、铁、铝、镁、锌等金属离子螯合,从而将难溶磷转化成可溶性磷[9–10]。范丙全等[12]研究表明,草酸青霉菌P8在溶解磷矿粉时主要分泌苹果酸 (56.6 mg/L)、乙酸 (69.3 mg/L) 和丙酸 (42 mg/L);Yin等[11]研究结果与之相似,其还检测到大量柠檬酸 (98.24 mg/L) 和少量其他7种少量有机酸,Li等[7]测定溶磷菌An2在5种难溶磷源中主要分泌的有机酸为草酸、酒石酸和琥珀酸。不同难溶磷种类影响菌株M2分泌总有机酸的含量,草酸青霉M2在磷酸钙、磷酸铝和磷矿粉培养液中检测到7种有机酸,其中含量最高的为草酸 (653.46 mg/L) 和柠檬酸 (269.61 mg/L),有机酸的总含量为778.27~999.95 mg/L。已有报道显示,溶磷菌株分泌有机酸的含量范围为12~1620 mg/L[8, 12, 53]。溶磷菌An2产生酒石酸的含量为880 mg/L,酒石酸为二羧酸,其溶磷能力高低取决于有机酸中羧基多少、螯合能力强弱[54]。本研究中菌株M2分泌的大量柠檬酸为三羧酸,此酸能大量螯合难溶磷中的金属离子,从而有效释放水溶性磷,是影响菌株M2高效溶磷能力的主要因素。
研究表明,溶磷微生物作为一类促生菌,能够分泌一定量的植物激素 (主要为生长素IAA、赤霉素GA和细胞分裂素),促进作物生长[45]。本研究利用LC-MS法测定菌株M2在磷酸三钙、磷酸铝和磷矿粉供应条件下分泌生长素和玉米素的含量最大值分别为66.17 mg/L和0.07 mg/L。已报道的溶磷菌株分泌IAA含量较高的菌种包括Penicillium、Aspergillus、Pseudomonas、Rhizopu和Trichoderma等,其中溶磷真菌分泌生长素的含量为2.5~42.53 mg/L[13, 45],且大多研究中仅测定溶磷菌株接入一种难溶磷培养液中吲哚乙酸 (IAA) 的分泌量,菌株M2分泌生长素的能力高于已报道的溶磷菌株。目前,还未见溶磷青霉菌分泌玉米素的报道。溶磷菌株M2具有高效的溶磷作用和分泌生长素、玉米素,从而表现出对玉米和花生良好的促生增产效果。因此,具有溶磷、促生和耐盐多功能的草酸青霉M2,有望不远的将来,在农业生产中发挥巨大的增产节肥作用。
4 结论1) 筛选到一株耐盐、高效溶磷真菌M2,鉴定为草酸青霉菌 (Penicillium oxalicum),该菌株在7.5% NaCl的固体培养基中生长正常。
2) 液体培养条件下,草酸青霉菌M2能够溶解磷酸三钙、磷酸铝和5种磷矿粉,溶解率达48.20%~60.26%,溶解能力显著高于已报道的具有高效溶磷能力的菌株斜卧青霉菌P83。
3) 玉米盆栽试验中,北京石灰性潮土、安徽黏性潮土、安徽水稻土和山东沿海盐潮土4种土壤中分别施入磷酸三钙、磷酸铝和开阳磷矿粉,土壤有效磷提高21.80~25.28 mg/kg,增长率达204.5%~311.0%。接种M2菌剂的玉米鲜重提高26.4%~99.2%,干重提高20.0%~262.9%。菌剂M2与4种土壤的适配性均高于菌剂P83,菌株M2与黏性潮土适配性最好。
4) 菌株M2在磷酸三钙、磷酸铝和磷矿粉3种难溶磷培养液中,分泌的有机酸总含量为778.27~999.95 mg/L,主要为草酸和柠檬酸;生长素含量为32.38~66.17 mg/L;玉米素含量为0.05~0.07 mg/L。有机酸是菌株M2溶磷的主要机制,生长素和玉米素是促进作物生长和增产的主要因素。
5) 接种M2菌剂显著提高花生产量,比拜莱青霉菌 (ATCC20851) 菌剂增产8.4%,比斜卧青霉菌P83菌剂增产5.4%,比不接菌 (CK) 增产23.3%。
| [1] | Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root induced chemical changes: A review[J]. Plant and Soil, 2001, 237(2): 173–195. DOI:10.1023/A:1013351617532 |
| [2] |
吴鹏飞, 张冬明, 郝丽虹, 等. 解磷微生物研究现状及展望[J].
中国农业科技导报, 2008, 10(3): 40–46.
Wu P F, Zhang D M, Hao L H, et al. Status guo and prospects of phosphate soluble microorganisms[J]. Journal of Agricultural Science and Technology, 2008, 10(3): 40–46. |
| [3] | Singh H, Reddy M. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils[J]. European Journal of Soil Biology, 2011, 47(1): 30–34. DOI:10.1016/j.ejsobi.2010.10.005 |
| [4] | Son H, Park G, Cha M, et al. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere [J]. Bioresource Technology, 2006, 97(2): 204–210. DOI:10.1016/j.biortech.2005.02.021 |
| [5] | Yao Q, Li X, Feng G, et al. Mobilization of sparingly soluble inorganic phosphates by the external mycelium of an abuscular mycorrhizal fungus[J]. Plant and Soil, 2001, 230(2): 279–285. DOI:10.1023/A:1010367501363 |
| [6] | Mendes G, Freitas A, Pereira O, et al. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources[J]. Annals of Microbiology, 2014, 64(1): 239–249. DOI:10.1007/s13213-013-0656-3 |
| [7] | Li X, Luo L, Yang J, et al. Mechanisms for solubilization of various insoluble phosphates and activation of immobilized phosphates in different soils by an efficient and salinity-tolerant Aspergillus niger strain An2 [J]. Applied Biochemistry and Biotechnology, 2015, 175(5): 2755–2768. DOI:10.1007/s12010-014-1465-2 |
| [8] | Illmer P, Schinner F. Solubilization of inorganic calcium phosphates-solubilization mechanisms[J]. Soil Biology and Biochemistry, 1995, 27(3): 257–263. DOI:10.1016/0038-0717(94)00190-C |
| [9] | Hussein K A, Joo J H. Isolation and characterization of rhizomicrobial isolates for phosphate solubilization and indole acetic acid production[J]. Journal of the Korean Society for Applied Biological Chemistry, 2015, 58(6): 847–855. DOI:10.1007/s13765-015-0114-y |
| [10] | Stalstrom V. Beitrag Zur Kenntrusder einwinsking sterilizer and in garung befindlieher striffe any dil loslieshkeit der phosphorus are destrical cum phosphours[J]. Zbt Bakt Abt II, 1903, 11: 724–732. |
| [11] | Yin Z, Shi F, Jiang H, et al. Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil [J]. Canadian Journal of Microbiology, 2015, 61(12): 913–923. DOI:10.1139/cjm-2015-0358 |
| [12] |
范丙全, 金继运, 葛诚. 溶磷草酸青霉菌筛选及其溶磷效果的初步研究[J].
中国农业科学, 2002, 35(5): 525–530.
Fan B Q, Jin J Y, Ge C. Isolation of Penicillium oxalicum P8 and its effect on solubilization of insoluble phosphate under different conditions [J]. Scientia Agricultural Sinica, 2002, 35(5): 525–530. |
| [13] | Narsian V, Patel H. Aspergillus aculeatus as a rock phosphate solubilizer [J]. Soil Biology and Biochemistry, 2000, 32(4): 559–565. DOI:10.1016/S0038-0717(99)00184-4 |
| [14] | Kumar K, Manigundan K, Amaresan N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions [J]. Journal of Basic Microbiology, 2017, 57(2): 141–150. DOI:10.1002/jobm.201600369 |
| [15] | Sunil M, Richa A, Tsering S, et al. Solubilization of insoluble inorganic phosphates by a novel temperature-, pH-, and salt-tolerant yeast, Rhodotorula sp. PS4, isolated from seabuckthorn rhizosphere, growing in cold desert of Ladakh, India [J]. World Journal of Microbiology, 2011, 27(10): 2387–2396. DOI:10.1007/s11274-011-0708-4 |
| [16] | New M, Yu S, Latt Z. Study on phosphate solubilization of salt tolerant soil yeast isolates and effects on maize germination and growth[J]. International Journal of Innovation and Applied, 2013, 2(3): 524–533. |
| [17] | Morales A, Alvear M, Valenzuela E. Screening, evaluation and selection of phosphate-solubilising fungi as potential biofertilizer[J]. Journal of Soil Science and Plant Nutrition, 2011, 11(4): 89–103. DOI:10.4067/S0718-95162011000400007 |
| [18] | Naik S, Maurya S, Kumar R, et al. Evaluation of rhizospheric fungi from acid soils of Jharkhand on phosphate solubilization[J]. The Bioscan, 2013, 8(3): 875–880. |
| [19] | Kavita S. Inorganic phosphate solubilization by fungi isolated from agriculture soil[J]. Journal of Phytology, 2011, 3(4): 11–12. |
| [20] | Vyas P, Rahi P, Chauhan A, et al. Phosphate solubilization potential and stress tolerance of Eupenicillium parvum from tea soil [J]. Mycological Research, 2007, 11(8): 931–938. |
| [21] | Posada R, Heredia–Abarca G, Sieverding E, et al. Solubilization of iron and calcium phosphates by soil fungi isolated from coffee plantations[J]. Archives of Agronomy and Soil Science, 2013, 59(2): 185–196. DOI:10.1080/03650340.2011.610030 |
| [22] |
张梦琦, 陈云云, 张熙, 等. 多功能植物根际促生菌DD3的功能特性及对大蒜幼苗的促生效果[J].
植物营养与肥料学报, 2017, 23(3): 748–756.
Zhang M Q, Chen Y Y, Zhang X, et al. Characterizations of rhizobacteria DD3 and their growth promoting effect on garlic seedlings[J]. Journal of Plant Nutrition and Fertilizer, 2017, 23(3): 748–756. DOI:10.11674/zwyf.16153 |
| [23] | Mahamuni S, Wani P, Patil A, et al. Isolation of phosphate solubilizing fungi from rhizosphere of sugarcane and sugar beet using TCP and RP solubilization[J]. Asian Journal of Biochemical Pharmaceutical Research, 2012, 2(1): 237–244. |
| [24] | Sharma K, Pandey K, Dubey S. Exploitation of phosphate solublization potential of certain species of Penicillium for agriculture [J]. Bioscience Biotechnology Research Communication, 2010, 2(2): 209–210. |
| [25] | Kanimozhi G, Panneerselvam A. Isolation and screening of phosphate solubilizing fungi from mangrove soils of Muthupettai, Thiruvarur District, India[J]. Asian Journal Microbiology, Biotechnology and Environmental Sciences, 2010, 12(12): 359–361. |
| [26] | Malviya J, Singh K, Joshi V. Effect of phosphate solubilizing fungi on growth and nutrient uptake of ground nut (Arachis hypogaea) plants [J]. Advances in Bioresearch, 2011, 2(2): 110–113. |
| [27] | Scervino J, Papinutti V, Godoy M, et al. Medium pH, carbon and nitrogen concentrations modulate the phosphate solubilization efficiency of Penicillium purpurogenum through organic acid production [J]. Journal of Applied Microbiology, 2011, 110(5): 1215–1223. DOI:10.1111/jam.2011.110.issue-5 |
| [28] |
史发超, 殷中伟, 江红梅, 等. 一株溶磷真菌的筛选鉴定及其溶磷促生效果研究[J].
微生物学报, 2014, 54(11): 1333–1343.
Shi F C, Yin Z W, Jiang H M, et al. Screening, identification of P-dissolving fungus P83 strain and its effects on phosphate solubilization and plant growth promotion[J]. Acta Microbiologica Sinica, 2014, 54(11): 1333–1343. |
| [29] | Murumkar D, Borkar D, Chimote V. Diversity in phosphate solubilizing fungi (Aspergillus awamori and Penicillium digitatum) present in soils of Maharashtra [J]. Research Journal of Biotechnology, 2014, 9(8): 20–26. |
| [30] | Fenice M, Selbman L, Federici F, et al. Application of encapsulated Penicillium variabile P16 in solubilization of rock phosphate [J]. Bioresource Technology, 2000, 73(2): 157–162. DOI:10.1016/S0960-8524(99)00150-9 |
| [31] | Rice W, Olsen P, Leggett M, et al. Co-culture of Rhizobium meliloti and a phosphorus-solubilizing fungus (Penicillium bilaii) in sterile peat [J]. Soil Biology and Biochemistry, 1995, 27(4-5): 703–705. DOI:10.1016/0038-0717(95)98651-4 |
| [32] | Asea P, Kucey R, Stewart J. Inorganic phosphate solubilisation by two Penicillium species in solution culture and soil [J]. Soil Biology and Biochemistry, 1988, 20(4): 459–464. DOI:10.1016/0038-0717(88)90058-2 |
| [33] |
乔欢, 吴小芹, 魏伟. 两株解磷青霉对马尾松根际土壤的微生态效应及其促生作用[J].
南京林业大学学报, 2016, 40(6): 109–116.
Qiao H, Wu X Q, Wei W. Effects of two phosphate-solubilizing Penicillium strains on microbial community structure and their growth-promoting in masson pine rhizosphere [J]. Journal of Nanjing Forestry University(Natural Sciences Edition), 2016, 40(6): 109–116. |
| [34] | Chhonkar P, Subba–Rao N. Phosphate solubilization by fungi associated with legume root nodules[J]. Canadian Journal of Microbiology, 1967, 13(7): 749–753. DOI:10.1139/m67-099 |
| [35] |
龚明波, 范丙全, 王洪媛. 一株新的溶磷棘孢青霉菌Z32的分离、鉴定及其土壤定殖与溶磷特性[J].
微生物学报, 2010, 50(5): 580–585.
Gong M B, Fan B Q, Wang H Y. Isolation and identification of a novel phosphate-dissolving strain Penicillium aculeatum Z32 and its colonization and phosphate-dissolving characteristics in soil [J]. Acta Microbiologica Sinica, 2010, 50(5): 580–585. |
| [36] | Chatli A, Beri V, Sidhu B. Isolation and characterisation of phosphate solubilising microorganisms from the cold desert habitat of Salix alba Linn in trans Himalayan region of Himachal Pradesh [J]. Indian Journal of Microbiology, 2008, 48(2): 267–273. DOI:10.1007/s12088-008-0037-y |
| [37] | Bhattacharya S, Barman S, Ghosh R, et al. Phosphate solubilizing activity of Emericella nidulans V1 isolated from vermicompost [J]. Indian Journal of Experimental Biology, 2013, 51(10): 840–848. |
| [38] | Naveenkumar K, Thippeswamy B, Banakar S, et al. Lignolytic and phosphate solubilizing efficiency of fungal species isolated from Arecanut husk waste[J]. Journal of Research Biology, 2012, 2(2): 143–151. |
| [39] | Rinu K, Malviya M, Sati P, et al. Response of cold–tolerant Aspergillus spp. to solubilization of Fe and Al phosphate in presence of different nutritional sources [J]. ISRN Soil Science, 2013: 135–143. |
| [40] |
李海云, 牛世全, 孔维宝, 等. 猪粪堆肥中一株溶磷菌的筛选鉴定及溶磷能力初步测定[J].
环境科学学报, 2015, 35(5): 1464–1470.
Li H Y, Niu S Q, Kong W B, et al. Screening and identification of a phosphate solubilizing strain isolated from pig manure compost and determination of its phosphate solubilizing capacity[J]. Acta Scientiae Circumstantiae, 2015, 35(5): 1464–1470. |
| [41] | Nath R, Sharma G, Barooah M. Efficiency of tricalcium phosphate solubilization by two different endophytic Penicillium sp. isolated from tea (Camellia sinensis L.) [J]. European Journal of Experimental Biology, 2012, 2(4): 1354–1358. |
| [42] | Chai B, Wu Y, Liu P, et al. Isolation and phosphate-solubilizing ability of a fungus, Penicillium sp. from soil of an alum mine [J]. Journal of Basic Microbiology, 2011, 51(1): 5–14. DOI:10.1002/jobm.v51.1 |
| [43] |
范延辉, 王君, 刘雪红, 等. 一株耐盐解磷真菌的筛选、鉴定及其发酵优化[J].
土壤通报, 2015, 46(2): 362–367.
Fan Y H, Wang J, Liu X H, et al. Isolation, identification and fermentation optimization of a halotolerant strain with phosphate-solubilizing activity[J]. Chinese Journal of Soil Science, 2015, 46(2): 362–367. |
| [44] | Srinivasan R, Alagawadi A, Yandigeri M, et al. Characterization of phosphate-solubilizing microorganisms from salt-affected soils of India and their effect on growth of sorghum plants[J]. Annals of Microbiology, 2012, 62(1): 93–105. DOI:10.1007/s13213-011-0233-6 |
| [45] |
李学平, 任加云, 邹美玲, 等. 一株耐盐解磷菌的解磷能力及对玉米敏感期生长的影响[J].
水土保持研究, 2015, 22(5): 276–278.
Li X P, Ren J Y, Zou M L, et al. Phosphate-solubilizing ability of a saline-alkali fungus strain and effects on the growth of corn in sensitive period[J]. Research of Soil and Water Conservation, 2015, 22(5): 276–278. |
| [46] | Gupta R, Singal R, Shankar A, et al. A modified plate assay for screening phosphate solubilizing microorganisms[J]. Journal of General and Applied Microbiology, 1994, 40(3): 255–260. DOI:10.2323/jgam.40.255 |
| [47] | Hoagland D, Arnon D. The water-culture method for growing plants without soil[J]. California Agricultural Experiment Station Circular, 1950, 347(2): 2–32. |
| [48] | Peay K, Kennedy P, Bruns T. Fungal community ecology: a hybrid beast with a molecular master[J]. Biology Science, 2008, 58(9): 799–810. |
| [49] | Rivas R, Trujillo M, Sanchez M, et al. Microbacterium ulmi sp.nov.a xylanolytic, phosphate-solubilizing bacterium isolated from sawdust of Ulmus nigra [J]. International Journal of Systematic and Evolutionary Microbiology, 2004, 54(2): 513–517. DOI:10.1099/ijs.0.02724-0 |
| [50] |
刘文干, 何园球, 张坤, 等. 一株红壤溶磷菌的分离、鉴定及溶磷特性[J].
微生物学报, 2012, 52(3): 326–333.
Liu W G, He Y Q, Zhang K, et al. Isolation, identification and characterization of a strain of phosphate-solubilizing bacteria from red soil[J]. Acta Microbiologica Sinica, 2012, 52(3): 326–333. |
| [51] |
乔志伟, 洪坚平, 谢英荷, 等. 一株石灰性土壤强溶磷真菌的分离鉴定及溶磷特性[J].
应用与环境生物学报, 2013, 19(5): 873–877.
Qiao Z W, Gong J P, Xie Y H, et al. Screening, identification and dissolving characteristics of a strong phosphorus solubilizing fungi in calcareous soil[J]. Chinese Journal of Applied and Environmental Biology, 2013, 19(5): 873–877. |
| [52] | Xiao C, Chi R, He H, et al. Isolation of phosphate-solubilizing fungi from phosphate mines and their effect on wheat seedling growth[J]. Applied Biochemistry and Biotechnology, 2009, 159(2): 330–342. DOI:10.1007/s12010-009-8590-3 |
| [53] | Mittal V, Singh O, Nayyar H, et al. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2) [J]. Soil Biology and Biochemistry, 2008, 40(3): 718–727. DOI:10.1016/j.soilbio.2007.10.008 |
| [54] | Sperber J. The incidence of apatite-solubilizing organisms in the rhizosphere and soil[J]. Australian Journal of Agricultural Research, 1958, 9(6): 778–781. DOI:10.1071/AR9580778 |
| [55] | Wang H, Appan A, Gulliver J, et al. Modeling of phosphorus dynamics in aquatic sediments: II-examination of model performance[J]. Water Research, 2003, 37(16): 3939–3953. DOI:10.1016/S0043-1354(03)00305-1 |
 2018, Vol. 24
2018, Vol. 24  doi:
doi: 

