植物营养与我国的粮食安全密切相关,是关系到国家粮食安全和环境质量的重大科技问题[1],我国的粮食安全大致分为三个阶段:第一阶段为国民经济发展水平较低时期,大致为1980年以前,这一时期的基本特征是粮食还没有满足消费需求,整个社会面临的是温饱问题,因此总量保障是这一时期粮食安全的重点;第二阶段是国民经济快速发展时期,大致为1980年到2000年,这一时期的基本特征是粮食总量已能满足社会需求,社会已经摆脱了粮食短缺的困扰,人们的选择性明显加强,小康社会的种种特征日益明显;第三阶段是国民经济发展到工业化水平时期,大致为2000年以后,这一时期的基本特征是粮食生产的潜力得到了较充分的发挥,人们除了关注总量,更关心营养品质[2],如硝酸盐累积、重金属超标和农药残留等,这与人体健康密切相关,因此国家也开始关注和重视农业供给侧改革[3]。
广义的植物营养品质涉及到诸多方面,除了营养成分外,还包括如外观品质和口感味道等,狭义的植物营养品质主要是指植物的营养成分,实际上植物的营养与成分也决定着外观品质和口感味道等,本文主要是针对狭义上的植物营养品质。不同的作物、不同的生长环境以及不同的管理措施对植物营养品质的影响巨大,因此植物营养品质的表征成为现代社会中人们十分关注的问题。近60年以来,传统的植物营养品质分析主要以化学分析为主,即通过各种提取和分离手段,进行显色和比色,这些化学分析方法为植物营养品质的表征做出了重大贡献,并还将继续发挥重要作用。但随着社会的发展,人们对分析的时效性和成本提出了越来越高的要求,并推动了现代仪器分析技术的发展,而现代光谱技术则是现代仪器分析技术最重要表现之一[4]。
1 现代光谱技术光谱分析是基于物质与电磁辐射相互作用,这种相互作用取决于该物质的性质,这种方法主要体现辐射能与物质组成和结构之间的关系。电磁辐射按频率 (波长) 可分为不同的区域 (图1),形成了不同光谱分析法,其中红外光谱是迄今最为重要的分析方法之一[5];该方法最大的特点在于几乎所有形态的样本均可以采用这种方法进行研究,即不论是固态、液态和气态样本还是糊状、块状和粉状样本,都可以采用不同的检则附件进行研究。随着现代科学技术的发展和制造技术的进步,很多新型的检测附件不断被开发和应用,使得不同形态的样本能得到更为有效的检测[6]。
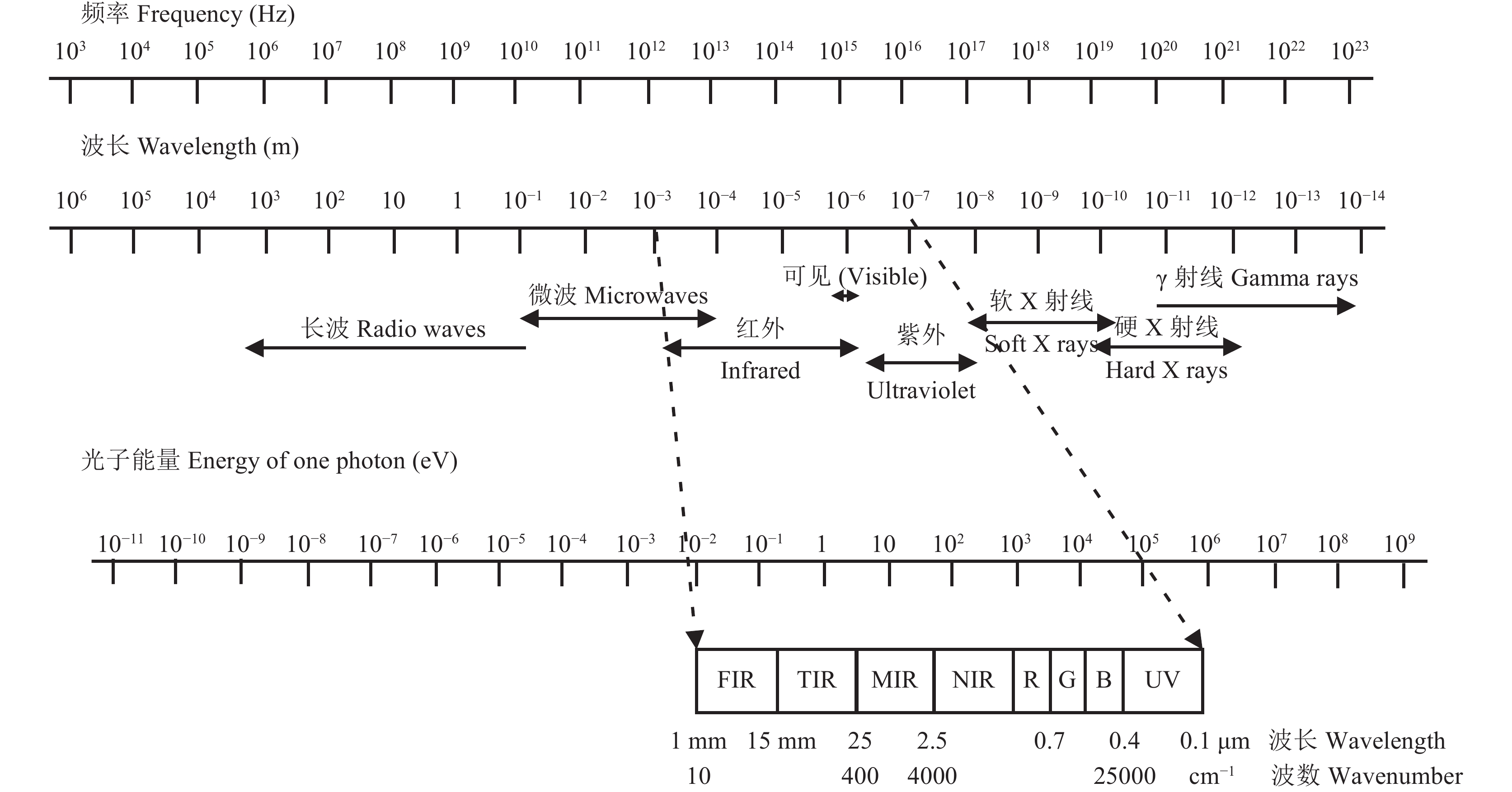 |
| 图1 电磁波谱 Fig. 1 Electromagnetic wave spectrum |
红外光照射物质后,其中某些波长的光被物质吸收,将通过物质吸收后的红外辐射强度按波长逐一记录下来即为该物质的红外光谱,测定这种光谱的仪器称为红外光谱仪。从上个世纪50年代以来,红外光谱仪不断得到改进,已从色散型红外光谱仪发展到了干涉型傅里叶转换红外光谱仪,这两种光谱仪在不同的分析领域均得到广泛的应用,后者分辨率高、扫描速度快且波长范围宽,但价格较昂贵。分子是保持物质理化性质的基本微粒,在物质的结构中,分子由若干个原子组成。分子在不断地运动,在外界条件的作用下,不同物质的分子通过扩散、碰撞、能量传递而发生理化反应。在一定的条件下分子的运动会达到一种平衡状态,并可以产生稳定的光谱吸收。分子的光谱吸收除了包含有原子吸收的特征外,还具有自己的吸收特征,更多理论上的阐述参见相关参考文献[5–7]。
现代红外光谱分析技术是近十年来分析化学领域迅猛发展的高新分析技术,引起了国内外分析专家的注目,在分析化学领域广泛应用,被誉为分析“巨人”,它的出现带来了又一次分析技术的革命。本世纪以来,红外光谱在各领域中的应用全面展开,在植物营养分析中,有关红外光谱的研究及应用文献几乎呈指数增 长(图2a),成为发展最快、最引人注目的一门独立的分析技术。我国的植物营养品质分析中,红外光谱技术的研究及应用起步较晚,但近10年以来发展迅速,将现代光谱测量技术、计算机技术、化学计量学技术与基础测试技术有机结合,呈现出多学科的交叉与融合。相关研究论文已跃居世界第一 (图2b),取得了显著的进步,甚至明显超过美国,但在原始创新、研究质量和技术应用等方面与美国等发达国家还存在相当大距离。
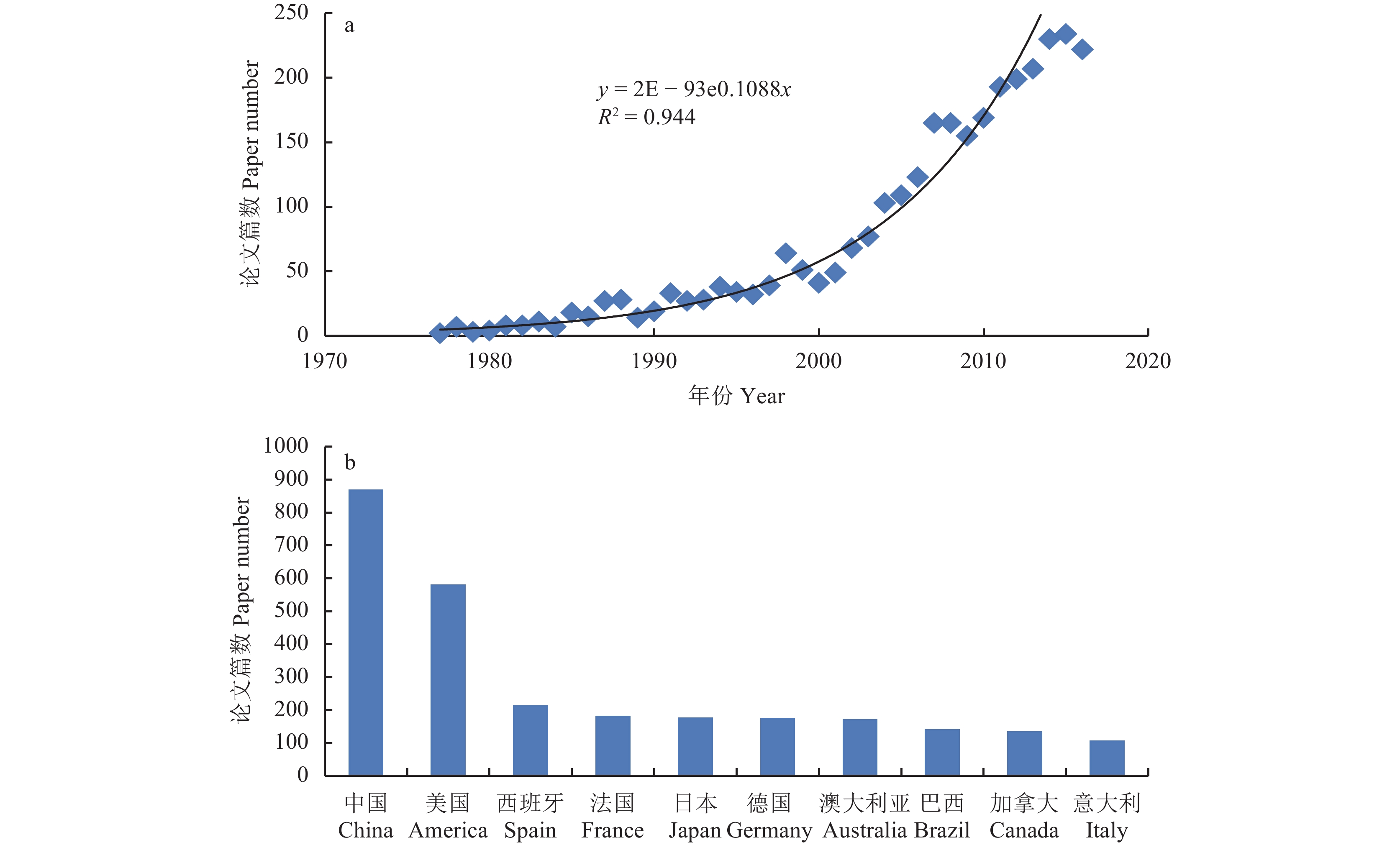 |
| 图2 近40年来植物营养品质分析中与红外光谱技术相关论文发表情况 Fig. 2 Published papers using technique of infrared spectroscopy in plant nutritional analysis in last 40 years [注(Note): 数据来源于 web of science Data source from web of science;主题词为红外光谱、植物 Keywords were infrared spectroscopy, plant.] |
红外光谱反映样品分子键的振动信息,包括C-H、O-H、N-H、S-H、C-C、N-O等化学键的信息,因此分析范围几乎可覆盖所有含分子键的有机或无机样本[5–6]。它是采用化学计量学方法建立校正模型,进而预测未知样品的一种分析方法,在植物营养品质分析中开始发挥越来越大的作用[8–9]。
2 红外光谱在植物营养品质分析中的应用红外光谱已被广泛应用于各类植物品质营养分析,包括各类粮食作物、水果、蔬菜以及经济作物 (表1)。在这些分析中,绝大部分采用的是近红外光谱,同时,小部分分析也应用了中红外光谱,尽管中红外光谱应用明显较少,但整体上,其效果等同或优于近红外。由于红外光谱分析是多参数分析,往往需要采用多元校正的化学计量学方法,其中偏最小二乘法 (partial least square,PLS) 是应用最广泛的一种方法,这种方法表达的主要是一种线性关系,而对于非线性关系则可能出现较大预测误差,因此非线性的方法也经常被应用,如支持向量机 (support vector machine,SVM)、人工神经网络 (artificial neural networks,ANN) 等。此外,还有很多其它算法以及不同算法的联用,在实际应用中可以进行选择和优化。在植物营养品质参数上,红外光谱分析几乎涉及人们所关心的所有参数,如矿质养分、氨基酸、蛋白质、脂肪、有机酸、多糖、淀粉、微量元素、类胡萝卜素、纤维、三聚氰胺、油酸、硬脂酸、维生素E、多酚、黄酮、花青素、植物甾醇、儿茶素、咖啡因、芥酸、硫甙等。不同的植物、不同的手段以及不同的算法在分析精度和准确性上表现出显著的差异,因此在实际应用中需要结合需求进行选择使用。
| 表1 红外光谱在植物营养品质分析中的应用 Table 1 Application of infrared spectroscopy in plant nutritional analysis |
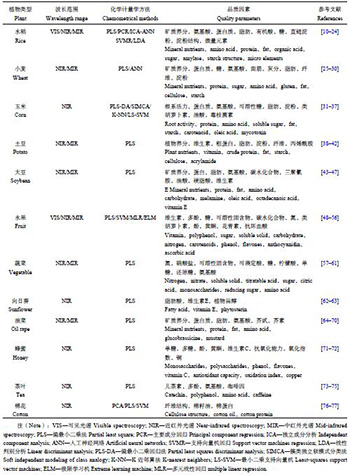 |
除了以上常见的植物营养参数,红外光谱表达的是样品的整体信息,因此,红外光谱本身能在植物样品品质判别鉴定、植物样品溯源和道地性上发挥独特的作用,如虫草真假的鉴定[78]、品种差异的鉴别[79],中药材的道地性等[80]。同时,融合光谱参数,可以对一些难以直接测定主观性比较强的指标进行更客观的分析,如茶叶、葡萄酒的口感和品味[56, 75, 81]。
3 化学计量学方法在自然科学研究中经常要获取各种数据,尤其是实验科学,需要根据研究目的采用各种方法获取或测定相关数据,然后在所获取的数据基础上进行分析和总结,提出、证明、修正或推翻某一个结论、假说或理论。在一些实验科学中,如土壤学和生物学,经常会处理海量的数据,因此在数据处理时必须借助计算机通过相关分析软件进行处理,从数据中挖掘目标信息[82]。
3.1 数据预处理在红外光谱分析中,由于系统或环境干扰,原始光谱中含有与样品组成和结构无关的信息,即噪声,使得近红外光谱变得不稳定,并可能发生漂移或重叠,所以首先有必要对光谱进行预处理,以消除噪声干扰,优化光谱信号,提高光谱分辨率和校正模型的分析精度和稳定性。光谱预处理方法有很多种,比如光谱平滑,其基本思想是在平滑点的前后各取若干点进行平均或拟合,求得平滑点的最佳估计值,消除随机噪声,这一方法的前提是随机噪声的增均值为零。常用的平滑方法有Savitzky-Golay卷积平滑法、傅里叶变换滤波以及小波变换滤波[83–84]。平滑处理带有一定的经验性,如果平滑处理不合适有可能导致有用信息的丢失,红外光谱多采用小波滤波进行平滑化处理,本文以此为例简要介绍小波分析的方法和原理。
小波理论是上个世纪80年代后期发展起来的应用数学分支,其思想来源于伸缩与平移,既保持了傅里叶变换的优点又具有多分辨率、方向选择性和自动调焦的特点,被称为数学上的显微镜[83]。数据的标准化是将数据按一定比例缩放,使之落入一个特定的区间。由于指标体系的各个指标度量单位不同,为了平衡选择指标权重,通过函数变换将其数值映射到某个数值区间,使得基本度量单位能统一起来,从而有利于进一步的定性与定量分析。常见的标准化方法包括线性标准化法 (linear normalization algorithm) 和非线性标准化法 (nonlinear normalization algorithm) 两大类。
对于相对复杂样本如植物样本的红外分析,其红外光谱是很多种不同组分吸收的叠加,因此不同组分之间的相互干扰很强,而将光谱进行微分能提高光谱分析的分辨率和灵敏度,但随着导数阶数的增加,信噪比变低,预测误差增加。微分处理不仅成为解析光谱的强有力工具,而且在相当程度上改善了多重共线性,使校正模型的性能有了显著的改善,但微分处理对微小的噪声具有强调作用,因此在实际应用中,一般采用一阶和二阶微分光谱,三阶或三阶以上的微分光谱则很少采用。此外,反卷积 (deconvolution) 也可以起到分离光谱信号的作用,但反卷积过程中,随机高频干扰信号可能会被放大,因此需要适当抑制噪声[85]。
3.2 模型构建光谱分析因涉及多元校正,因此要依赖于化学计量学 (chemometrics)。化学计量学是应用数学、统计学与计算机科学的工具和手段,设计或选择最优化学量测方法,并通过解析化学量测数据以最大限度地获取化学及其相关信息[86]。化学计量学是化学、分析化学与数学、统计学及计算机科学之间的“接口”,是多学科融合的产物。化学计量学之所以得以迅速发展的主要原因是计算机科学的发展不仅使大量化学测量仪器操作实现了自动化,并使得大量数据的自动采集和传输成为事实。计算机科学的迅猛发展,对近代数学也产生了巨大影响,过去难以适用的复杂数学方法可在计算机上实现,为解决复杂的数据处理与目标信息提取提供了可能。正是有了现代化学测量手段的进步和数学解析手段的发展,多元校正的分析方法成了化学计量学中最活跃、最有生气的一个分支。
植物样品的红外光谱包含了组成与结构的信息,在样品的红外光谱和其理化性质参数间也必然存在着内在的关系。使用化学计量学这种数学方法对光谱和理化性质进行关联,可确立这两者间的定量或定性关系,即校正模型。进而通过测量未知样品的近红外光谱,选择正确模型或者构建自适应模型[87],就可以预测样品的理化性质参数。因此,红外光谱分析方法包括了预处理、校正和预测三个过程。由于校正模型的复杂性和经验性,红外光谱分析又称“黑匣子”分析技术,即间接测量技术。
化学计量学是综合使用数学、统计学和计算机科学等方法,并结合应用领域专业知识,从测量数据中提取信息的一门新兴的应用交叉学科。大量化学计量学方法被写成软件,并成为分析仪器 (尤其是红外光谱仪) 的重要组成部分,这些软件的出现使得应用化学计量学方法解决实际复杂体系的分析问题成为现实。这些方法的基本原理、算法和功能可参考有关文献[86, 88]。在常规植物营养品质分析中,从每个样本中所获取的数据多是具体的和点式的,如蛋白质含量,仅仅就是单个数据点,而每一个植物样本的红外光谱其数据是二维的,即含波长和吸收强度,一条数据往往含有数百乃至数千个带式数据点,携带着十分丰富的样本信息,但是很多信息是蕴藏在众多的信息之中,相互干扰和遮盖,因此需要进行信息提取或数据挖掘 (data mining)。光谱数据挖掘涉及到大量运算,其复杂程度远高于点式数据运算,现代计算机技术和化学计量学的发展为复杂的数据挖掘提供了可能的手段。
4 展望 4.1 新型光谱技术目前,可见光、紫外光和红外光在植物营养品质分析中得到广泛的应用,相应的仪器设备也在分析能力、分辨率、分析精度以及便携性上都不断发展。从单样品分析到多样品自动分析,从模拟信号到高精度数字信号,从单通道到多通道,从台式机到手持式机等。同时,随着技术和检测手段的进步,不断产生新型光谱技术,其中开始应用的包括红外光声光谱、拉曼光谱和激光诱导击穿光谱。
 |
| 图3 红外光声光谱原理示意图 Fig. 3 Schematic description of the photoacoustic spectroscopy setup |
Bell于1880年在研究光纤通讯时发现了光声效应[89–90],但直到20世纪80年代,由于傅利叶红外光谱仪、低噪音高灵敏度微音器以及计算机技术的发展,光声光谱开始成为非常有价值的分析方法[91]。一束红外光入射到光声附件,通过KBr窗口照射到光声池中的样品,样品受到红外光照射后产生热效应,并将热传导给样品池中的气体 (通常为氦气),气体受热后会膨胀与收缩,从而产生热波,热波可被敏感的麦克风 (微音器) 检测,即为光声信号,光声信号转化成电信号,经过放大后就得到红外光声光谱 (图3)。热波在样品光激发处产生并开始传递,但衰减很快,这个衰减过程也决定了探测深度,因此不同的调制频率就可以探测到不同深度的样品,当调制频率增加时,样品探测深度减小,反之增大[92]。红外光声光谱分析不需要或者需要很少的样品前处理,而且能直接获取样品不同深度的信息,这种直接快速的分析方法适用于具有很弱和很强吸收的样品,同时也适用于不同形态植物样品的分析。
1928年印度物理学家拉曼在实验中发现,当用波长比试样粒径小得多的单色光照射气体、液体或透明试样时,发现还有一系列对称分布的若干条谱线,强度很弱且与入射光频率发生位移,这种现象称为拉曼效应,拉曼也因此获得了1930年的诺贝尔物理学奖。由于拉曼谱线的数目、位移的大小、谱线的长度直接与试样分子振动或转动能级有关,与红外吸收光谱类似,拉曼光谱可以得到有关分子振动或转动的信息,已广泛应用于物质的鉴定以及分子结构的解析[93]。目前的拉曼技术包括单道检测的拉曼光谱分析技术、以CCD为代表的多通道探测器的拉曼光谱分析技术、采用傅立叶变换技术的拉曼光谱分析技术、共振拉曼光谱分析技术和表面增强拉曼效应分析技术。拉曼光谱的分析方法不需要样品前处理,也没有样品的制备过程,并且具有分析过程操作简便、测定时间短和灵敏度高等优点。
原子光谱是利用基于不同原子的特征光谱进行元素分析,传统的原子光谱分析需要进行样品前处理,通常包括湿消解和干消解,因而无法获得样品本身的原子光谱,而要获得样品本身的原子光谱就要求不能进行复杂的样品前处理,包括消解或提取。激光诱导击穿光谱 (laser-induced breakdown spectroscopy,LIBS) 是一种利用高能量脉冲激光烧蚀样品材料,使材料表面的微量样品瞬间气化形成高温、高密度的等离子体,测量等离子体中原子发射光谱的谱线波长和强度,进而完成样品材料所含化学元素的定性和定量分析的光谱检测技术 (图4)。近10年来LIBS的研究得到了快速发展,相关研究论文逐年增多,应用领域也逐渐扩大[94]。LIBS具备许多独特的优点,如样品预处理简单或无需预处理,适合于各种形态 (气态、液态、固态、颗粒) 物质的分析,激光激发样品无二次污染,近似于无损检测,类似于激光探针,可进行快速实时现场分析,能够完成表面和逐层原位检测,可以实现非接触式远距离探测,能够应对恶劣环境下的在线分析,仪器操作简单方便。但LIBS在植物营养品质分析中的应用还非常有限[95],因此具有广阔的发展前景。
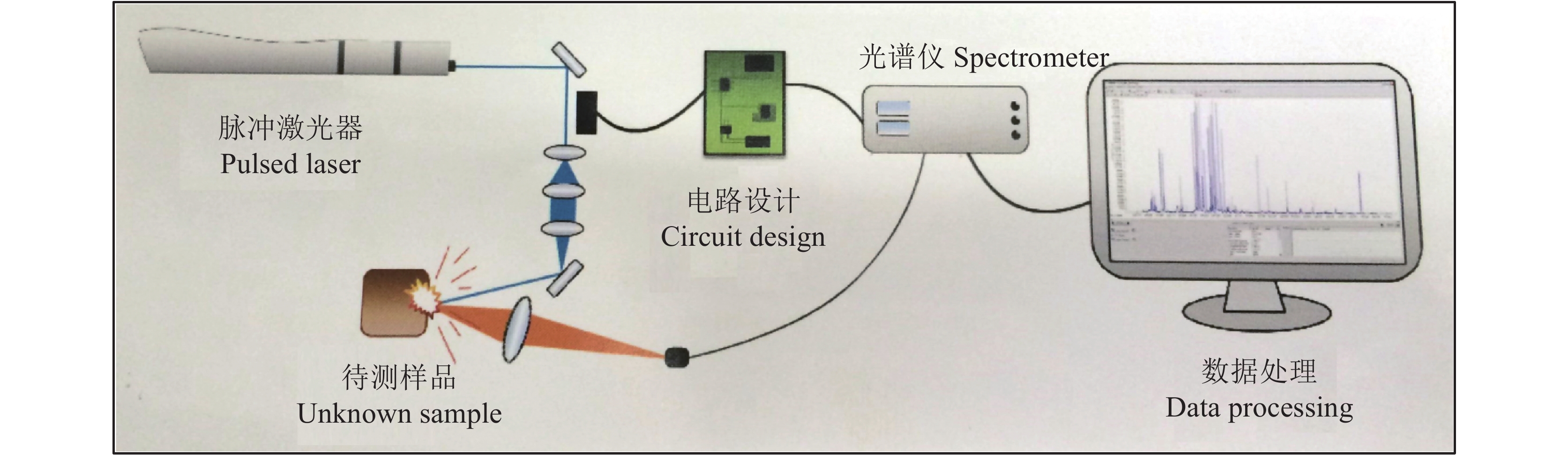 |
| 图4 激光诱导击穿光谱工作原理图 Fig. 4 Principle of laser induced breakdown spectroscopy (LIBS) |
除了以上光谱外,还有同步辐射、各类核磁共振 (nuclear magnetic resonance,NMR)、质谱 (mass spectroscopy,MS)、CT等,但这些光谱学方法设备昂贵,难以在常规植物营养品质分析中应用,本文就不作介绍,进一步了解可参阅有关文献[96–98]。
4.2 光谱融合与现代信息技术光谱根据波长可分为X光、可见近红外光谱、中红外光谱等;根据光的种类可分为红外、拉曼、荧光等;根据振动的类型可为原子光谱、分子光谱等;根据信号获取方法可分为吸收光谱、光声光谱等。以上各种光谱具有各自的特点和优势,如红外光谱主要是响应极性分子振动,而拉曼光谱则响应非极性分子振动,无疑两种光谱数据的融合能获得更多样品的信息,从而为样品的表征提供更好的技术支撑[4]。又如,分子光谱反映的是分子键的振动,主要表现为结构组成特征,而原子光谱反映的是原子的特征吸收,主要表现元素组成特征,两者的结合也是信息的互补,因此光谱融合具有重要应用前景[93]。但光谱数据的融合涉及数据的权重、数据的连接、信息的冗余以及干扰或噪音的引入,因此需要选择或优化不同的光谱数据处理方法和模型[81],否则,光谱的融合不但不能发挥作用,反而可能使分析的精度和准确度降低。
当前信息技术发展迅猛,智能手机广泛使用,互联网和云技术开始应用于诸多行业,如工业、商业和服务业等,在农业中已开始运用,但还相当薄弱[99]。现代光谱技术以互联网和云技术 (云贮存和云计算) 为平台,通过智能终端 (如手机),结合植物营养专业知识,将植物营养品质分析与鉴定常规化 (图5),在满足人们对植物营养品质信息需求的同时,也能进一步规范市场,促进植物营养品质的提升。
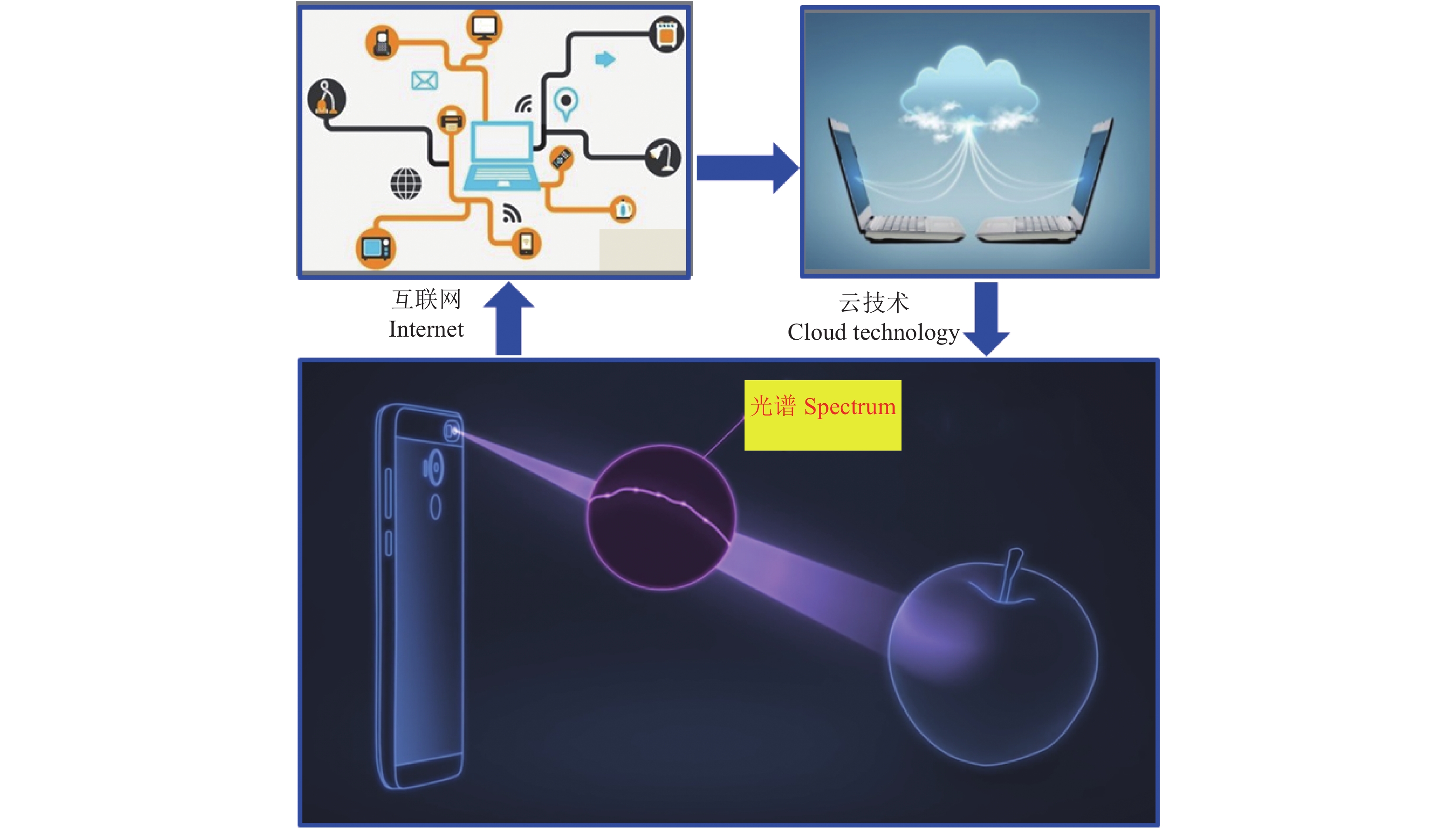 |
| 图5 植物营养品质信息的适时快速获取示意图 Fig. 5 In situ rapid obtaining of plant nutrition quality parameters combining information technology |
| [1] |
朱兆良, 金继运. 保障我国粮食安全的肥料问题[J].
植物营养与肥料学报, 2013, 19(2): 259–273.
Zhu Z L, Jin J Y. Fertilizer use and food security in China[J]. Journal of Plant Nutrition and Fertilizer, 2013, 19(2): 259–273. |
| [2] |
张士康, 山丽杰, 吴林海. 中国农产品消费的形态特征、关注度与农产品品质的安全供给分析[J].
世界农业, 2010, 376: 49–52.
Zhang S K, Shan L J, Wu H L. Food quality and security in China regarding a new consumption perspective[J]. World Agriculture, 2010, 376: 49–52. |
| [3] | Luo B L. The key points, difficulties and direction of agricultural supply side reforms[J]. Agricultural Economics, 2017, 1: 1–10. |
| [4] | Santosh L, Sangdae L, Hoonsoo L, et al. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration[J]. Trends in Food Science and Technology, 2015, 46: 85–98. |
| [5] | Stuart B. Infrared spectroscopy: Fundamentals and applications [M]. New Jersey: John Wiley & Sons, 2004. |
| [6] | Gunzler H, Gremlich H U. IR spectroscopy: an introduction [M]. Weinheim: Wiley-VCH, 2002. |
| [7] |
杜昌文. 土壤红外光声光谱原理与应用[M]. 北京: 科学出版社, 2012.
Du C W. Soil infrared photoacoustic spectroscopy: principal and application[M]. Beijing: Science Press, 2012. |
| [8] | Dixon R, Coates D. Near infrared spectroscopy of faeces to evaluate the nutrition and physiology of herbivores[J]. Journal of Near Infrared Spectroscopy, 2009, 17(1): 1–31. |
| [9] | Lu X N, Rasco B A. Determination of antioxidant content and antioxidant activity in foods using infrared spectroscopy and chemometrics: A review[J]. Critical Reviews in Food Science and Nutrition, 2012, 52(10): 853–875. |
| [10] |
张玉森, 姚霞, 田永超, 等. 应用近红外光谱预测水稻叶片氮含量[J].
植物生态学报, 2010, 34(6): 704–712.
Zhang Y S, Yao X, Tian Y C, et al. Estimating leaf nitrogen content with near infrared reflectance spectroscopy in rice[J]. Chinese Journal of Plant Ecology, 2010, 34(6): 704–712. |
| [11] | Shen F, Niu X Y, Yang D T, et al. Determination of amino acids in Chinese rice wine by Fourier transform near-infrared spectroscopy[J]. Journal of Agricultural and Food Chemistry, 2010, 58: 9809–9816. |
| [12] | Escuredo O, Martín M I G, Moncada G W, et al. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques [J]. Journal of Cereal Science, 2014, 60: 67–74. |
| [13] | Torit B B, Srigopal S, Krishnendu C. Development of NIRS models to predict protein and amylose content of brown rice and proximate compositions of rice bran[J]. Food Chemistry, 2016, 191: 21–27. |
| [14] | Zhang B, Rong Z. Prediction of the amino acid composition in brown rice using different sample status by near-infrared reflectance spectroscopy[J]. Food Chemistry, 2011, 127: 275–281. |
| [15] | Chuang Y K, Hua Y P, Yang I C. Integration of independent component analysis with near infrared spectroscopy for evaluation of rice freshness[J]. Journal of Cereal Science, 2014, 60: 238–242. |
| [16] | Chen K J, Huang M. Prediction of milled rice grades using Fourier transform near-infrared spectroscopy and artificial neural networks[J]. Journal of Cereal Science, 2010, 52: 221–226. |
| [17] | Pornarree S, Kazuhiro N, Sirichai K, et al. Eating quality evaluation of Khao Dawk Mali 105 rice using near infrared spectroscopy[J]. LWT - Food Science and Technology, 2017, 79: 70–77. |
| [18] | Xie L H, Tang S Q, Chen N, et al. Optimization of near-infrared reflectance model in measuring protein and amylose content of rice flour[J]. Food Chemistry, 2014, 142: 92–100. |
| [19] | Xu S X, Shi X Z, Wang M Y, et al. Determination of rice root density at the field level using visible and near-infrared reflectance spectroscopy[J]. Geoderma, 2016, 267: 174–184. |
| [20] | Fan D M, Ma W R, Wang L Y, et al. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy[J]. Starch, 2012, 64: 598–606. |
| [21] | Shao Y N, Cen Y L, He Y, et al. Infrared spectroscopy and chemometrics for the starch and protein prediction in irradiated rice[J]. Food Chemistry, 2011, 126: 1856–1861. |
| [22] | Shen F, Ying Y B, Li B B, et al. Prediction of sugars and acids in Chinese rice wine by mid-infrared spectroscopy[J]. Food Research International, 2011, 44: 1521–1527. |
| [23] | Shao N, Y Y. Visible/near infrared spectroscopy and chemometrics for the prediction of trace element (Fe and Zn) levels in rice leaf[J]. Sensors, 2013, 13: 1872–1883. |
| [24] | Cozzolino D. An overview of the use of infrared spectroscopy and chemometrics in authenticity and traceability of cereals[J]. Food Research International, 2014, 60: 262–265. |
| [25] | Ingrid V, Martin E, Nicolas V, et al. Monitoring nitrogen leaf resorption kinetics by near-infrared spectroscopy during grain filling in durum wheat in different nitrogen availability conditions[J]. Crop Science, 2013, 53: 284–296. |
| [26] | Ayse C M, Ismail H B, Huseyin E G, et al. Prediction of wheat quality parameters using near-infrared spectroscopy and artificial neural networks[J]. Europe Food Research and Technology, 2011, 233: 267–274. |
| [27] | Johannes H, Michael P, Lukas D, et al. A comparison between near-infrared (NIR) and mid-infrared (ATR-FTIR) spectroscopy for the multivariate determination of compositional properties in wheat bran samples[J]. Food Control, 2016, 60: 365–369. |
| [28] | Fontaine J, Schirmer B, Horr J. Near-infrared reflectance spectroscopy (NIRS) enables the fast and accurate prediction of essential amino acid contents. 2. Results for wheat, barley, corn, triticale, wheat bran/middlings, rice bran, and sorghum[J]. Journal of Agricultural and Food Chemistry, 2002, 50(14): 3902–3911. |
| [29] |
姚霞, 王雪, 黄宇, 等. 应用近红外光谱法估测小麦叶片糖氮比[J].
应用生态学报, 2015, 26(8): 2371–2378.
Yao X, Wang X, Huang Y, et al. Estimation of sugar to nitrogen ratio in wheat leaves with near infrared spectrometry[J]. Chinese Journal of Applied Ecology, 2015, 26(8): 2371–2378. |
| [30] |
姚霞, 汤守鹏, 曹卫星, 等. 应用近红外光谱预测小麦叶片氮含量[J].
植物生态学报, 2011, 35(8): 844–852.
Yao X, Tang S P, Cao W X, et al. Estimating the nitrogen content in wheat leaves by near-infrared reflectance spectroscopy[J]. Chinese Journal of Plant Ecology, 2011, 35(8): 844–852. |
| [31] | Lidia E A, David D E, Susan D, et al. Feasibility of near infrared spectroscopy for analyzing corn kernel damage and viability of soybean and corn kernels[J]. Journal of Cereal Science, 2012, 55: 160–165. |
| [32] | Jiang H Y, Zhu Y J, Wei L M, et al. Analysis of protein, starch and oil content of single intact kernels by near infrared reflectance spectroscopy (NIRS) in maize (Zea mays L.) [J]. Plant Breeding, 2007, 126: 492–497. |
| [33] | Oreste B, Nicola B. Application of near-infrared reflectance spectroscopy (NIRS) to the evaluation of carotenoids content in maize[J]. Journal of Agricultural and Food Chemistry, 2004, 52: 5577–5582. |
| [34] | Tesfaye M B, Tom C P, Settles A M. Development of a calibration to predict maize seed composition using single kernel near infrared spectroscopy[J]. Journal of Cereal Science, 2006, 43: 236–243. |
| [35] | Nicola B, Vincenza P, Paola B, et al. Rapid detection of kernel rots and mycotoxins in maize by near-infrared reflectance spectroscopy[J]. Journal of Agricultural and Food Chemistry, 2005, 53: 8128–8134. |
| [36] | Jasper G T, Natalia P, Paul R A. Prediction of maize seed attributes using a rapid single kernel near infrared instrument[J]. Journal of Cereal Science, 2009, 50: 381–387. |
| [37] |
魏良明, 严衍禄, 戴景瑞. 近红外反射光谱测定玉米完整籽粒蛋白质和淀粉含量的研究[J].
中国农业科学, 2004, 37(5): 630–633.
Wei L M, Yan Y L, Dai J R. Determining protein and starch contents of whole maize kernel by near infrared reflectance spectroscopy (NIRS)[J]. Scientia Agricultura Sinica, 2004, 37(5): 630–633. |
| [38] | Sylwester M, Roman S, Agnieszka K. Application of infrared reflection and Raman spectroscopy for quantitative determination of fat in potato chips[J]. Journal of Molecular Structure, 2016, 1126: 213–218. |
| [39] | Oluwatosin E A, Suzanne D J, Van-Den T, et al. Development and validation of a near-infrared spectroscopy method for the prediction of acrylamide content in french-fried potato[J]. Journal of Agricultural and Food Chemistry, 2016, 64: 1850–1860. |
| [40] | Timm B, Bernd T, Wolfgang F, et al. Development of near-infrared reflection spectroscopy calibrations for crude protein and dry matter content in fresh and dried potato tuber samples[J]. Potato Research, 2016, 59: 149–165. |
| [41] | Ainara L, Silvia A, Ignacio G, et al. Review of the application of near-infrared spectroscopy for the analysis of potatoes[J]. Journal of Agricultural and Food Chemistry, 2013, 61: 5413–5424. |
| [42] | Xu S X, Zhao C, Shi X Z, et al. Rapid determination of carbon, nitrogen, and phosphorus contents of field crops in China using visible and near-infrared reflectance spectroscopy[J]. Crop Science, 2017, 57: 475–489. |
| [43] | Daniela S F, Juliana A L P, Ronei J P. Fourier transform near-infrared spectroscopy (FT-NIRS) application to estimate Brazilian soybean (Glycine max (L.) Merril) composition [J]. Food Research International, 2013, 51: 53–58. |
| [44] | Simon A H, Stewart F G, Emmanuelle C, et al. The application of near-infrared reflectance spectroscopy (NIRS) to detect melamine adulteration of soya bean meal[J]. Food Chemistry, 2013, 136: 1557–1561. |
| [45] | Wang L, Wang Q, Liu H Z, et al. Determining the contents of protein and amino acids in peanuts using near-infrared reflectance spectroscopy[J]. Journal of the Science of Food Agriculture, 2013, 93: 118–124. |
| [46] | Zhang G Y, Li P H, Zhang W F, et al. Analysis of multiple soybean phytonutrients by near-infrared reflectance spectroscopy[J]. Analytical and Bioanalytical Chemistry, 2017, 409: 3515–3525. |
| [47] | Ferreira D S, Galão O F, Pallone J A L, et al. Comparison and application of near-infrared (NIR) and mid-infrared (MIR) spectroscopy for determination of quality parameters in soybean samples[J]. Food Control, 2014, 35: 227–232. |
| [48] | Audrey P, Juan A F P, Vincent B, et al. Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using near-infrared spectroscopy[J]. Journal of Agricultural and Food Chemistry, 2013, 93: 238–244. |
| [49] | Qi S Y, Seiichi O, Yoshio M, et al. Influence of sampling component on determination of soluble solids content of Fuji apple using near-infrared spectroscopy[J]. Applied Spectroscopy, 2017, 71(5): 856–865. |
| [50] | Mark W D, Wouter S, Ellen H, et al. Application of visible and near-infrared reflectance spectroscopy (Vis/NIRS) to determine carotenoid contents in banana (Musa spp.) fruit pulp [J]. Journal of Agricultural and Food Chemistry, 2009, 57: 1742–1751. |
| [51] | Nicoletta S, Anna S, Valentina D E, et al. Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy [J]. Postharvest Biology and Technology, 2008, 50: 31–36. |
| [52] | Bei D R, Fuentes S, Sullivan W, et al. Rapid measurement of total non-structural carbohydrate concentration in grapevine trunk and leaf tissues using near infrared spectroscopy[J]. Computers and Electronics in Agriculture, 2017, 136: 176–183. |
| [53] | Cortés V, Ortiz C, Aleixos N, et al. A new internal quality index for mango and its prediction by external visible and near-infrared reflection spectroscopy[J]. Postharvest Biology and Technology, 2016, 118: 148–158. |
| [54] | Wang J, Zhao H B, Shen C W, et al. Determination of nitrogen concentration in fresh pear leaves by visible/near-infrared reflectance spectroscopy[J]. Agronomy Journal, 2014, 106(5): 1867–1872. |
| [55] | Jiang H, Zhu W X. Determination of pear internal quality attributes by Fourier transform near infrared (FT-NIR) spectroscopy and multivariate analysis[J]. Food Analytical Methods, 2013, 6: 569–577. |
| [56] | Cozzolino D. The role of visible and infrared spectroscopy combined with chemometrics to measure phenolic compounds in grape and wine samples[J]. Molecules, 2015, 20: 726–737. |
| [57] | Li C Y, Du C W, Zeng Y, et al. Two-dimensional visualization of nitrogen distribution in leaves of Chinese cabbage (Brassica rapa subsp. chinensis) by the Fourier transform infrared photoacoustic spectroscopy technique [J]. Journal of Agricultural and Food Chemistry, 2016, 64: 7696–7701. |
| [58] | Katherine F, María-Teresa S, Dolores P, et al. Feasibility in NIRS instruments for predicting internal quality in intact tomato[J]. Journal of Food Engineering, 2009, 91: 311–318. |
| [59] | Steven V, Katrien B, Peter M, et al. Sequential injection ATR-FTIR spectroscopy for taste analysis in tomato[J]. Sensors and Actuators B, 2009, 137: 715–721. |
| [60] | Huseyin A, Andrea S, Didem P. Monitoring multicomponent quality traits in tomato juice using portable mid-infrared (MIR) spectroscopy and multivariate analysis[J]. Food Control, 2016, 66: 79–86. |
| [61] | Iwona S C, Maryse R, Sylvie B, et al. Mid-infrared spectroscopy as a tool for rapid determination of internal quality parameters in tomato[J]. Food Chemistry, 2015, 125: 1390–1397. |
| [62] | Pérez-Vicha B, Velascob L, Fernández-Martíneza J M. Composition in sunflower through the analysis of intact seeds, husked seeds, meal and oil by near-infrared reflectance spectroscopy[J]. JAOCS, 1998, 75: 547–555. |
| [63] | Sato T, Takahata Y, Noda T, et al. Nondestructive determination of fatty acid composition of husked sunflower (Helianthus annuus L.) seeds by near-infrared spectroscopy [J]. Journal of American Oil Chemistry Society, 1995, 72: 1177–1183. |
| [64] | Chen G L, Zhang B, Wu J G, et al. Nondestructive assessment of amino acid composition in rapeseed meal based on intact seeds by near-infrared reflectance spectroscopy[J]. Animal Feed Science and Technology, 2011, 165: 111–119. |
| [65] | Rafael F, Mercedes D R, Elena C, et al. Quantification of glucosinolates in leaves of leaf rape (Brassica napus ssp. pabularia) by near-infrared spectroscopy [J]. Phytochemistry, 2005, 66: 175–185. |
| [66] | Tkachuk R. Oil and protein analysis of whole rapeseed kernels by near infrared reflectance spectroscopy[J]. JAOCS, 1981, 8: 819–822. |
| [67] |
陆宇振, 杜昌文, 余常兵, 等. 红外光谱在油菜籽快速无损检测中的应用[J].
植物营养与肥料学报, 2013, 19(5): 1257–1263.
Lu Y Z, Du C W, Yu C B, et al. Advances in the application of infrared spectroscopy in the rapid and non-destructive characterization of rapeseeds[J]. Journal of Plant Nutrition and Fertilizer, 2013, 19(5): 1257–1263. |
| [68] | Lu Y Z, Du C W, Yu C B, et al. Use of FTIR-PAS combined with chemometrics to quantify nutritional information in rapeseeds (Brassica napus) [J]. Journal of Plant Nutrition and Soil Science, 2014, 177(6): 927–933. |
| [69] | Lu Y Z, Du C W, Yu C B, et al. Fourier transform mid-infrared photoacoustic spectroscopy (FTIR-PAS) coupled with chemometrics for non-destructive determination of oil content in rapeseed[J]. Transactions of the ASABE, 2015, 58(5): 1403–1407. |
| [70] | Gotor A A, Farkas E, Berger M, et al. Determination of tocopherols and phytosterols in sunflower seeds by NIR spectrometry[J]. European Journal of Lipid Science and Technology, 2007, 109(5): 525–530. |
| [71] | Ruoff K, Luginbuhl W, Bogdanov S, et al. Quantitative determination of physical and chemical measurands in honey by near-infrared spectrometry[J]. European Food Research and Technology, 2007, 225: 415–423. |
| [72] | Escuredo O, Seijo M C, Salvador J, et al. Near infrared spectroscopy for prediction of antioxidant compounds in the honey[J]. Food Chemistry, 2013, 141: 3409–3414. |
| [73] | Ren G X, Wang S P, Ning J M, et al. Quantitative analysis and geographical traceability of black tea using Fourier transform near-infrared spectroscopy (FT-NIRS)[J]. Food Research International, 2013, 53: 822–826. |
| [74] | Chen Q S, Zhao J W, Sumpun C, et al. Simultaneous analysis of main catechins contents in green tea (Camellia sinensis L.) by Fourier transform near infrared reflectance (FT-NIR) spectroscopy [J]. Food Chemistry, 2009, 113: 1272–1277. |
| [75] | Jiang H, Chen Q S. Chemometric models for the quantitative descriptive sensory properties of green tea (Camellia sinensis L.) using Fourier transform near infrared (FT-NIR) spectroscopy [J]. Food Analytical Methods, 2015, 8: 954–962. |
| [76] | Liu Y L. Recent progress in Fourier transform infrared (FTIR) spectroscopy study of compositional, structural and physical attributes of developmental cotton fibers[J]. Materials, 2013, 6: 299–313. |
| [77] | Huang Z R, Sha S, Rong Z Q, et al. Feasibility study of near infrared spectroscopy with variable selection for non-destructive determination of quality parameters in shell-intact cottonseed[J]. Industrial Crops and Products, 2013, 43: 654–660. |
| [78] | Du C W, Zhou J M, Liu J F. Identification of Chinese medicinal fungus Cordyceps sinensis by depth-profiling mid-infrared photoacoustic spectroscopy[J]. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy, 2017, 173: 489–494. |
| [79] | Andreia M S, Mawsheng C, Jeemeng L, et al. Combining multivariate analysis and monosaccharide composition modeling to identify plant cell wall variations by Fourier Transform Near Infrared spectroscopy[J]. Plant Methods, 2011, 7: 26. |
| [80] | Sun S Q, Chen J B, Zhou Q, et al. Application of mid-Infrared spectroscopy in the quality control of traditional Chinese medicines[J]. Planta Medica, 2010, 76: 1987–1996. |
| [81] | Eva B, Joan F, Ricard B, et al. Data fusion methodologies for food and beverage authentication and quality assessment-A review[J]. Analytica Chimica Acta, 2015, 891: 1–14. |
| [82] | Du C W, Zhou J M. Application of infrared photoacoustic spectroscopy in soil analysis[J]. Applied Spectroscopy Reviews, 2011, 46: 405–422. |
| [83] | Michel M, Yves M, Georges O, et al. Wavelet toolbox for use with Matlab®[M]. The MathWorks, Inc., 2002. |
| [84] | Pascal C, Serge W, Michel U. Combined wavelet transform-artificial neural network use in tablet active content determination by near-infrared spectroscopy[J]. Analytica Chimica Acta, 2007, 591: 219–224. |
| [85] |
邹谋炎. 反卷积和信号复原[M]. 北京: 国防工业出版社, 2001.
Zhou M Y. Deconvolution and signal recovery [M]. Beijing: National Defense Industry Press, 2001. |
| [86] | Richard G B. Applied chemometrics for scientist[M]. Chichester, UK: John Wiley & Sons, Ltd, 2007. |
| [87] | Ma F, Du C W, Zhou J M, et al. A self-adaptive model for the prediction of soil organic matter using mid-infrared photoacoustic spectroscopy[J]. Soil Science Society of America Journal, 2016, 80(1): 238–246. |
| [88] | Howard M, Jerry W. Chemometrics in spectroscopy [M]. London, UK: Academic Press, Elsevier Ltd., 2007. |
| [89] | Bell A G. On the production and re-production of sound by light[J]. American Journal of Science, 1880, 20: 305–324. |
| [90] | Brunn J, Grosse P, Wynands R. Quantitative analysis of photoacoustic IR spectra[J]. Applied Physics B, 1988, 47: 343–348. |
| [91] | Rosencwaig A, Gersho A. Theory of photoacosutic effect with solids[J]. Journal of Applied Physics, 1976, 47: 64–69. |
| [92] | Du C W, Zhou J M, Wang H Y, et al. Determination of soil properties using Fourier transform mid-infrared photoacoustic spectroscopy[J]. Vibrational Spectroscopy, 2009, 49: 32–37. |
| [93] | Xing Z, Du C W, Zeng Y, et al. Characterizing typical farmland soils in China using Raman spectroscopy[J]. Geoderma, 2016, 268: 147–155. |
| [94] | Motea M, James R, Simon F, et al. Evaluation of elemental profiling methods, including laser-induced breakdown spectroscopy (LIBS), for the differentiation of Cannabis plant material grown in different nutrient solutions [J]. Forensic Science International, 2015, 251: 95–106. |
| [95] | Banu S, Gonca B, Ismail H B. Laser-induced breakdown spectroscopy based protein assay for cereal samples[J]. Journal of Agricultural and Food Chemistry, 2016, 64: 9459–9463. |
| [96] | Kovrlija R, Rondeau-Mouro C. Multi-scale NMR and MRI approaches to characterize starchy products[J]. Food Chemistry, 2017, 236: 2–14. |
| [97] | Lim S, Lee J G, Lee E J. Comparison of fruit quality and GC-MS-based metabolite profiling of kiwifruit 'Jecy green': Natural and exogenous ethylene-induced ripening[J]. Food Chemistry, 2017, 234: 81–92. |
| [98] | Mohoric A, Vergeldt F, Gerkema E, et al. The effect of rice kernel microstructure on cooking behaviour: A combined mu-CT and MRI study[J]. Food Chemistry, 2009, 115: 1491–1499. |
| [99] | Ojha T, Misra S, Raghuwanshi N S. Sensing-cloud: Leveraging the benefits for agricultural applications[J]. Computers and Electronics in Agriculture, 2017, 135: 96–107. |
 2017, Vol. 23
2017, Vol. 23  doi:
doi: 
