文章信息
- 表观弥散系数与胶质瘤IDH-1/1p19q基因型的相关性研究
- Correlation Between Apparent Diffusion Coefficient and IDH-1/1p19q Genotype of Glioma
- 肿瘤防治研究, 2023, 50(3): 271-275
- Cancer Research on Prevention and Treatment, 2023, 50(3): 271-275
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2023.22.0698
- 收稿日期: 2022-06-22
- 修回日期: 2022-09-29
2. 445000 恩施,恩施土家族苗族自治州中心医院重症医学科
2. Department of Critical Care Medicine, The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi 445000, China
脑胶质瘤是中枢神经系统最常见的原发性恶性脑肿瘤,占原发性恶性脑肿瘤的80%[1]。2016版中枢神经系统肿瘤WHO分类将异柠檬酸脱氢酶(isocitrate dehydrogenase, IDH)和1p19q等基因检测纳入弥漫星形细胞瘤和少突胶质细胞瘤的诊断标准,而2021年WHO更新了中枢神经系统肿瘤分类,将成人弥漫性胶质瘤分为星形细胞瘤-IDH突变型、少突胶质细胞瘤-IDH突变伴1p19q联合缺失型及胶质母细胞瘤-IDH野生型,并提出了组织学和分子病理学的整合诊断理念。目前胶质瘤分子病理学研究主要依赖于病理活检或手术病理,但部分患者无法手术或拒绝有创方法。弥散加权成像(diffusion weighted imaging, DWI)是MRI常用的功能性成像技术,通过定量检测肿瘤组织内水分子的自由扩散信息,反映肿瘤异质性和细胞增殖状况等。表观弥散系数(apparent diffusion coefficient, ADC)是DWI的定量参数,能够客观反映胶质瘤的细胞增殖、细胞外间隙等病理学特征,从而有望应用于无创性评价胶质瘤的分子病理学信息,进而指导临床治疗并预测患者预后。研究[2-4]表明DWIADC值与胶质瘤基因型(IDH/1p19q)间存在相关性、且与胶质瘤的预后相关,然而有关DWI与Ⅱ/Ⅲ级胶质瘤基因型间的相关性研究较少。本研究旨在观察ADC值与Ⅱ/Ⅲ级胶质瘤基因型(IDH-1、1p19q)间的相关性,探讨ADC值在无创性预测胶质瘤分子分型中的临床价值,从而为ADC辅助制定治疗方案、预测患者预后提供依据。
1 资料与方法 1.1 研究对象收集2013年3月—2020年12月在兰州大学第二医院就诊且临床资料完整的69例胶质瘤患者的临床资料。其中男34例、女35例,年龄18~72岁(42.24±14.73)岁;WHOⅡ级胶质瘤33例,WHO Ⅲ级胶质瘤36例。分子病理学检测:IDH-1基因检测48例(WHOⅡ级22例、Ⅲ级26例)、1p19q基因检测27例(WHOⅡ级13例、Ⅲ级14例)。所有患者术前均行常规MRI、DWI、FLAIR及MR增强检查。该研究中胶质瘤的诊断标准基于2007版和2016版WHO中枢神经系统肿瘤分类。本研究获得兰州大学第二医院医学伦理委员会批准(编号:2022A-497)。
1.2 MR设备与扫描方法采用Siemens 3.0 T Verio MR扫描仪。所有患者术前均行常规T1WI、T2WI、FLAIR序列及DWI序列扫描,MRI平扫后行增强扫描。扫描序列及参数:(1)GRE T1WITR550 ms,TE 11 ms,层厚5.0 mm,层间距1.5 mm,视野(FOV)260 mm×260 mm,矩阵256×256;(2)TSE T2WITR2200 ms,TE 96 ms,回波时间10 ms,回波链长度8,激励次数2;(3)DWI(SE序列)TR4500 ms,TE 98 ms,层厚5.0 mm,层距1.5 mm,矩阵256×256,b=0、1000 s/mm2;(4)FLAIR TR 9000 ms,TE 110.0 ms,层厚5.0 mm,层间距1.5 mm;(5)MR增强扫描:经肘静脉团注钆喷葡胺注射液(Gd-DTPA),剂量0.1 mmol/kg、流速3 ml/s。
1.3 DWI数据测量与分析 1.3.1 ROI的界定肿瘤实性部分或正常脑白质区勾画ROI区,避开肿瘤囊变、坏死、出血及水肿等区域;ROI面积取10~20 mm2。
1.3.2 ADC值的测量在ST-PACS医学图像工作站(北京思创贯宇科技开发有限公司)上选取肿瘤最大层面相邻的三个层面,利用自由形标记工具在ADC图上手工绘制ROI,在b=1 000s/mm2下测量平均ADC值(ADCmean)、最小ADC值(ADCmin),每个层面测量3次,并计算平均值。相对ADC值(rADC)=瘤体区ADC值/对侧脑白质ADC值,见图 1。
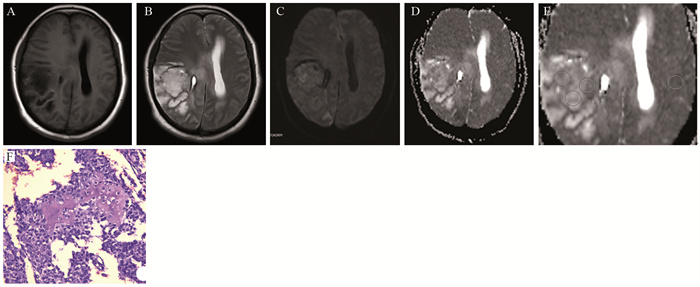
|
| A, B: the lesions in the right temporal-parietal lobe showed heterogeneous low signal and high signal on T1WI and T2WI, respectively; C, D: DWI presented as an inhomogeneous equal-slightly higher signal (mild diffusion restricted) (C) and ADC map showed an inhomogeneous low-slightly higher signal (D); E: the measurement method of ADC value to select three ROI (10-20 mm2) in lesions and take their average values as well as select the contralateral normal brain white matter to calculate rADC; F: tumor cells were arranged in diffuse pieces, the nucleus with obvious atypia was large, and numerous necrotic regions were found in the lesions (HE ×200). 图 1 少突胶质细胞瘤(WHOⅢ级;IDH-1突变/1p19q共缺失)MRI和病理学表现 Figure 1 MRI and pathological manifestations of oligodendrocytoma (WHO gradeⅢ) with IDH-1 mutation/1p19q co-deletion |
采用SPSS23.0统计学软件进行统计分析。计量资料组间比较采用独立样本t检验,计数资料组间比较采用卡方检验。胶质瘤ADC值/rADC值与IDH-1/1p19q的相关性采用卡方检验及独立样本t检验。采用受试者工作特征曲线(receiver operator characteristic curve, ROC)评价ADC值(ADCmean、ADCmin、rADCmean、rADCmin)对胶质瘤基因型的诊断性能。利用统计软件获得ROC曲线下面积(AUC),AUC值越接近1提示诊断效能越好,并分别计算敏感度、特异性等;采用Medcalc统计软件分析ADC参数值间诊断效能的差异性。P < 0.05为差异有统计学意义。
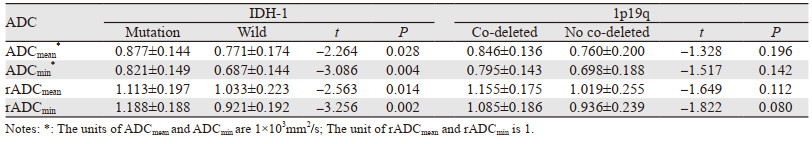
2 结果IDH-1突变组ADCmean、ADCmin、rADCmean、rADCmin值均显著高于IDH-1野生组(P < 0.05),而1p19q共缺失与非共缺失间的ADC值差异无统计学意义(P > 0.05),见表 1。
|
ROC曲线:ADCmean、ADCmin、rADCmean和rADCmin诊断胶质瘤IDH-1基因型的阈值及其敏感度、特异性、PPV、NPV及AUC见表 2、图 2,其中rADCmin诊断胶质瘤IDH-1基因型的效能最高。Medcalc软件分析显示ADCmean、ADCmin、rADCmean、rADCmin在诊断胶质瘤IDH-1基因型效能方面各组间比较差异无统计学意义(P > 0.05),见表 3。

|
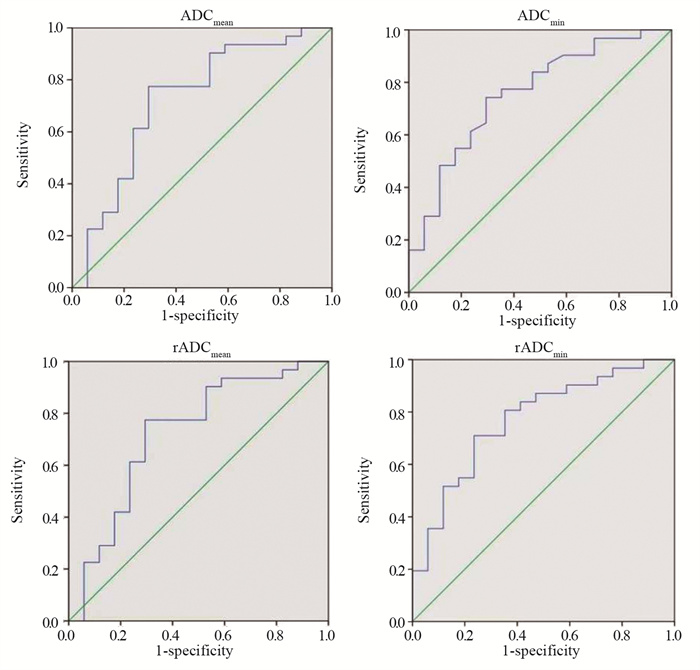
|
| 图 2 ADCmean、ADCmin、rADCmean、rADCmin预测胶质瘤IDH-1基因型的ROC曲线图 Figure 2 ROC curve of ADCmean, ADCmin, rADCmean, and rADCminvalues predicting IDH-1 genotypes of glioma |

|
最大程度地安全切除并辅以放疗、化疗为主的综合治疗是脑胶质瘤的标准治疗策略,而脑胶质瘤的WHO分级、分子分型影响其治疗决策和预后[5]。WHOⅠ、Ⅱ级为低级别胶质瘤(low-grade glioma, LGG),Ⅲ、Ⅳ级为高级别胶质瘤(high-grade glioma, HGG)。HGG具有高度侵袭性,中位生存期仅14.6个月,而LGG中位生存期可达13.0年[6]。少突胶质细胞瘤占所有原发性中枢神经系统肿瘤的2%~5%、占胶质细胞瘤的5%~20%[7],其预后相对较好,WHOⅡ级少突胶质细胞瘤、少突星形胶质瘤的5年生存率分别为84%和68%,而Ⅲ级少突胶质细胞瘤、星形细胞瘤的5年生存率仅为66%和32%[8]。
IDH是细胞能量代谢过程中的重要限速酶,参与细胞代谢、表观遗传调节、氧化还原和DNA损伤修复,可分为IDH-1、IDH-2和IDH-3三个亚型。IDH突变可改变IDH酶的活性,导致胶质瘤细胞代谢和微观结构发生一系列改变,从而影响胶质瘤的治疗疗效和预后。IDH-1主要分布于细胞质和过氧化物酶体,而IDH-2主要分布于线粒体[9];IDH-1突变型HGG的预后显著好于IDH野生型,中位生存期分别为24个月和10个月[10]。WHOⅡ级少突胶质细胞瘤lp/19q共缺失阳性率高达80%~90%,而WHOⅢ级阳性率为50%~70%,且1p19q共缺失型胶质瘤对烷化剂等化疗药物敏感[11]。此外,IDH-1突变型和1p19q共缺失型Ⅱ级弥漫型胶质瘤预后好于IDH-1野生型,且IDH-1突变型和1p19q共缺失型少突胶质细胞瘤患者较IDH-1突变型和1p19q未缺失/单缺失型预后更好[2]。另外,研究[12-13]表明ADC值在预测脑胶质瘤基因型及预后方面具有重要的临床价值。因此,探讨预测Ⅱ /Ⅲ级胶质瘤IDH/1p19q基因型的常规DWI技术,有助于术前无创性评估胶质瘤IDH/1p19q基因型,进而辅助临床制定有效的治疗方案。
WHOⅡ级弥漫型胶质瘤DWI与基因型间的相关性研究[2]证实低ADC值与IDH野生型独立相关,且IDH野生型胶质瘤伴低ADCmin时临床预后更差,提示IDH突变状况联合ADC值可以更准确预测Ⅱ级弥漫型胶质瘤的临床预后。标准临床DWI序列可评价胶质瘤IDH基因型和临床预后,如IDH野生型胶质瘤的rADCmean显著低于IDH突变型,且不论WHO分级,低rADCmean(< 1.08)的IDH突变型和野生型胶质瘤的预后显著差于高rADCmean(> 1.08)的IDH突变型和野生型,而低rADCmean的IDH突变型与野生型胶质瘤间的mOS无差异[3]。另有研究[4]显示基于标准临床DWI序列,肿瘤平均ADC值/正常脑白质ADC值联合形态学特征、年龄能够准确预测Ⅱ/Ⅲ级胶质瘤的IDH突变状况。鉴于胶质瘤的异质性,肿瘤局部ADC值预测IDH突变状况可能有一定的局限性。研究[13]证实肿瘤区域标准化ADCmean在预测Ⅱ ~Ⅲ级实性胶质瘤IDH突变状况方面不劣于容积标准化ADCmean,但就非实性胶质瘤而言,容积ADC值优于区域ADC值。因此,临床实践中ADC值测量中ROI的选择至关重要,可能影响ADC值预测胶质瘤基因型的准确性。
Park等[14]证实IDH-1突变/1p/19q共缺失Ⅱ级胶质瘤较IDH-1突变/1p/19q非共缺失胶质瘤呈现出高-中ADC混杂模式,说明ADC值可以预测IDH-1突变低级别胶质瘤的1p/19q基因型。目前,有关ADC值在预测胶质瘤1p/19q基因型方面的价值存在争议[14-16]。本研究显示1p19q共缺失型与1p19q单缺失或不缺失型胶质瘤的ADC值间差异无统计学意义,分析原因可能与肿瘤区水肿、新生血管生成及ADC值测量的兴趣区选择、肿瘤异质性等因素相关,该假设有待于临床研究证实。此外,本研究提示ADC值可以鉴别IDH-1突变型和IDH-1野生型胶质瘤,与文献[2, 13, 16]报道一致。
近年来,ADC直方图分析和MR影像组学在胶质瘤基因型预测中的研究倍受关注,其能够较全面反映胶质瘤的异质性。刘丹等[17]探讨了Ⅱ/Ⅲ级弥漫性胶质瘤的ADC直方图特征,结果显示IDH突变型胶质瘤ADC(75%、90%、95%,Max、范围、标准差及不均一性)显著低于IDH野生型,而IDH突变型胶质瘤ADCmin和峰度显著高于IDH野生型;IDH突变/1p19q未缺失型胶质瘤ADC(Mean,5%、10%、25%、50%、75%,众数)显著高于IDH突变/1p19q缺失型。此外,ADC不均一性鉴别IDH突变型和IDH野生型胶质瘤的效能最高,而众数鉴别IDH突变/1p19q未缺失型和IDH突变/1p19q缺失型胶质瘤的效能最高。同样,亦有研究[15]证实低级别胶质瘤ADC值与IDH-1突变状态显著相关。另有学者评价了ADC直方图图像分割方式对Ⅱ/Ⅲ级弥漫型胶质瘤基因型分类的影响,结果表明ADC直方图有助于分类IDH野生型和突变型胶质瘤,尤其去除囊变/坏死时更有助于评价肿瘤异质性和分类IDH野生型胶质瘤,然而在预测1p19q基因型方面价值有限[16]。多序列MRI整合影像组学能够预测胶质瘤IDH和1p/19q状况,如基于T1WI增强和ADC的影像组学预测IDH突变型胶质瘤的效能最佳,而T1WI增强影像组学预测1p/19q共缺失型胶质瘤的效能最佳[18]。
本研究的主要局限性在于仅探讨了肿瘤局部,如胶质瘤最大层面的ADC值与IDH-1/1p19q基因型间的相关性。鉴于胶质瘤的异质性,肿瘤局部DWI(ADC值)特征可能并不能反映肿瘤整体弥散特征,进而影响ADC值预测胶质瘤基因型的价值。此外,本研究纳入的样本量较小亦可能影响结论的客观性。综上所述,常规临床DWI技术(ADC值)一定程度上可以预测WHOⅡ /Ⅲ级胶质瘤的IDH-1/1p19q突变状况,尽管ADC直方图分析、MR多模态影像组学可能更能有效预测IDH-1/1p19q基因型,然而ADC值不失为预测IDH- 1/1p19q基因型和预后的实用影像学生物标志物,值得临床推广。
作者贡献:
孙鹏飞:研究设计和论文撰写
牟福玲、马莉:临床资料收集和测量、分析、汇总
付正丰:图像资料整理
| [1] |
Hanif F, Muzaffar K, Perveen K, et al. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment[J]. Asian Pac J Cancer Prev, 2017, 18(1): 3-9. |
| [2] |
Villanueva-Meyer JE, Wood MD, Choi BS, et al. MRI Features and IDH Mutational Status of Grade Ⅱ Diffuse Gliomas: Impact on Diagnosis and Prognosis[J]. AJR Am J Roentgenol, 2018, 210(3): 621-628. DOI:10.2214/AJR.17.18457 |
| [3] |
Wu CC, Jain R, Radmanesh A, et al. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from The Cancer Genome Atlas[J]. AJNR Am J Neuroradiol, 2018, 39(10): 1814-1820. DOI:10.3174/ajnr.A5794 |
| [4] |
Maynard J, Okuchi S, Wastling S, et al. World Health Organization Grade Ⅱ /Ⅲ Glioma Molecular Status: Prediction by MRI Morphologic Features and Apparent Diffusion Coefficient[J]. Radiology, 2020, 296(1): 111-121. DOI:10.1148/radiol.2020191832 |
| [5] |
张宇, 何堃宇, 冯世宇. 脑胶质瘤诊疗进展[J]. 肿瘤防治研究, 2022, 49(6): 528-534. [Zhang Y, He KY, Feng SY. Current Progress in Treatment of Glioma[J]. Zhong Liu Fang Zhi Yan Jiu, 2022, 49(6): 528-534.] |
| [6] |
Morshed RA, Young JS, Hervey-Jumper SL, et al. The management of low-grade gliomas in adults[J]. J Neurosurg Sci, 2019, 63(4): 450-457. |
| [7] |
Van Den Bent MJ, Bromberg JE, Buckner J. Low-grade and anaplastic oligodendroglioma[J]. Handb Clin Neurol, 2016, 134: 361-380. |
| [8] |
Miller KD, Ostrom QT, Kruchko C, et al. Brain and other central nervous system tumor statistics, 2021[J]. CA Cancer J Clin, 2021, 71(5): 381-406. DOI:10.3322/caac.21693 |
| [9] |
Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities[J]. Cancer Discov, 2013, 3(7): 730-741. DOI:10.1158/2159-8290.CD-13-0083 |
| [10] |
卫力文, 姚冰, 孙佩欣, 等. 63例高级别胶质瘤中IDH-1和TERT突变状态的预后价值[J]. 现代肿瘤医学, 2020, 28(14): 2412-2416. [Wei LW, Yao B, Sun PX, et al. Prognostic significance of IDH1 and TERT promotor mutations in 63 patients with high-grade gliomas[J]. Xian Dai Zhong Liu Yi Xue, 2020, 28(14): 2412-2416. DOI:10.3969/j.issn.1672-4992.2020.14.010] |
| [11] |
Jansen M, Yip S, Louis DN. Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers[J]. Lancet Neurol, 2010, 9(7): 717-726. DOI:10.1016/S1474-4422(10)70105-8 |
| [12] |
Wang QP, Lei DQ, Yuan Y, et al. Accuracy of ADC derived from DWI for differentiating high-grade from low-grade gliomas: Systematic review and meta-analysis[J]. Medicine (Baltimore), 2020, 99(8): e19254. DOI:10.1097/MD.0000000000019254 |
| [13] |
Thust SC, Maynard JA, Benenati M, et al. Regional and Volumetric Parameters for Diffusion-Weighted WHO Grade Ⅱ and Ⅲ Glioma Genotyping: A Method Comparison[J]. AJNR Am J Neuroradiol, 2021, 42(3): 441-447. DOI:10.3174/ajnr.A6965 |
| [14] |
Park YW, Han K, Ahn SS, et al. Prediction of IDH1-Mutation and 1p/19q-Codeletion Status Using Preoperative MR Imaging Phenotypes in Lower Grade Glioma[J]. AJNR Am J Neuroradiol, 2018, 39(1): 37-42. DOI:10.3174/ajnr.A5421 |
| [15] |
Gihr GA, Horvath-Rizea D, Hekeler E, et al. Histogram Analysis of Diffusion Weighted Imaging in Low-Grade Gliomas: in vivo Characterization of Tumor Architecture and Corresponding Neuropathology[J]. Front Oncol, 2020, 10: 206. DOI:10.3389/fonc.2020.00206 |
| [16] |
Liu D, Gao SX, Liao HF, et al. A Comparative Study of 2 Different Segmentation Methods of ADC Histogram for Differentiation Genetic Subtypes in Lower-Grade Diffuse Gliomas[J]. Biomed Res Int, 2020, 2020: 9549361. |
| [17] |
刘丹, 徐婧梅, 廖鸿帆, 等. 表观扩散系数直方图鉴别弥漫性较低级别脑胶质瘤分子亚型的价值[J]. 中国医学科学院学报, 2020, 42(4): 444-451. [Liu D, Xu JM, Liao HF, et al. Diffusion Coefficient Histogram Analysis: Differentiation of Genetic Subtypes of Diffuse Lower-grade Gliomas[J]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2020, 42(4): 444-451.] |
| [18] |
Yan J, Zhang B, Zhang ST, et al. Quantitative MRI-based radiomics for noninvasively predicting molecular subtypes and survival in glioma patients[J]. NPJ Precis Oncol, 2021, 5(1): 72-75. DOI:10.1038/s41698-021-00205-z |
 2023, Vol. 50
2023, Vol. 50
