文章信息
- 碘难治性分化型甲状腺癌诱导再分化治疗研究进展
- Progress in Re-differentiating Therapy of Radioiodine-refractory Differentiated Thyroid Cancer
- 肿瘤防治研究, 2022, 49(10): 1086-1092
- Cancer Research on Prevention and Treatment, 2022, 49(10): 1086-1092
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2022.22.0011
- 收稿日期: 2022-01-05
- 修回日期: 2022-03-30
2. 430079 武汉,湖北省肿瘤医院头颈外科
2. Department of Head and Neck Surgery, Hubei Cancer Hospital, Wuhan 430079, China
甲状腺癌(thyroid carcinoma, TC)是近年发病率增长最快的实体内分泌肿瘤,我国是发病率增长最快的国家之一[1]。其中,以分化型甲状腺癌(differentiated thyroid carcinoma, DTC)最常见,占所有甲状腺癌的95%,大多数DTC经手术、放射性碘(radioactive iodine, RAI)治疗和(或)促甲状腺激素(thyroid stimulating hormone, TSH)抑制治疗后预后良好[2]。但是即便如此,仍有15%的DTC患者在其自然病程或治疗过程中丧失摄碘能力或碘摄取不足,成为碘难治性分化型甲状腺癌(radioiodine-refractory differentiated thyroid cancer, RAIR-DTC),该病预后差[3]。目前RAIR-DTC治疗方式有限且疗效欠佳,随着对RAIR-DTC发生、发展及分化等相关分子机制研究的不断深入,以诱导再分化治疗为代表的新型治疗策略已被尝试用于治疗RAIR-DTC并取得了一定成效。本文就RAIR-DTC的诱导再分化治疗的临床研究进展进行综述。
1 碘难治性分化型甲状腺癌2015年美国甲状腺协会(American Thyroid Association, ATA)指南界定RAIR-DTC为在TSH刺激及无外源性碘负荷干扰的低碘状态下,病灶在经RAI治疗后完全或部分丧失摄碘能力,或虽然病灶存在碘摄取,但经RAI治疗后仍出现进展[4]。该界定基于患者摄碘特征及临床进程,而缺乏病理和分子基础;此外,碘难治性病灶的判断会受到RAI的剂量、显像时间、显像设备的分辨率等因素影响,病灶的碘摄取情况与RAI治疗疗效也并非完全匹配,RAI全身显像(RAI-whole body scan, RAI-WBS)的局限性、病灶的影像异质性、RAI治疗的累计剂量问题等都使得RAIR-DTC的定义存在争议,但分子病理及分子影像等方面的研究进展有助于澄清争议[5]。当前RAIR-DTC治疗手段主要有手术、化疗、放疗、靶向治疗,但手术往往无法彻底切除,传统化疗和放疗疗效欠佳,虽然靶向药物络氨酸激酶抑制剂(tyrosine kinase inhibitor, TKI)索拉非尼(sorafenib)和仑伐替尼(lenvatinib)被批准用于RAIR-DTC治疗,但二者治疗RAIR-DTC的总生存期(overall survival, OS)获益尚未得到证实,而长期靶向治疗常伴有严重不良反应。研究显示RAIR-DTC病情进展快,RAIR-DTC患者中位OS不足5年,10年生存率(survival rate, SR)仅10%,而对RAI治疗敏感的转移性DTC患者10年SR可达60%[6]。
甲状腺碘代谢相关特异基因表达产物包括Na/I转运体(sodium iodide symporter, NIS)、促甲状腺激素受体(thyroid stimulating hormone receptor, TSHR)等[7],TSHR与TSH结合后激活效应蛋白NIS表达进而完成甲状腺摄碘功能。TSHR与NIS的表达异常可能是RAIR-DTC对RAI治疗不敏感的原因,其表达受不同机制调控,其中包括参与DTC发生的主要信号通路、表观遗传修饰、基因转录调控等[8]。
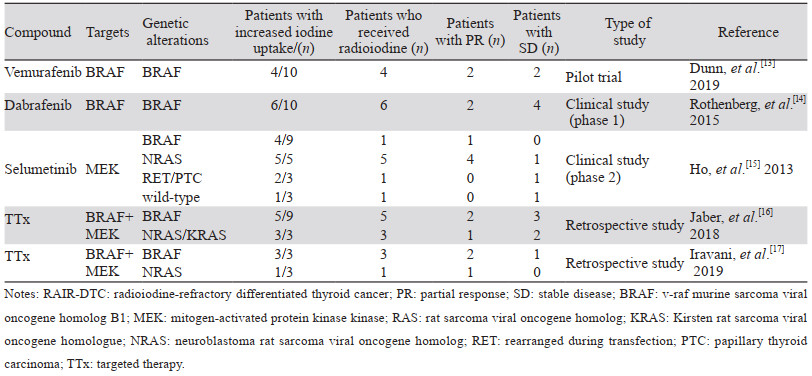
2 信号通路调控 2.1 丝裂原激活蛋白激酶通路大鼠肉瘤病毒致癌基因同源物(rat sarcoma viral oncogene homolog, RAS)/RAF激酶(rapidly accelerated fibrosarcoma, RAF)/丝裂原激活蛋白激酶激酶(mitogen-activated protein kinase kinase, MEK)/丝裂原激活蛋白激酶(mitogen-activated protein kinase, MAPK)级联通路在甲状腺癌发生、发展及分化过程中发挥重要作用[9]。RAS基因位于MAPK信号通路的上游,该基因突变后可激活MAPK信号通路导致细胞异常增殖,从而促使肿瘤的发生。鼠类肉瘤病毒癌基因同源物B1(v-raf murine sarcoma viral oncogene homolog B1, BRAF)属于RAF基因家族,是一种重要的原癌基因,其编码丝氨酸/苏氨酸蛋白激酶位于MAPK信号通路的入口,该基因突变后可持续稳定地活化MEK使MAPK通路激活,进而导致细胞周期和循环失控,对肿瘤的生长增殖和侵袭转移至关重要。MEK是MAPK通路的一个重要靶标,是BRAF下游信号级联的一部分[10],MEK抑制剂可阻断MAPK通路产生显著的抗肿瘤作用。BRAF/RAS突变可通过激活MAPK通路,导致NIS的缺乏和RAI摄取降低,引起对RAI治疗不敏感[11]。体外研究证明了作用于MAPK通路上的BRAF、RAS、MEK等位点的分子靶向药物可提高甲状腺碘代谢基因的表达水平和细胞摄碘率[12],临床研究显示MAPK通路抑制剂可诱导RAIR-DTC再分化提高摄碘率,见表 1、图 1,目前研究用于临床诱导RAIR-DTC再分化的靶向药物主要有BRAF抑制剂和MEK抑制剂。
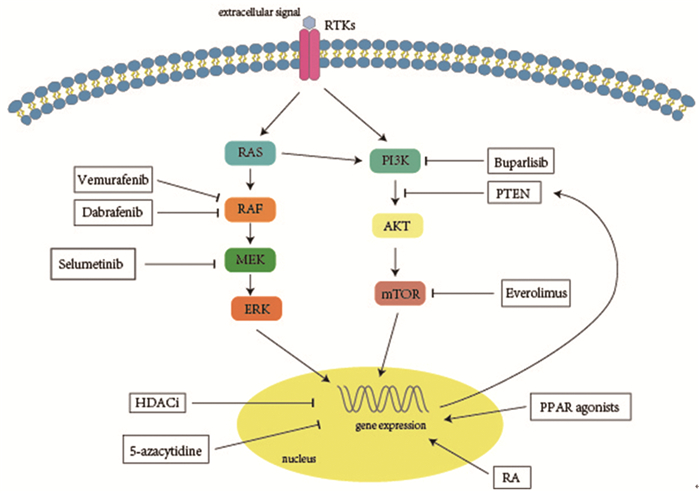
|
| 图 1 RAIR-DTC诱导再分化治疗药物及作用途径 Figure 1 Drugs and relevant pathways involved in the re-differentiating therapy of RAIR-DTC |
BRAF抑制剂可通过抑制MAPK通路恢复RAI摄取,Dunn等开展了一项体内预实验,采用BRAF抑制剂维莫非尼诱导RAIR-DTC再分化后,10例患者中有4例RAI摄取增加,经RAI治疗后,2例获得部分缓解(partial response, PR),2例病情稳定(stable disease, SD)[13]。Rothenberg则采用BRAF抑制剂达拉非尼(dabrafenib)进行诱导再分化,在该研究中,10例患者接受达拉非尼治疗6周后行全身显像(WBS),发现6例患者RAI摄取增加,该6例患者经RAI治疗后,2例患者获得PR,4例SD[14],表明BRAF抑制剂可恢复BRAF突变型RAIR-DTC患者对RAI的摄取并提高RAI治疗疗效,是RAIR-DTC诱导再分化治疗的可选择药物。
Hayes等研究采用MEK抑制剂司美替尼治疗RAIR-DTC后,BRAF V600E突变型患者的PFS较野生型长,表明MEK抑制剂司美替尼(selumetinib)治疗可能对BRAF V600E突变患者的效果更好,并有望增强晚期甲状腺癌对RAI的摄取[18]。随之Ho等开展临床研究采用司美替尼诱导RAIR-DTC再分化后,20例患者中有12例RAI摄取增加,8例在RAI摄取达到一定阈值后接受RAI治疗,其中5例患者PR,3例患者SD,值得注意的是,纳入的病例中神经母细胞瘤RAS病毒致癌基因同源物(neuroblastoma RAS viral oncogene homolog, NRAS)突变患者5例,经司美替尼治疗后RAI摄取均增加,接受RAI治疗后4例PR,表明司美替尼对RAS突变型RAIR-DTC患者的再分化治疗有效率更高[15],这启发了研究者对信号通路调节的研究和实行个体化治疗的进一步思考。基于现有研究的样本量较少,英国启动了一项大样本、多中心临床研究来进一步证实司美替尼在诱导再分化治疗中的可行性[19],我们也期待研究结果的发布。
对使用MAPK通路抑制剂(BRAF和(或)MEK抑制剂)治疗的RAIR-DTC患者进行回顾性分析,也进一步证实了选择性靶向治疗可诱使BRAF/RAS突变的RAIR-DTC患者再分化对RAI治疗的敏感度[16-17]。有个案研究报道了达拉非尼和曲美替尼治疗RAIR-DTC的再分化潜力,因其较轻的不良反应被考虑作为BRAF突变的RAIR-DTC患者的靶向治疗方案之一[20]。由此可见,BRAF抑制剂和MEK抑制剂均可提高RAIR-DTC患者的RAI摄取,进一步证明了MAPK通路在甲状腺细胞中调节RAI摄取的作用。然而,它们恢复RAI摄取的临床有效性仍然有限,BRAF抑制剂的诱导再分化治疗结果虽然喜人,但只是小样本研究,仍需大样本研究结果进一步证实其疗效;司美替尼虽然显示出了良好的诱导再分化能力,但它似乎对RAS突变的RAIR-DTC患者疗效最好,因此进一步明确潜在获益人群、探索针对特定突变的个体化疗法、治疗临床最常见的与预后相关的BRAF突变型患者仍然是临床一大难题。
最近,Saqcena等开展的一项新的研究发现,染色质重塑复合体(SWItch/Sucrose Non-Fermentable, SWI/SNF)在维持TC分化中发挥重要作用,其基因突变导致表达降低可对抗MAPK通路抑制剂的再分化治疗作用[21],这项研究为联合诱导提高RAIR-DTC再分化治疗疗效提供了新的思路。
2.2 磷酸肌醇-3羟激酶/蛋白激酶B/雷帕霉素靶蛋白通路磷酸肌醇-3羟激酶(phosphoinositide 3-kinase, PI3K)/蛋白激酶B(protein kinase B, PKB, 又称AKT)/雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)(PI3K/AKT/mTOR)信号通路的异常激活与DTC的发生、发展、转移、恶性转归等密切相关[9],是潜在的治疗靶点。mTOR是一种丝氨酸/苏氨酸蛋白激酶,属于磷脂酰肌醇激酶相关激酶(phosphatidy-linositol kinase-related kinase, PIKK)蛋白家族成员,是PI3K/AKT通路下游的效应靶蛋白,可调节NIS表达及RAI摄取,在PI3K通路中起较为主导的作用[7]。研究表明抑制PI3K通路可恢复RAIR-DTC对RAI的敏感度,靶向抑制AKT、mTOR可诱导甲状腺肿瘤细胞再分化,提高RAI摄取,见图 1[22]。内源性NIS表达增加与抑制PI3K/AKT/mTOR信号通路有关[23]。
AKT抑制剂可以通过提高NIS介导的碘转运率来增加RAI摄取,且在非甲状腺肿瘤中未发现AKT介导的碘摄取,表明AKT抑制剂及其衍生物可能是选择性增加RAI摄取的潜在靶点。在BARF和同源性磷酸酶-张力蛋白(phosphatase and tension homolog, PTEN)突变患者中,抑制mTOR可通过提高转录终止因子(transcription termination factor 1, TTF1)的转录来恢复RAI摄取[11]。体外实验进一步靶向PI3K、AKT、MEK、BRAF等基因,发现PI3K抑制剂在增加RAI摄取方面优于其他抑制剂[24],但体内试验并没有进一步佐证PI3K抑制剂带来的临床获益,临床研究表明依维莫斯(Everolimus)作为一种mTOR抑制剂,对晚期DTC患者具有抗肿瘤活性[25],但观察到疾病控制率较低。Borson等研究了泛PI3K抑制剂布帕里西布(Buparlisib)对RAIR-DTC的疗效,也没有发现其明显的临床获益,但却降低了肿瘤生长速率,提示致癌途径和(或)肿瘤逃逸机制可能受到不完全抑制[26]。mTOR磷酸化与RAS突变密切相关,MAPK和PI3K通路之间通过RAS的交叉作用也已被证实[27],这解释了肿瘤细胞从已知的PI3K抑制剂逃逸的机制,考虑其临床观察到的疾病控制率低,有必要进一步研究PI3K抑制剂在RAIR-DTC患者的序贯或联合治疗中的疗效。
3 表观遗传修饰 3.1 组蛋白去乙酰化酶抑制剂表观遗传调控失调正成为致癌和肿瘤进展的一个重要因素,组蛋白修饰在基因表达调控中发挥着重要作用,这些修饰包括乙酰化、甲基化、磷酸化和泛素化等,其中以乙酰化修饰尤为重要。组蛋白乙酰化是调节肿瘤发生、发展的主要机制,也是调节甲状腺碘代谢基因表达的机制之一[28]。组蛋白的乙酰化状态主要由2种酶决定:组蛋白乙酰化转移酶(histone acetyltransferase, HAT)和组蛋白去乙酰化酶(histone deacetylase, HDAC)。组蛋白去乙酰化酶抑制剂(histone deacetylase inhibitors, HDACi)如丙戊酸[29]、帕比司他[28, 30]、伏立诺他[30]、曲古抑菌素A[30]等可在甲状腺细胞中重新诱导NIS的mRNA水平表达,提高碘摄取,但临床试验并未见较好疗效[31-32]。基于MAPK通路可下调组蛋白乙酰化,从而导致DTC患者中碘代谢基因的异常沉默,Fu等尝试将HDACi与MAPK通路抑制剂联合用于TC细胞的诱导再分化,发现MAPK抑制剂增强了TC细胞的再分化效果,为体内和体外临床研究使用HDACi联合MAPK通路抑制剂诱导RAIR-DTC再分化治疗提供了理论基础[28]。Cheng等研究在MAPK通路的BRAF抑制剂联合HDACi治疗基础上,加用TSH刺激疗法,发现三种疗法联合作用增强TC细胞RAI摄取的功能明显高于单药或双药联合[33],但该研究尚处于细胞实验阶段,仍需临床试验进一步证实其疗效。
3.2 DNA甲基化酶抑制剂有研究表明TSHR、NIS基因启动子甲基化导致NIS mRNA表达下降,DTC细胞摄碘功能下降[34]。甲状腺转录因子-1(thyroid transcription factor-1, TTF-1)基因的甲基化使TTF-1表达减少,TTF-1与启动子的结合减少,可导致TSHR、NIS等甲状腺特异基因的沉默,此外,NIS表达也与部分5-胞嘧啶-磷酸-鸟嘌呤-3(5-C-phosphate-G-3, CpG)岛甲基化程度负相关[35]。Massimino等发现DNA甲基化酶抑制剂5-氮杂胞苷(5-azacytidine, 5-azaC)可抑制TC细胞的增殖潜能,提高肿瘤细胞凋亡率,恢复低分化TC的NIS表达和RAI摄取[36]。但Tuncel等研究未发现阳性结果,他们发现经5-azaC药物处理后,甲状腺正常细胞和肿瘤细胞中NIS基因的表达均未见提高,肿瘤细胞的表达水平甚至出现降低[37],这样的阴性结果可能是由转录后修饰等一系列复杂机制引起的。到目前为止,仍缺乏进一步的去甲基化药物诱导RAIR-DTC再分化作用的体内外研究来支持去甲基化药物的潜在临床价值。
4 基因转录调控 4.1 维甲酸类药物维甲酸(retinoic acid, RA)是维生素A的活性代谢产物,通过经典RA受体通路激活靶基因转录,参与细胞分化、增殖和凋亡等[38]。RA类药物通过作用于RA受体等核受体提高NIS表达水平从而提高TC细胞的摄碘率,同时降低正常细胞的摄碘率[39]。体外实验表明RA具有促使TC滤泡细胞再分化的作用[40],但后续RA类药物诱导RAIR-DTC再分化治疗的临床效果却存在争议,Short等开展的一项Ⅱ期临床研究纳入了16例患者,经RA类药物治疗8周后,仅1例患者检测到少量的RAI摄取增加[41]。Liu等进行的一项开放、前瞻性研究虽发现RA类药物可增加RAI摄取,但却没有转化为临床获益[42]。为了进一步确定RA类药物的再分化治疗疗效,Pak等的一项Meta分析,显示经RA类药物诱导再分化后,RAI摄取增加的概率为27.6%,再分化行RAI治疗后疾病缓解率为17%,表明RA类药物在少数RAIR-DTC患者中的再分化治疗有效[43],因此,RA也是一种RAIR-DTC诱导再分化的治疗选择,其具体的获益人群仍有待进一步探索。
4.2 过氧化物酶体增殖物激活受体激动剂过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptor, PPAR)属于转录因子的核受体家族,包括PPAR-α、PPAR-δ和PPAR-γ3类受体,其中PPAR-γ对维持甲状腺细胞增殖及分化具有重要作用[44]。PPAR激动剂(如罗格列酮、吡格列酮和曲格列酮等)与PPAR-γ结合,后者结合靶基因启动子区过氧化物酶体增殖物反应元件(PPAR-γ response element, PPRE),从而促进靶基因PTEN的表达,进而抑制TC细胞中异常激活的PI3K通路[45]。
在体内研究中,Tepmongkol等发现罗格列酮可提高PPAR-γ高表达患者的RAI摄取,PPAR-γ低表达或不表达患者极少会重新摄碘[46]。Kebebew等进行的Ⅱ期临床试验结果却与之不同,该研究发现罗格列酮治疗后RAI摄取状态与PPAR mRNA和蛋白表达水平之间没有相关性[47],随后的研究结果与Tepmongkol相似,发现罗格列酮治疗可诱导RAIR-DTC患者的RAI摄取[48],但以上研究在随访中均未发现肿瘤负荷减少。Rosenbaum等在此基础上延长了罗格列酮用药诱导时间,对9例患者进行了研究,发现4例患者在治疗后肿瘤消退,其中3例患者获PR[49],表明罗格列酮也是一种潜在的诱导再分化药物,但需要进一步大样本临床研究证实其疗效。其他PPAR激动剂中以曲格列酮效果最佳,但其提高RAI摄取的作用目前尚处于体外研究阶段[50],需要进一步的体内研究证实能否临床获益。
5 小结与展望RAIR-DTC的治疗是目前临床面临的一大难题,本文介绍了目前临床尝试用于RAIR-DTC诱导再分化治疗的信号通路抑制剂、HDACi、DNA甲基化酶抑制剂、RA类药物及PPAR激动剂的研究进展。虽然HDACi、DNA甲基化酶抑制剂、RA类药物及PPAR激动剂等药物在体外试验中可以诱导RAIR-DTC再分化,不同程度地提高细胞摄碘率,但其临床疗效总体欠佳。相比之下,MAPK和PI3K通路抑制剂诱导RAIR-DTC再分化重新摄碘并介导RAI治疗效果较好。尽管相关临床证据有限,但是目前看来分子靶向诱导联合RAI治疗可能具有一定的临床应用前景。目前临床研究中尚缺乏长期随访数据,靶向诱导的优势获益人群筛选、个体化疗法的探索、联合诱导再分化治疗疗效评价等诸多问题仍有待解决,以优化诱导再分化治疗方案。随着越来越多针对不同靶点的新药涌现,RAIR-DTC的诱导再分化治疗将迎来新的希望。
作者贡献:
张亚奇、朱锡群、樊倩妤:文献检索、论文撰写
陈健:指导及修改论文
| [1] |
Miranda-Filho A, Lortet-Tieulent J, Bray F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study[J]. Lancet Diabetes Endocrinol, 2021, 9(4): 225-234. DOI:10.1016/S2213-8587(21)00027-9 |
| [2] |
田文, 张浩. 分化型甲状腺癌术后管理中国专家共识(2020版)[J]. 中国实用外科杂志, 2020, 40(9): 1021-1028. [Tian W, Zhang H. Expert consensus on postoperative management of differentiated thyroid cancer (2020 edition)[J]. Zhongguo Shi Yong Wai Ke Za Zhi, 2020, 40(9): 1021-1028. DOI:10.19538/j.cjps.issn1005-2208.2020.09.04] |
| [3] |
Worden F. Treatment strategies for radioactive iodine-refractory differentiated thyroid cancer[J]. Ther Adv Med Oncol, 2014, 6(6): 267-279. DOI:10.1177/1758834014548188 |
| [4] |
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer[J]. Thyroid, 2016, 26(1): 1-133. DOI:10.1089/thy.2015.0020 |
| [5] |
Mu ZZ, Zhang X, Lin YS. Identification of Radioactive Iodine Refractory Differentiated Thyroid Cancer[J]. Chonnam Med J, 2019, 55(3): 127-135. DOI:10.4068/cmj.2019.55.3.127 |
| [6] |
Jayarangaiah A, Sidhu G, Brown J, et al. Therapeutic options for advanced thyroid cancer[J]. Int J Clin Endocrinol Metab, 2019, 5(1): 26-34. DOI:10.17352/ijcem.000040 |
| [7] |
Zhang L, Xu S, Cheng X, et al. Curcumin enhances the membrane trafficking of the sodium iodide symporter and augments radioiodine uptake in dedifferentiated thyroid cancer cells via suppression of the PI3K-AKT signaling pathway[J]. Food Funct, 2021, 12(18). |
| [8] |
Riesco-Eizaguirre G, Wert-Lamas L, Perales-Patón J, et al. The miR-146b-3p/PAX8/NIS Regulatory Circuit Modulates the Differentiation Phenotype and Function of Thyroid Cells during Carcinogenesis[J]. Cancer Res, 2015, 75(19): 4119-4130. DOI:10.1158/0008-5472.CAN-14-3547 |
| [9] |
Laha D, Nilubol N, Boufraqech M. New Therapies for Advanced Thyroid Cancer[J]. Front Endocrinol (Lausanne), 2020, 11: 82. DOI:10.3389/fendo.2020.00082 |
| [10] |
Ullmann TM, Liang H, Moore MD, et al. Dual inhibition of BRAF and MEK increases expression of sodium iodide symporter in patient-derived papillary thyroid cancer cells in vitro[J]. Surgery, 2020, 167(1): 56-63. DOI:10.1016/j.surg.2019.04.076 |
| [11] |
Oh JM, Ahn BC. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS[J]. Theranostics, 2021, 11(13): 6251-6277. DOI:10.7150/thno.57689 |
| [12] |
Faria M, Domingues R, Bugalho MJ, et al. MAPK Inhibition Requires Active RAC1 Signaling to Effectively Improve Iodide Uptake by Thyroid Follicular Cells[J]. Cancers (Basel), 2021, 13(22): 5861. DOI:10.3390/cancers13225861 |
| [13] |
Dunn LA, Sherman EJ, Baxi SS, et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers[J]. J Clin Endocrinol Metab, 2019, 104(5): 1417-1428. DOI:10.1210/jc.2018-01478 |
| [14] |
Rothenberg SM, McFadden DG, Palmer EL, et al. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib[J]. Clin Cancer Res, 2015, 21(5): 1028-1035. DOI:10.1158/1078-0432.CCR-14-2915 |
| [15] |
Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer[J]. N Engl J Med, 2013, 368(7): 623-632. DOI:10.1056/NEJMoa1209288 |
| [16] |
Jaber T, Waguespack SG, Cabanillas ME, et al. Targeted Therapy in Advanced Thyroid Cancer to Resensitize Tumors to Radioactive Iodine[J]. J Clin Endocrinol Metab, 2018, 103(10): 3698-3705. DOI:10.1210/jc.2018-00612 |
| [17] |
Iravani A, Solomon B, Pattison DA, et al. Mitogen-Activated Protein Kinase Pathway Inhibition for Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer: An Evolving Protocol[J]. Thyroid, 2019, 29(11): 1634-1645. DOI:10.1089/thy.2019.0143 |
| [18] |
Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase Ⅱ efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements[J]. Clin Cancer Res, 2012, 18(7): 2056-2065. DOI:10.1158/1078-0432.CCR-11-0563 |
| [19] |
Brown SR, Hall A, Buckley HL, et al. Investigating the potential clinical benefit of Selumetinib in resensitising advanced iodine refractory differentiated thyroid cancer to radioiodine therapy (SEL-I-METRY): protocol for a multicentre UK single arm phase Ⅱtrial[J]. BMC Cancer, 2019, 19(1): 582. DOI:10.1186/s12885-019-5541-4 |
| [20] |
Jafri S, Yaqub A. Redifferentiation of BRAF V600E-Mutated Radioiodine Refractory Metastatic Papillary Thyroid Cancer After Treatment With Dabrafenib and Trametinib[J]. Cureus, 2021, 13(8): e17488. |
| [21] |
Saqcena M, Leandro-Garcia LJ, Maag JLV, et al. SWI/SNF Complex Mutations Promote Thyroid Tumor Progression and Insensitivity to Redifferentiation Therapies[J]. Cancer Discov, 2021, 11(5): 1158-1175. DOI:10.1158/2159-8290.CD-20-0735 |
| [22] |
Liu J, Liu Y, Lin Y, et al. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy[J]. Endocrinol Metab (Seoul), 2019, 34(3): 215-225. DOI:10.3803/EnM.2019.34.3.215 |
| [23] |
Oh JM, Baek SH, Gangadaran P, et al. A Novel Tyrosine Kinase Inhibitor Can Augment Radioactive Iodine Uptake Through Endogenous Sodium/Iodide Symporter Expression in Anaplastic Thyroid Cancer[J]. Thyroid, 2020, 30(4): 501-518. DOI:10.1089/thy.2018.0626 |
| [24] |
Lakshmanan A, Scarberry D, Green JA, et al. Modulation of thyroidal radioiodide uptake by oncological pipeline inhibitors and Apigenin[J]. Oncotarget, 2015, 6(31): 31792-31804. DOI:10.18632/oncotarget.5172 |
| [25] |
Hanna GJ, Busaidy NL, Chau NG, et al. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A PhaseⅡ Study[J]. Clin Cancer Res, 2018, 24(7): 1546-1553. DOI:10.1158/1078-0432.CCR-17-2297 |
| [26] |
Borson-Chazot F, Dantony E, Illouz F, et al. Effect of Buparlisib, a Pan-Class I PI3K Inhibitor, in Refractory Follicular and Poorly Differentiated Thyroid Cancer[J]. Thyroid, 2018, 28(9): 1174-1179. DOI:10.1089/thy.2017.0663 |
| [27] |
Shorning BY, Dass MS, Smalley MJ, et al. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling[J]. Int J Mol Sci, 2020, 21(12): 4507. DOI:10.3390/ijms21124507 |
| [28] |
Fu H, Cheng L, Jin Y, et al. MAPK Inhibitors Enhance HDAC Inhibitor-Induced Redifferentiation in Papillary Thyroid Cancer Cells Harboring BRAFV600E: An In VitroStudy[J]. Mol Ther Oncolytics, 2019, 12: 235-245. DOI:10.1016/j.omto.2019.01.007 |
| [29] |
Haghpanah V, Malehmir M, Larijani B, et al. The Beneficial Effects of Valproic Acid in Thyroid Cancer Are Mediated through Promoting Redifferentiation and Reducing Stemness Level: An In Vitro Study[J]. J Thyroid Res, 2014, 2014: 218763. |
| [30] |
Wächter S, Damanakis AI, Elxnat M, et al. Epigenetic Modifications in Thyroid Cancer Cells Restore NIS and Radio-Iodine Uptake and Promote Cell Death[J]. J Clin Med, 2018, 7(4): 61. DOI:10.3390/jcm7040061 |
| [31] |
程凌霄, 陈立波. 131Ⅰ难治性分化型甲状腺癌的再分化治疗[J]. 中国癌症杂志, 2016, 26(1): 35-42. [Cheng LX, Chen LB. Re-differentiating therapy of radioiodine-refractory differentiated thyroid cancer[J]. Zhongguo Ai Zheng Za Zhi, 2016, 26(1): 35-42.] |
| [32] |
Nilubol N, Merkel R, Yang L, et al. A phaseⅡ trial of valproic acid in patients with advanced, radioiodine-resistant thyroid cancers of follicular cell origin[J]. Clin Endocrinol (Oxf), 2017, 86(1): 128-133. DOI:10.1111/cen.13154 |
| [33] |
Cheng W, Liu R, Zhu G, et al. Robust Thyroid Gene Expression and Radioiodine Uptake Induced by Simultaneous Suppression of BRAF V600E and Histone Deacetylase in Thyroid Cancer Cells[J]. J Clin Endocrinol Metab, 2016, 101(3): 962-971. DOI:10.1210/jc.2015-3433 |
| [34] |
D'Agostino M, Sponziello M, Puppin C, et al. Different expression of TSH receptor and NIS genes in thyroid cancer: role of epigenetics[J]. J Mol Endocrinol, 2014, 52(2): 121-131. |
| [35] |
Galrão AL, Camargo RY, Friguglietti CU, et al. Hypermethylation of a New Distal Sodium/Iodide Symporter (NIS) enhancer (NDE) is associated with reduced NIS expression in thyroid tumors[J]. J Clin Endocrinol Metab, 2014, 99(6): E944-E952. DOI:10.1210/jc.2013-1450 |
| [36] |
Massimino M, Tirrò E, Stella S, et al. Effect of Combined Epigenetic Treatments and Ectopic NIS Expression on Undifferentiated Thyroid Cancer Cells[J]. Anticancer Res, 2018, 38(12): 6653-6662. DOI:10.21873/anticanres.13032 |
| [37] |
Tuncel M, Aydin D, Yaman E, et al. The comparative effects of gene modulators on thyroid-specific genes and radioiodine uptake[J]. Cancer Biother Radiopharm, 2007, 22(3): 443-449. DOI:10.1089/cbr.2006.319.A |
| [38] |
Ghyselinck NB, Duester G. Retinoic acid signaling pathways[J]. Development, 2019, 146(13): dev167502. DOI:10.1242/dev.167502 |
| [39] |
Tang M, Hou YL, Kang QQ, et al. All-trans-retinoic acid promotes iodine uptake via up- regulating the sodium iodide symporter in medullary thyroid cancer stem cells[J]. Asian Pac J Cancer Prev, 2014, 15(4): 1859-1862. DOI:10.7314/APJCP.2014.15.4.1859 |
| [40] |
Schmutzler C, Köhrle J. Retinoic acid redifferentiation therapy for thyroid cancer[J]. Thyroid, 2000, 10(5): 393-406. DOI:10.1089/thy.2000.10.393 |
| [41] |
Short SC, Suovuori A, Cook G, et al. A phaseⅡ study using retinoids as redifferentiation agents to increase iodine uptake in metastatic thyroid cancer[J]. Clin Oncol (R Coll Radiol), 2004, 16(8): 569-574. DOI:10.1016/j.clon.2004.06.018 |
| [42] |
Liu YY, Stokkel MP, Pereira AM, et al. Bexarotene increases uptake of radioiodide in metastases of differentiated thyroid carcinoma[J]. Eur J Endocrinol, 2006, 154(4): 525-531. DOI:10.1530/eje.1.02123 |
| [43] |
Pak K, Shin S, Kim SJ, et al. Response of Retinoic Acid in Patients with Radioactive Iodine-Refractory Thyroid Cancer: A Meta-Analysis[J]. Oncol Res Treat, 2018, 41(3): 100-104. DOI:10.1159/000484206 |
| [44] |
Raman P, Koenig RJ. Pax-8-PPAR-γ fusion protein in thyroid carcinoma[J]. Nat Rev Endocrinol, 2014, 10(10): 616-623. DOI:10.1038/nrendo.2014.115 |
| [45] |
de Biase D, Visani M, Pession A, et al. Molecular diagnosis of carcinomas of the thyroid gland[J]. Front Biosci (Elite Ed), 2014, 6(1): 1-14. |
| [46] |
Tepmongkol S, Keelawat S, Honsawek S, et al. Rosiglitazone effect on radioiodine uptake in thyroid carcinoma patients with high thyroglobulin but negative total body scan: a correlation with the expression of peroxisome proliferator-activated receptor-gamma[J]. Thyroid, 2008, 18(7): 697-704. DOI:10.1089/thy.2008.0056 |
| [47] |
Kebebew E, Peng M, Reiff E, et al. A phaseⅡ trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer[J]. Surgery, 2006, 140(6): 960-967. DOI:10.1016/j.surg.2006.07.038 |
| [48] |
Kebebew E, Lindsay S, Clark OH, et al. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer[J]. Thyroid, 2009, 19(9): 953-956. DOI:10.1089/thy.2008.0371 |
| [49] |
Rosenbaum-Krumme SJ, Freudenberg LS, Jentzen W, et al. Effects of rosiglitazone on radioiodine negative and progressive differentiated thyroid carcinoma as assessed by 124I PET/CT imaging[J]. Clin Nucl Med, 2012, 37(3): e47-e52. DOI:10.1097/RLU.0b013e3182443ca6 |
| [50] |
Wen G, Fischer J, Most E, et al. Decreased All- trans Retinoic Acid-Induced Expression of Sodium-Iodide Transporter in Mammary Epithelial Cells Caused by Conjugated Linoleic Acid Isomers[J]. J Agric Food Chem, 2019, 67(16): 4493-4504. DOI:10.1021/acs.jafc.9b00673 |
 2022, Vol. 49
2022, Vol. 49