文章信息
- 替雷利珠单抗联合化疗在尿路上皮癌中的疗效及不良反应分析
- Efficacy and Adverse Reaction of Tislelizumab Combined with Chemotherapy on Urothelial Carcinoma
- 肿瘤防治研究, 2022, 49(7): 698-702
- Cancer Research on Prevention and Treatment, 2022, 49(7): 698-702
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2022.21.1340
- 收稿日期: 2021-11-22
- 修回日期: 2022-03-10
尿路上皮癌是泌尿系统中最常见的恶性肿瘤,最新的研究显示[1]其发病率在所有肿瘤中排第7位,而在男性中为第4位,成为了一个重大的公共卫生问题。对于晚期尿路上皮癌患者,免疫检查点抑制剂如帕博利珠单抗、阿特利珠单抗、纳武利尤单抗等对晚期尿路上皮癌表现出良好的疗效[2-3],但这些药物在中国尚未获批用于晚期尿路上皮癌的治疗。2020年国家药品监督管理局批准了国产新药替雷利珠单抗(商品名:百泽安)的晚期尿路上皮癌适应证,但国内尚缺乏对替雷利珠单抗联合化疗在尿路上皮癌的真实世界研究报告。本文将替雷利珠单抗联合化疗治疗尿路上皮癌患者的疗效及安全性评估的结果报道如下。
1 资料与方法 1.1 一般资料本研究纳入2020年4月至2021年10月重庆医科大学附属第一医院泌尿外科32例接受替雷利珠单抗联合化疗(吉西他滨/顺铂或紫杉醇)治疗的尿路上皮癌患者,根据治疗方案不同,15例患者因不耐受铂类化疗(其中10例ECOG评分≥2分,4例肾功能异常,1例拒绝铂类化疗)纳入替雷利珠单抗联合紫杉醇组,17例患者纳入替雷利珠单抗联合GC方案组。基本资料见表 1。本研究通过了重庆医科大学附属第一医院伦理委员会审核,并免除了患者知情同意书。
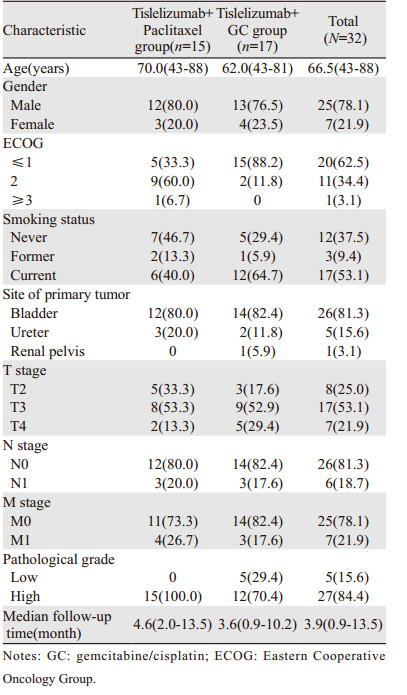
|
替雷利珠单抗联合紫杉醇(或白蛋白结合型紫杉醇),输注方案为替雷利珠单抗200 mg D1+紫杉醇135~175 mg/m2(或白蛋白结合型紫杉醇260 mg/m2)D1,每3周输注一次;替雷利珠单抗联合GC(吉西他滨+顺铂)方案,输注方案为替雷利珠单抗200 mg D1+吉西他滨1000 mg/m2 D1、D8,顺铂70 mg/m2 D2,每3周输注一次。
1.3 疗效及安全性评价每9~12周进行影像学检查,包括CT或MRI,对基线评估后至少有1次影像学检查的患者进行疗效分析。疗效评估标准按照实体肿瘤疗效评价标准1.1版(RECIST v1.1)实施,疗效分为完全缓解(completed response, CR)、部分缓解(partial response, PR)、疾病稳定(stable disease, SD)、疾病进展(progressive disease, PD)。评价指标有客观缓解率(objective response rate, ORR)为CR+PR,疾病控制率(disease control ate, DCR)为CR+PR+SD。中位PFS及中位OS因随访时间较短无法评估。
安全性分析纳入所有接受过替雷利珠单抗治疗的患者。安全性评估根据2019中国临床肿瘤学会(Chinese Society of Clinical Oncology, CSCO)免疫检查点抑制剂相关的毒性管理指南实施,每3周复查血常规、肝肾功能、电解质、尿常规、甲功全套、心肌酶谱、心电图及心脏彩超。不良事件的统计及分级根据美国卫生及公共服务部常见不良事件评价标准(CTCAE)5.0版实施,由主管医生判断不良事件是否与免疫检查点抑制剂相关。
2 结果 2.1 疗效评价截至2021年10月,32例接受替雷利珠单抗联合化疗的尿路上皮癌患者,1例因肠梗阻去世、1例因疾病进展去世和3例在治疗过程中失访而未评估影像学,3例基线影像学资料为院外资料无法评估疗效,余24例患者均纳入疗效评估,见表 2。

|
2例典型患者在使用免疫治疗联合化疗后持续反应为完全缓解,见图 1~2。

|
| A: CT enhancement before treatment showed that the space-occupying lesion was located on the right posterior wall and involved the entrance of the right ureter; B: after two months of treatment, re-examination of CT indicated that there was no obvious space-occupying lesions on the right posterior wall; C: after two months of treatment, only a few calcification lesions remained at the original site by cystoscopy. 图 1 替雷利珠单抗联合紫杉醇方案到达完全缓解的尿路上皮癌病例 Figure 1 One patient with urothelial carcinoma treated with tislelizumab combined with paclitaxel achieved complete response |
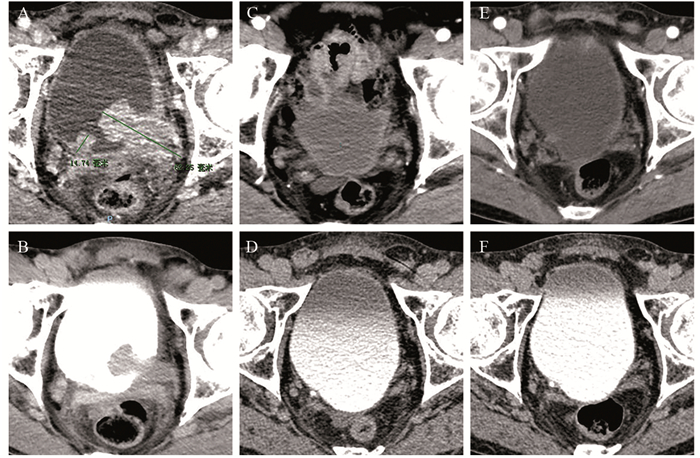
|
| CT-enhanced arterial phase (A) and excretory phase (B) before treatment showed that multiple space-occupying lesions were located on the left and right walls of the bladder; after two months of treatment, re-examination of CT (C, D) showed no space-occupying lesions in the bladder; after six months of treatment, re-examination of CT (E, F) showed no space-occupying lesions in the bladder. 图 2 替雷利珠单抗联合吉西他滨+顺铂方案达到完全缓解的尿路上皮癌病例 Figure 2 One patient with urothelial carcinoma treated with tislelizumab combined with GC regimen achieved complete response |
替雷利珠单抗联合化疗患者耐受性良好,未发生输注反应。截至2021年10月,所有患者均发生了治疗相关不良反应,但大多数为1级或2级不良反应,见表 3。常见不良反应包括贫血(56.3%)、食欲下降(53.1%)、皮肤瘙痒(50.0%)、恶心(40.6%)、乏力(40.6%)、血清白蛋白下降(31.3%)。其中7例(21.8%)患者出现了3级及以上的治疗相关不良反应,分别是骨髓抑制(4例),免疫性结肠炎(2例),消化道反应(1例)。替雷利珠单抗联合紫杉醇方案组骨髓抑制和胃肠道反应率分别为6.7%与13.3%,显著低于替雷利珠单抗联合GC方案组的41.2%与64.7%。免疫相关不良反应,见表 4。3例患者出现了甲状腺功能异常,其中1例在给药3周后出现了甲状腺功能亢进(1级),继续随访发展为甲状腺功能减退(2级),予以左甲状腺钠片替代治疗后继续免疫治疗。2例患者出现了3级的免疫性结直肠炎,予以激素治疗后病情得到了控制。
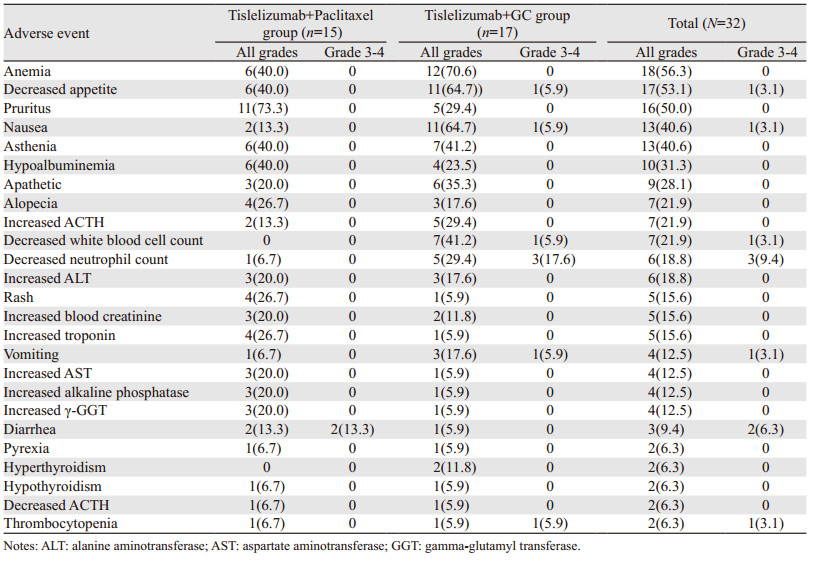
|
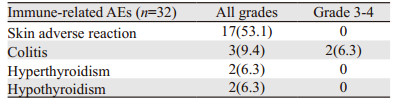
|
几十年来,晚期尿路上皮癌的金标准疗法一直是含铂类药物的化疗。近年来,随着免疫治疗的出现,晚期尿路上皮癌患者的治疗方式有了更多的选择,也得到很大程度的获益。最新的美国国家综合癌症网络(NCCN)指南推荐免疫检查点抑制剂帕博利珠单抗、阿特利珠单抗、纳武利尤单抗、度伐利尤单抗和阿维鲁单抗用于局部晚期或转移性尿路上皮细胞癌的一线或二线治疗[4]。尽管这些免疫检查点抑制剂(ICIs)对晚期尿路上皮癌表现出良好的疗效,但这些药物在中国尚未获批用于晚期尿路上皮癌的治疗。国内最新的一项Ⅱ期临床研究[5]显示替雷利珠单抗在单药治疗PD-L1阳性的晚期尿路上皮癌患者中ORR达24%,DCR为38%,中位无进展生存时间为2.1月,中位总生存时间为9.8月。基于此项研究,2020年4月国家药品监督管理局批准国产免疫检查点抑制剂替雷利珠单抗在含铂类化疗失败的PD-L1高表达的晚期尿路上皮癌的适应证,开启了国内泌尿系统肿瘤免疫治疗的新时代。
而以铂类为主的传统化疗方案,有效率可达到45%左右,因此对于晚期的尿路上皮癌患者,免疫治疗单药治疗方案暂时还不能替代标准的化疗方案,但为不能耐受标准化疗的患者提供了治疗选择。针对部分不能从免疫检查点抑制剂单药治疗中获益的实体肿瘤患者,既往的研究显示联合化疗则可能给患者带来更好的疗效获益,替雷利珠单抗联合化疗作为一线方案已经在晚期肺癌中取得了良好的效果[6]。临床试验有着严格的入组标准,疗效及安全性不能完全反映药物在真实世界中的情况,目前国内还没有替雷利珠单抗联合化疗应用于尿路上皮癌患者的真实世界研究报道。因此本研究中,我们重点探讨替雷利珠单抗联合化疗在真实世界中尿路上皮癌患者的疗效,结果显示在替雷利珠单抗联合化疗中ORR为54.2%,DCR为83.3%。有效率与IMvigor 130[7]、KEYNOTE361[8]研究结果相似。根据IMvigor 130研究,阿特利珠单抗联合铂类化疗的客观缓解率为47%,而单用阿特利珠单抗为23%,单用铂类化疗为44%。同样在KEYNOTE361研究中,帕博利珠单抗联合化疗组、帕博利珠单抗单药组和化疗组的客观缓解率分别为54.7%、30.3%和44.9%,疾病控制率分别为80.3%、47.2%和75.9%。目前的研究均证实免疫治疗联合化疗可以提高尿路上皮癌的反应率,原因可能是化疗导致的肿瘤微环境改变,增加肿瘤细胞免疫原性和T淋巴细胞浸润作用,从而增强了免疫检查点抑制剂的治疗效果[9]。
晚期尿路上皮癌对铂类为主方案的化疗比较敏感,研究[10]显示吉西他滨联合顺铂方案(GC方案)的客观缓解率为49.4%,但有部分患者因年龄大、肾功能损害、身体状况差或合并其他疾病等原因而无法耐受顺铂为主的化疗。对于此类人群,既往研究表明不能耐受顺铂的人群对紫杉醇拥有良好的耐受性[11-12],紫杉醇作为晚期尿路上皮癌的二线化疗方案,单药的客观缓解率为27.7%[13]。因此在本研究中,不能耐受吉西他滨联合顺铂方案的患者我们选择替雷利珠单抗联合紫杉醇的化疗方案,该治疗组的中位年龄为70.0(43~88)岁,ECOG评分≥2分的占66.7%,而在替雷利珠联合GC方案组中位年龄为62.0(43~81)岁,ECOG评分≥2分的占11.8%。研究结果显示在紫杉醇联合替雷利珠单抗组和GC联合替雷利珠单抗组中ORR分别为50.0%与58.3%,DCR分别为75.0%和91.7%,而在不良反应方面,替雷利珠单抗联合紫杉醇方案组骨髓抑制和胃肠道反应率显著低于替雷利珠单抗联合GC方案组。这一研究结果初步显示替雷利珠单抗联合紫杉醇在不能耐受顺铂的尿路上皮癌患者中耐受性良好,并且取得了不错的疗效,但这一结论还需进一步的临床试验来证实。
本研究,最常见的免疫相关不良反应(irAEs)为皮肤毒性(53.1%),暂未观察到3~4级的皮肤毒性。截至目前,尚未观察到免疫性心肌炎、免疫性肺炎等危险度较高的免疫相关不良反应,但在免疫检测点抑制剂治疗过程中仍要加强心脏功能及肺功能的检测[14]。
本研究有以下不足:(1)本研究为小样本观察性研究,病例数较少,同时纳入了术前新辅助及晚期膀胱癌患者;(2)未能对比替雷利珠单抗联合化疗与单用化疗或免疫单药治疗的疗效;(3)本研究随访时间较短,未能达到中位OS等。本研究的结论还需后续大规模的临床研究来证实。
综上所述,国产免疫检测点抑制剂替雷利珠单抗联合化疗在尿路上皮癌中的疗效显著,不良反应可控,而对于不能耐受铂类化疗的患者,替雷利珠单抗联合紫杉醇方案可考虑为一种治疗选择。
作者贡献:
危宗杰:数据收集、随访、统计分析、论文撰写
匡幼林、陈勇:论文修改
王璐、陈潇:数据收集、随访
苟欣:课题设计、论文修改
| [1] |
Siegeal RL, Miller KD, Fuchs HE, et al. Cancer statistis, 2022[J]. CA Cancer J Clin, 2022, 72(1): 7-33. DOI:10.3322/caac.21708 |
| [2] |
Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma[J]. N Engl J Med, 2017, 376(11): 1015-1026. DOI:10.1056/NEJMoa1613683 |
| [3] |
Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2017, 18(3): 312-322. DOI:10.1016/S1470-2045(17)30065-7 |
| [4] |
Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2020, 18(3): 329-354. DOI:10.6004/jnccn.2020.0011 |
| [5] |
Ye D, Liu J, Zhou A, et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma[J]. Cancer Sci, 2020, 112(1): 305-313. |
| [6] |
Wang Z, Zhao J, Ma Z, et al. A Phase 2 Study of Tislelizumab in Combination With Platinum-Based Chemotherapy as First-line Treatment for Advanced Lung Cancer in Chinese Patients[J]. Lung Cancer, 2020, 147: 259-268. DOI:10.1016/j.lungcan.2020.06.007 |
| [7] |
Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2020, 395(10236): 1547-1557. DOI:10.1016/S0140-6736(20)30230-0 |
| [8] |
Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2021, 22(7): 931-945. DOI:10.1016/S1470-2045(21)00152-2 |
| [9] |
Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors[J]. Ann Oncol, 2019, 30(2): 219-235. |
| [10] |
Von Der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase Ⅲ study[J]. J Clin Oncol, 2000, 18(17): 3068-3077. DOI:10.1200/JCO.2000.18.17.3068 |
| [11] |
Calabrò F, Lorusso V, Rosati G, et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma[J]. Cancer, 2009, 115(12): 2652-2659. DOI:10.1002/cncr.24313 |
| [12] |
Vaughn DJ, Broome CM, Hussain M, et al. Phase Ⅱ trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer[J]. J Clin Oncol, 2002, 20(4): 937-940. DOI:10.1200/JCO.2002.20.4.937 |
| [13] |
Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study[J]. Lancet Oncol, 2013, 14(8): 769-776. |
| [14] |
Li L, Li G, Rao B, et al. Landscape of immune checkpoint inhibitor-related adverse events in Chinese population[J]. Sci Rep, 2020, 10(1): 15567. |
 2022, Vol. 49
2022, Vol. 49


