文章信息
- 儿童Xp11.2易位/TFE3基因融合相关性肾癌的影像学表现
- Imaging Characteristics of Renal Cell Carcinoma Associated with Xp11.2 Translocation/TFE3 Gene Fusions in Children
- 肿瘤防治研究, 2021, 48(9): 883-887
- Cancer Research on Prevention and Treatment, 2021, 48(9): 883-887
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2021.21.0786
- 收稿日期: 2021-07-06
- 修回日期: 2021-08-03
2. 430016 武汉,华中科技大学同济医学院附属武汉儿童医院影像中心
2. Medical Imaging Center, Wuhan Children's Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan 430016, China
Xp11.2易位/TFE3基因融合相关性肾癌简称Xp11.2易位性肾癌(Xp11.2 translocation renal cell carcinoma, Xp11.2 tRCC),于2004年首次被世界卫生组织(WHO)归为肾细胞癌的一种罕见亚型,2016年被WHO泌尿生殖系统肿瘤分类中归入MiT家族tRCC[1]。Xp11.2 tRCC主要见于儿童和青少年,约占所有儿童RCC的50%,但在成人病例中仅占1%[2]。术前正确诊断对患者术式选择尤为重要。本文回顾性分析华中科技大学同济医学院附属武汉儿童医院5例经手术病理证实为Xp11.2 tRCC患儿的临床及影像学表现,旨在提高影像科医师的相关认识。
1 资料与方法 1.1 临床资料回顾性分析本院2015年1月—2020年12月经手术病理证实为Xp11.2 tRCC的5例患儿临床及影像学资料,其中男3例、女2例; 年龄0.8~12.8岁,中位年龄9.4岁,平均年龄8.0岁。临床表现主要为血尿(3/5)、腰/腹痛(2/5)、腹部包块(1/5)或无明显症状(1/5),除1例患者伴有恶心、呕吐外,其余患者均无发热、呕吐等。实验室检查表现为炎性指标(中性粒细胞或CRP)升高(3/5),肿瘤标志物CA199升高(2/5)及血肌酐升高(1/5)。其中,5例患者均行CT平扫和增强扫描,1例患者行MRI平扫、增强和DWI检查。
本组病例均进行术后临床随访。随访时间6~26月,1例术前腹膜后淋巴结转移者,进行放化疗2个疗程后淋巴结缩小,未发现新转移灶。其他患者均无远处转移。
1.2 检查方法CT检查采用德国Siemens Somatom definition AS+64排128层螺旋CT。扫描参数:管电压100~120 KV,管电流180~200 mAs,层厚8 mm,层间距8 mm,螺距1.4,扫描范围从膈顶至膀胱水平,增强扫描采用2.0 ml/kg碘海醇注射液(含碘300 mgI/ml)静脉团注,流速为0.5~2.0 ml/s,分别于注射对比剂后行皮质期(25~30 s)、实质期(80~120 s)和排泄期(5~8 min)扫描。原始图像采用1 mm薄层重建,后利用多平面重组等进行图像后处理。
MRI检查采用GE Discovery 750 3.0T MRI扫描仪,8通道心脏相控线圈。扫描序列和参数:平扫采用横轴位、冠状位抑脂及非抑脂呼吸触发FSE T2WI(TR 9 600 ms, TE 110 ms),视野:38 cm×38 cm,矩阵:512×512、层厚5 mm、层间距1 mm、激励次数2。横轴位及冠状位屏气脂肪抑制快速三维容积采集(liver acquisition volume acceleration, LAVA)(TR 4.1 ms, TE Min Full),视野:40 cm×40 cm,矩阵:256×192、层厚4 mm、层间距0 mm、激励次数1。DWI序列采用单次激发自旋平面回波(SS-EPI)序列,b值设置为0及800 s/mm2。增强扫描采用0.2 ml/kg Gd-DTPA静脉团注,分别行横轴位及冠状位LAVA扫描。依从性较差患儿采取10%水合氯醛(0.3~0.5 ml/kg)口服镇静。
1.3 图像分析患儿图像由两名高年资放射科医师共同阅片,主要观察病灶的部位(左肾、右肾或双肾,皮质型、髓质型或皮髓质型,内生型或外生型)、大小(最大横截面直径)、形态(类圆形或不规则形)、边界、性质(囊性、实性或囊实性)、成分(钙化、出血、囊变及坏死)、强化方式及程度(实质期CT值较平扫增加10~20 Hu为轻度强化,增加21~40 Hu为中度强化,增加40 Hu以上为明显强化)、与肾门及邻近大血管关系(有无侵犯,推移或包绕)及转移情况(有或无,邻近转移或远处转移)等,综合分析达成一致诊断意见。
2 结果 2.1 影像学表现CT表现:(1)部位:5例病灶均为单侧,左肾3例(图 1),右肾2例(图 2); 5例病灶均为皮髓质型,其中4例为内生型,1例为外生型; (2)大小:病灶最大横截面直径约25~96 mm,平均直径约为55 mm; (3)形态:4例病灶均为类圆形,仅1例表现为不规则型; (4)边界:3例病灶边界清晰,2例病灶边界欠清; (5)性质及成分:实性/囊实性病灶4例,钙化(1例病灶呈蛋壳样钙化,余病灶呈点、条状钙化)及坏死4例,出血1例,囊变1例; 囊性病灶1例,其内可见分隔及结节状钙化; 其中有囊变病灶及囊性病灶钙化均位于分隔影上; (6)强化方式及程度:4例实性病灶实性成分平扫为等(2例)或稍高(2例)密度,强化程度各期均低于正常肾实质,2例强化程度各期无明显差异,2例为渐进性强化,2例延迟期可见假包膜强化; 2例为轻度强化,1例中度强化,1例明显强化。1例囊性病灶囊性成分未强化,囊壁及分隔影可见轻-中度强化; (7)与肾门及邻近大血管关系:仅1例病灶出现同侧肾动、静脉包绕及下腔静脉受压推移; (8)转移情况:1例患者出现同侧腹膜后淋巴结钙化,1例患者出现同侧肾皮质、输尿管及腹膜后淋巴结转移,5例患者均无远处转移。
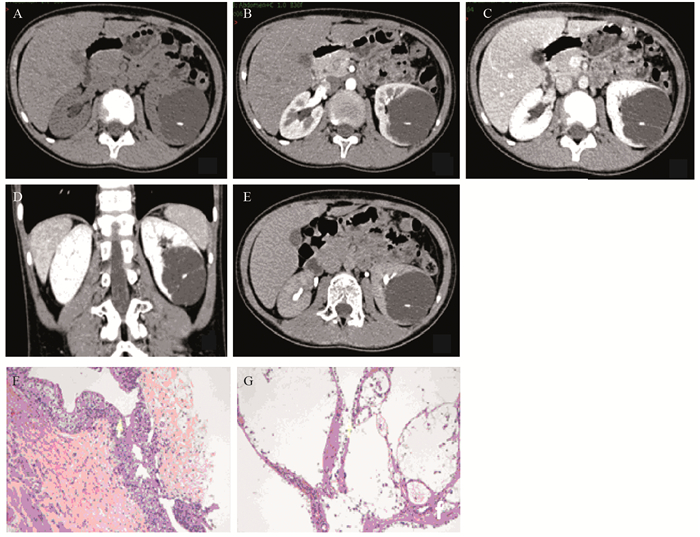
|
| A 12-year-old boy with left renal cystic mass detected for more than one week. A: axial unenhanced CT scan showed a mass in the middle and lower pole of left kidney, the center of the lesion with clear edges and small calcifications was in the renal medulla. The density of the lesion was slightly lower than the kidney parenchyma; B: the enhanced degree of the mass was lower than the normal kidney cortex during the cortical phase, with enhanced line-like septa; C, D: during the medullary phase, the mass was slightly enhanced, which was significantly lower than the normal renal parenchyma; E: the enhanced degree of the mass was slightly reduced during the delayed phase. It was lower than normal renal parenchyma; F, G: HE and IHC staining showed that the tumor cells were eosinophilic and arranged in papillary architecture, with round nuclei and small nucleoli. There were no tumor cells seen in the adipose tissues. TFE3(+), CK(+), CK8/18(++), Vimentin(+), WT-1(-), Ki67(index < 1%), CK34βe12(-), CD10(+), EMA(-). 图 1 Xp11.2易位/TFE3基因融合相关性肾癌(左肾) Figure 1 Left kidney of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion (Xp11.2 tRCC) patients |

|
|
A 6-years-old girl with hematuria for one week. "Right renal hamartoma" was suspected by ultrasound. A-C: Axial/coronal/sagittal unenhanced CT scan showed a solid, iso-to-hyperdense heterogeneous mass in the middle and upper right kidney. The center of the lesion was in the renal medulla. Irregular rings and striped calcifications were seen on the edge and inside of the mass. Coronal/sagittal CT showed the circumscribed mass was located in the posterior pole of the right kidney. D-F: axial enhanced CT scan showed during the early arterial phase(D), the enhanced degree of the mass was lower than the normal renal cortex, with discontinuous line-like enhancement on the edge. During the cortical phase(E), the mass was slightly enhanced, with obscure boundary. The enhanced degree was higher than the normal renal medulla but lower than the normal cortex. During the medullary phase(F), the uncircumscribed mass was enhanced, significantly lower than the normal renal parenchyma, with non-enhancing capsule. 图 2 Xp11.2易位/TFE3基因融合相关性肾癌(右肾) Figure 2 Right kidney of Xp11.2 tRCC patients |
MRI表现:仅囊性病灶患者行MRI扫描。平扫主要表现为长T1长T2信号,DWI序列可见弥散受限。增强扫描囊性部分未见明显强化,囊壁及分隔影可见强化,局部分隔影厚度欠均,并可见强化壁结节影,见图 3。
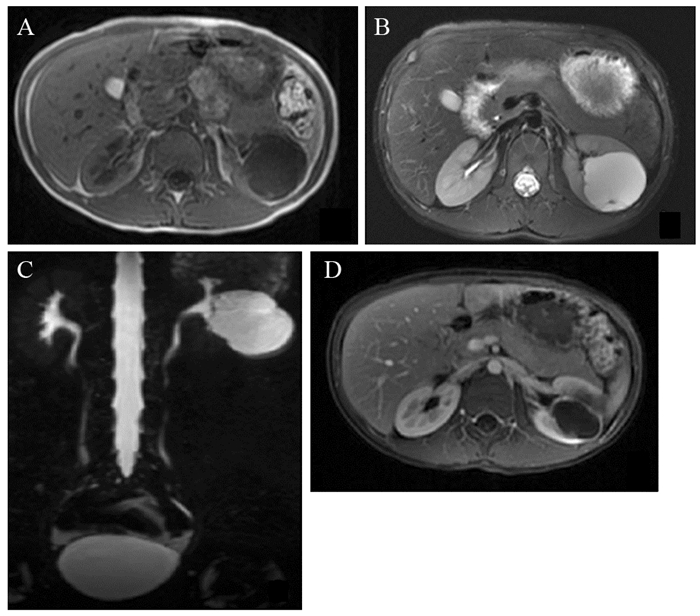
|
| A: axial SE T1-weighted MR imaging revealed a hypointense left renal mass with clear boundary; B: axial FS T2-weighted MR imaging showed a hyperintense mass with short T2 pseudocapsules on the edge; C: coronal MRCP showed that the middle and lower pole of the left kidney mass was slightly hyperintense, with clear boundary, slightly lobe, and strip-shaped septa; D: Axial enhanced FS LAVA T1WI scan showed that the tumor was non-enhancing, with the ring enhancement of pseudocapsule. 图 3 与图 1为同一患儿MRI图像 Figure 3 MRI image of the same patient in figure 1 |
大体观:肿块主要呈灰褐、灰白色组织,质软,与肾组织分界较清,大部分伴出血及坏死物质。镜下观:细胞边界清楚,胞质透亮或红染,核仁明显,透明细胞排列成乳头状结构,或伴有嗜酸性颗粒胞质的瘤细胞排列成巢状结构,内含砂粒体。免疫组织化学:所有患者均表现为TFE3(+),CK(+),CD10(+),EMA(-),Vimentin(+),Ki67(<1%~50%),见图 1F~G。
3 讨论 3.1 流行病学与临床Xp11.2 tRCC是一种罕见的肾脏恶性肿瘤,常见于45岁以下人群,儿童和青少年更为常见,57%患者小于25岁[3]。据报道,儿童Xp11.2 tRCC患者发病中位年龄约为9~12岁,且女性多见[4]。本组病例中位年龄(9.4岁)与文献报道类似,但存在1例10月龄婴幼儿病例,检索显示为目前报道最小患者。本组中男女比例约为3:2,可能与入组病例过少有关。约15%Xp11.2 tRCC患者有化疗病史,化疗后2年内最常见[3],但本组5例患者均无化疗相关病史。Xp11.2 tRCC患者极少出现典型的肉眼血尿、腹/腰痛和腹部包块肾癌三联征,大多表现为其中一种,以肉眼血尿最为常见,部分患者为偶然发现或仅表现为发热、盗汗、体重减轻等一般症状[5]。本组患者表现与之相符。
Xp11.2 tRCC预后较成人好,五年生存率约为60%~85%,无转移患者更高[5],Zhu等[2]证实高龄和远处转移是Xp11.2 tRCC不良预后的独立预测因子,虽然早期易出现淋巴结转移,但这些患者表现出惰性临床过程。本组2例患者出现淋巴结转移,5例患者均无远处转移,随访至今均存活。
3.2 影像学表现Xp11.2 tRCC不同于肾透明细胞癌,其起源于肾小管上皮细胞,因此小病灶多位于肾髓质,大病灶可突破肾包膜向外生长,并压迫邻近血管,呈浸润性生长,瘤体多呈类圆形或不规则形,边界一般较清,多位于肾脏一极,左肾与右肾发病率无明显统计学差异[3, 6]。本组病例中,左右肾发病率之比约为3:2。3例瘤体较小者肾被膜完整; 1例稍大者可累及肾周筋膜,但未突破; 1例瘤体直径9.6 cm,突破肾被膜向外生长,大于文献报道瘤体最大横截面直径多为4~7 cm[7]。
CT平扫,实性/囊实性Xp11.2 tRCC肿块的实性部分因蛋白成分、出血或含铁血黄素沉积多呈稍高或高密度,肿瘤中心或周围出现点状、斑片状钙化,认为是本病的重要特征性表现,囊变及坏死多见于瘤体较大者[8]。CT增强,瘤体实性部分多呈轻-中度强化,强化程度低于正常肾皮质[7]。目前该肿瘤强化主要有三种方式[8]:一是“快进慢出”的渐进式持续强化,本组3例; 二是“少进慢出”,即皮、髓质期病灶持续强化,延迟期强化程度减低; 三是各期强化无明显差异,本组2例; 延迟期的“假包膜征”是Xp11.2 tRCC的另一特征性表现[3]。本组4例实性病例中,体积由小到大密度逐渐减低(即CT表现由稍高密度到等密度),与肿瘤体积逐渐增大、坏死、囊变相关,且坏死区呈“轮辐样”改变; 较小者钙化更明显,呈现典型“蛋壳样”钙化[9],较大者多表现为斑片或砂砾样钙化。本组增强扫描4例为轻-中度强化,唯一一例明显强化者出现在瘤体较大病例,可能与瘤体较大、供血动脉增多有关,此征象与雷逸等[7]所见类似。陶磊等[10]认为,Xp11.2 tRCC瘤体大小与强化程度相关,提示瘤体生长速度快于供血血管建立,所以瘤体越大,囊变坏死越严重。另外,对于中、小瘤体各期强化程度无明显差异的原因,可能与延迟时间不足有关。本组病例无第二种强化方式,可能与病例较少、CT参数或造影剂剂量等相关。瘤体偏大者可见经典延迟期明显强化的“假包膜征”,该肿瘤假包膜发生率较高。实性或囊实性Xp11.2 tRCC瘤体典型MRI表现T1WI呈等或稍高信号,T2WI呈稍低信号,多为瘤体出血、含铁血黄素沉着以及蛋白含量较高所致[11]。T2WI上瘤体内可见“瘤中结节”及“条纹征”等表现,可作为鉴别诊断要点。DWI序列呈不均匀高信号,ADC值明显降低[6]。
Xp11.2 tRCC囊性肿块中,钙化、囊液成分、囊壁分隔厚度(不均匀增厚、最薄处囊壁或分隔厚度 > 1 mm)及壁结节是其主要的诊断要点[12],也是本组病例与复杂性肾囊肿鉴别的关键点。
3.3 鉴别诊断主要应与以下肿瘤鉴别:(1)肾母细胞瘤,是儿童最常见的肾脏恶性肿瘤,部分Xp11.2 tRCC影像学表现与之非常相似,极易误诊。肾母细胞瘤中位年龄及平均年龄较小,1~3岁常见,肾癌年龄偏大。肾癌多位于肾脏一极,一般体积较小,较少跨中线生长; 肾母细胞瘤往往瘤体较大,残肾呈“新月征”,具有一定特征性。肾癌钙化较肾母细胞瘤多见,囊变、坏死较其少,且Xp11.2 tRCC强化程度一般高于肾母细胞瘤[8, 13]; (2)肾透明细胞肉瘤,好发于幼儿,平均发病年龄3岁,瘤体通常很大,平均直径11 cm,不均质,含有较多囊性成分、血供较丰富、伴钙化,特别是发生骨转移征象时[14],多提示诊断; (3)肾恶性横纹肌样瘤,常见2岁以下,10%~15%儿童可同时或异时并发后颅窝脑肿瘤。肿瘤通常较大、坏死、出血、不均质、具有浸润性。约26%患者出现特发性高钙低磷血症,瘤体多位于肾中心,无包膜,多呈分叶状,70%可见包膜下积血,呈新月形,累及区域淋巴结、肺、脑和骨[15]; (4)肾透明细胞癌/乳头状肾细胞癌:临床出现肉眼血尿概率明显低于Xp11.2 tRCC,钙化极少,坏死囊变亦少见。CT增强幅度明显高于Xp11.2 tRCC[9]。
综上所述,儿童Xp11.2 tRCC临床罕见,若儿童出现以肾髓质为中心肿块,较小者可多位于肾轮廓内,较大者可突破肾被膜,坏死、囊变较多见。实性/囊实性病灶中心或周围出现点状、斑片状钙化,是一个重要征象。增强CT扫描延迟期见“假包膜征”,囊性病灶出现囊壁分隔不均匀增厚及强化壁结节,肿瘤早期出现淋巴结转移,则应高度警惕儿童Xp11.2 tRCC。一般认为该肿瘤生物学行为偏惰性,病情进展慢,对化疗和免疫治疗不敏感,根治性切除为主要治疗方法,预后相对较好。
作者贡献
彭飞:研究设计、病例收集、论文撰写
闫学强:参与研究设计、病例审核
邵剑波:研究设计审核、文章修改
| [1] |
Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours[J]. Eur Urol, 2016, 70(1): 93-105. DOI:10.1016/j.eururo.2016.02.029 |
| [2] |
Zhu Y, Pu X, Dong X, et al. Molecular Heterogeneity of Xp11.2 Translocation Renal Cell Carcinoma: The Correlation Between Split Signal Pattern in FISH and Prognosis[J]. Cancer Manag Res, 2021, 13: 2419-2431. DOI:10.2147/CMAR.S297457 |
| [3] |
Chen X, Zhu Q, Li B, et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE gene fusion: imaging findings in 21 patients[J]. Eur Radiol, 2017, 27(2): 543-552. DOI:10.1007/s00330-016-4421-4 |
| [4] |
Nakata K, Colombet M, Stiller CA, et al. Incidence of childhood renal tumours: an international population-based study[J]. Int J Cancer, 2020, 147(12): 3313-3327. DOI:10.1002/ijc.33147 |
| [5] |
van der Beek JN, Geller JI, Krijger R, et al. Characteristics and Outcome of Children with Renal Cell Carcinoma: A Narrative Review[J]. Cancers(Basel), 2020, 12(7): 1776. |
| [6] |
程瑾, 陈皓, 史景丽, 等. Xp11.2易位/TFE3基因融合相关性肾癌的CT和MRI表现[J]. 放射学实践, 2018, 33(8): 811-815. [Cheng J, Chen H, Shi JL, et al. MR and CT imaging features of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions[J]. Fang She Xue Shi Jian, 2018, 33(8): 811-815.] |
| [7] |
雷逸, 刘芳, 范承启, 等. Xp11.2易位/TFE3基因融合相关性肾癌CT表现及病理对照分析[J]. 放射学实践, 2017, 32(5): 504-508. [Lei Y, Liu F, Fang CQ, et al. CT imaging findings and pathologic correlation of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions[J]. Fang She Xue Shi Jian, 2017, 32(5): 504-508.] |
| [8] |
马秋红, 金科, 向永华, 等. 3例儿童Xp11.2易位TFE3基因融合相关性肾癌CT表现及文献复习[J]. 临床小儿外科杂志, 2020, 19(10): 909-915. [Ma QH, Jin K, Xiang YH, et al. Computed tomography features of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions: three cases report and literature review[J]. Lin Chuang Xiao Er Wai Ke Za Zhi, 2020, 19(10): 909-915.] |
| [9] |
张旭婷, 任基伟, 靳宏星, 等. Xp11.2易位/TFE3基因融合相关性肾癌的影像诊断与鉴别诊断[J]. 医学影像学杂志, 2019, 29(6): 997-1001. [Zhang XT, Ren JW, Jin HX, et al. CT and MRI Imaging in diagnosing renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion[J]. Yi Xue Ying Xiang Xue Za Zhi, 2019, 29(6): 997-1001.] |
| [10] |
陶磊, 崔文静, 刘丽, 等. Xp11.2易位/TFE3基因融合相关性肾癌影像表现及病理对照[J]. 临床放射学杂志, 2016, 35(8): 1190-1195. [Tao L, Cui WJ, Liu L, et al. The Comparative Study of Imaging Features of Renal Cell Carcinoma Associated with Xp11.2/TFE3 Gene Fusions and Pathological Results[J]. Lin Chuang Fang She Xue Za Zhi, 2016, 35(8): 1190-1195.] |
| [11] |
Prasad SR, Humphrey PA, Catena JR, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation[J]. Radiographics, 2006, 26: 1795-1806. DOI:10.1148/rg.266065010 |
| [12] |
Tse JR, Shen J, Shen L, et al. Bosniak Classification of Cystic Renal Masses Version 2019: Comparison of Categorization Using CT and MRI[J]. AJR Am J Roentgenol, 2021, 216(2): 412-420. DOI:10.2214/AJR.20.23656 |
| [13] |
谢起根, 苏诚, 高鹏飞, 等. 儿童肾癌的临床及CT和病理特点分析[J]. 中华小儿外科杂志, 2017, 38(10): 744-748. [Xie QG, Su C, Gao PF, et al. Clinical, computed tomography and pathologic features of pediatric renal cell carcinoma[J]. Zhonghua Xiao Er Wai Ke Za Zhi, 2017, 38(10): 744-748. DOI:10.3760/cma.j.issn.0253-3006.2017.10.005] |
| [14] |
朱明水, 唐文伟, 李小会, 等. 儿童肾透明细胞肉瘤的CT表现[J]. 实用放射学杂志, 2016, 32(9): 1418-1421. [Zhu MS, Tang WW, Li XH, et al. The CT features of clear cell sarcoma of the kidney in childrenJ][J]. Shi Yong Fang She Xue Za Zhi, 2016, 32(9): 1418-1421. DOI:10.3969/j.issn.1002-1671.2016.09.025] |
| [15] |
苗静, 周俊霖, 杨文萍, 等. 儿童肾脏恶性横纹肌样瘤的临床特征及CT诊断价值[J]. 中华放射学杂志, 2011, 45(2): 205-206. [Miao J, Zhou JL, Yang WP, et al. Malignant rhabdoid tumor of the kidney: clinical characteristics and CT findings[J]. Zhonghua Fang She Xue Za Zhi, 2011, 45(2): 205-206.] |
 2021, Vol. 48
2021, Vol. 48


