文章信息
- 放疗联合免疫检查点抑制剂在非小细胞肺癌中的临床研究进展
- Clinical Research Progress of Radiotherapy Combined with Immune Checkpoint Inhibitors on NSCLC
- 肿瘤防治研究, 2021, 48(10): 916-921
- Cancer Research on Prevention and Treatment, 2021, 48(10): 916-921
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2021.21.0382
- 收稿日期: 2021-04-07
- 修回日期: 2021-08-02
肺癌是世界范围内发病率第二和死亡率最高的恶性肿瘤[1],非小细胞肺癌(NSCLC)约占所有肺癌的85%[2],但临床可手术的NSCLC仅占20%~30%,约50%的NSCLC患者确诊时伴有远处转移,整体预后较差[3-4]。近年来靶向PD-1(programmed cell death 1)/PD-L1(programmed cell death ligand 1)通路和CTLA-4(cytotoxic T lymphocyte-associated antigen-4)的免疫检查点抑制剂(immune checkpoint inhibitors, ICIs)正在改变局部晚期和驱动基因阴性晚期NSCLC的治疗策略[5-6]。放疗作为传统治疗手段之一,在各期肺癌的治疗中均发挥着重要作用,不仅可以直接有效地杀伤肿瘤,还可以激活机体的抗肿瘤免疫[7]。在NSCLC的治疗中,放疗联合ICIs的治疗策略近来受到重视,二者联合的模式、疗效和不良反应成为关注的焦点。
1 放疗联合ICIs的生物学机制近来临床上观察到某一病灶接受放疗后,其他未受照射的远处转移灶也出现退缩,这一现象被称为“远隔效应”,并认为这种现象是放疗激活机体抗肿瘤免疫的结果[8];然而临床上可以观察到放疗“远隔效应”的发生率仅为10%左右,这提示放疗对于肿瘤的免疫激活机制可能非常复杂[9],受到多种因素的共同作用。研究发现放疗一方面可以诱导免疫原性细胞死亡(immunogenic cell death, ICD)产生T细胞特异性的肿瘤相关抗原(tumor associated antigen, TAA)、增加抗原提呈、释放趋化因子招募T细胞或破坏肿瘤血管屏障进而增加肿瘤内T细胞的浸润,调节肿瘤细胞表型如上调MHC-Ⅰ和死亡受体等改善CTL和NK细胞对肿瘤细胞的识别和杀伤效应[10];另一方面也可以适应性上调肿瘤细胞PD-L1表达、增加髓系抑制性细胞(myeloid-derived suppressor cells, MDSC)和调节性T细胞(regulatory T cells, Treg)等免疫抑制性细胞的肿瘤内浸润[11]。因此,理论上放疗联合ICIs不仅可以增强机体的抗肿瘤免疫反应,而且可以抑制放疗诱导的肿瘤微环境和肿瘤细胞的负向调节效应。目前为止,多项临床研究已经探索或正在探索放疗联合ICIs的协同效应。
2 放疗联合ICIs治疗早期NSCLC的临床研究早期NSCLC的标准治疗是手术切除,而体部立体定向放疗(stereotactic body radiotherapy, SBRT)被推荐为不可切除或不愿意手术的早期NSCLC患者优选治疗方式[12]。由于完全切除的早期NSCLC复发模式以远处转移为主[13],因此对早期肺癌治疗中引入ICI疗法的期望很高,其最终结果有待前瞻性的临床研究解答[14]。一项单中心、Ⅱ期的临床研究评价了原发灶SBRT联合Durvalumab对比单药Durvalumab新辅助治疗Ⅰ~ⅢA期可手术的NSCLC患者主要病理缓解率(major pathological response, MPR),结果表明SBRT联合Durvalumab组患者MPR率明显优于单药Durvalumab组患者(53.3% vs. 6.7%, P < 0.0001)[15]。ASTEROID是一项多中心、随机对照的Ⅱ期临床研究,旨在评估cT1~2N0M0的NSCLC患者SBRT后Durvalumab治疗的疗效和安全性,初步结果表明SBRT联合Durvalumab治疗的可行性[16]。目前已开展的临床研究涵盖了不同ICIs产品在不能手术或拒绝手术的早期NSCLC患者中联合SBRT的应用,见表 1。这些研究最终结果能否为免疫治疗时代下早期NSCLC的治疗带来更多选择,我们拭目以待。
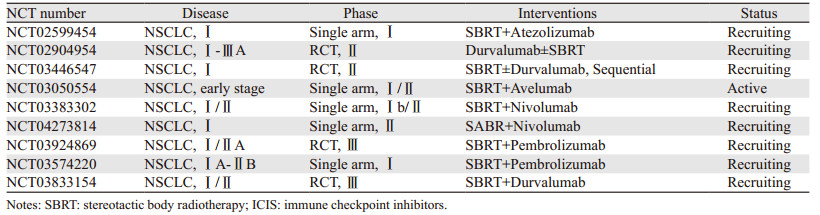
|
NSCLC确诊时20%~30%的患者为局部晚期NSCLC(locally advanced NSCLC, LA-NSCLC),同步放化疗是不能手术的LA-NSCLC的标准治疗方式[17],但中位生存时间一般不超过2年,5年生存率仅为20%。ICIs的应用使人们看到了改善LA-NSCLC预后的希望,放疗联合ICIs的临床研究逐渐证实了二者联合的有效性和安全性,尤其是PACIFIC研究取得了突破性进展并成功改写了指南[6]。
一项全球多中心Ⅲ期随机对照研究纳入了来自26个国家的713例不能手术的Ⅲ期NSCLC患者;在同步放化疗后1~42天,研究组接受为期1年的PD-L1抑制剂Durvalumab巩固治疗,对照组接受安慰剂治疗,结果研究组无进展生存(PFS)从5.6月提高到16.8月[18],患者的疾病进展或死亡风险降低了48%(P < 0.001; HR=0.52, 95%CI: 0.42~0.65)。截至2020年3月20日,研究组的中位生存(OS)达47.5月,明显高于对照组29.1月(P=0.0025; HR=0.68, 95%CI: 0.53~0.87),4年PFS和OS率分别为35.3%和49.6%,显著高于对照组的19.5%和36.6%[19]。研究组3级以上不良反应发生率为30.5%,对照组为26.1%;2组分别有15.4%和9.8%的患者因不良反应而终止治疗,最常见的为肺炎(研究组为4.8%,对照组为2.6%),其中放射性肺炎发生率均为1.3%[20]。Durvalumab的巩固治疗在改善患者预后的同时不仅未明显增加不良发应,而且也未影响患者的生活质量[21]。亚组分析发现,PFS在所有PD-L1表达状态下均有获益,而OS仅在PD-L1表达≥1%的患者中获益,并且PFS和OS在PD-L1表达≥25%的患者获益最为明显[22],这提示LA-NSCLC同步放化疗后的ICIs巩固治疗,检测PD-L1表达状态可能有助于筛选优势人群。
继PACIFIC研究后,相关拓展性研究也逐渐展开。LUN14-179是一项Ⅱ期单臂的研究,旨在评估不可手术Ⅲ期NSCLC患者同步放化疗后,PD-1抑制剂Pembrolizumab巩固治疗的安全性和有效性。目前报道无远处转移时间为22.4月,中位PFS为17.0月,中位OS未达到,2年的OS率为61.9%[23],有19.6%的患者因不良反应终止用药,主要的3/4级反应为肺炎(5.4%)、呼吸困难(5.4%)、乏力(4.3%)和腹泻(4.3%);该研究目前仍在进行中。我们对比PACIFIC和LUN14-179研究发现,LUN14-179的PFS和OS与PACIFIC研究相似,其不良反应发生情况相仿,但LUN14-179的远期疗效仍需要进一步随访验证。为了进一步扩大获益人群,启动了BTCRC LUN16-081临床研究,旨在评估PD-1抑制剂Nivolumab联合CTLA-4抑制剂Ipilumab治疗NSCLC过程中的安全性和疗效[24],2020年美国临床肿瘤学会(ASCO)报道了中期安全性数据分析,Nivolumab+Ipilumab组患者3级以上不良反应的发生率高达44%,3级以上肺炎的发生率为20%,约40%的患者因不良反应而中断治疗,因此该联合治疗模式有待于进一步探索优化。ETOP NICOLAS研究探讨了放化疗同步Nivolumab治疗Ⅲ期NSCLC患者的安全性和有效性;患者放疗开始即同步使用Nivolumab治疗并维持治疗1年。中期安全性分析发现,最常见的不良反应是贫血(41.4%)、呼吸困难(20.7%)和肺炎(19.0%),其中发生3级以上的概率分别为5.2%、1.7%和1.7%。前期21例随访患者在放疗后的3月内未发生3级以上放射性肺炎和其他未预料的不良反应,中位PFS达12.7月[25]。正在进行的AFT-16研究(NCT03102242)将评估同步放化疗联合Atezolizumab诱导和维持治疗不可切除Ⅲ期NSCLC的疗效;初步研究结果提示将免疫治疗时机提前安全可耐受,且能带来生存获益。多中心、回顾性的HOPE-005/CRIMSON研究报道了真实世界中LA-NSCLC放化疗后ICIs巩固治疗中,超过80%的患者发生治疗相关性肺炎,但多数为非症状性肺炎,V20≥25%是发生症状性肺炎的独立危险因素[26]。
综合现有的临床研究证据,同步放化疗后巩固ICIs的治疗策略显著改善了不可切除Ⅲ期NSCLC患者的PFS和OS,其不良反应注册临床研究和真实世界研究报道的结果有所不同,值得临床医师高度重视。
4 放疗联合ICIs治疗转移性NSCLC的临床研究约50%的NSCLC患者确诊时存在远处转移,针对驱动基因突变的靶向治疗是驱动基因阳性的晚期NSCLC患者治疗的首选[5],而ICIs已成为驱动基因阴性晚期NSCLC患者的一线治疗选择[27]。晚期NSCLC患者在以全身治疗为主的同时会选择性的辅以原发灶或(和)转移灶局部放疗,那么放疗联合ICIs是否可以改善晚期NSCLC患者的生存呢?KEYNOTE-001研究中驱动基因阴性的晚期NSCLC患者5年OS数据达到了新高度,无论PD-L1的表达状态,初治患者5年OS率可达23.2%,既往接受过放化疗患者的OS率为15.5%,而PD-L1表达≥50%的初治患者5年OS率达到了29.6%[28]。亚组分析中,既往接受过放疗患者的PFS较未接受过放疗的患者明显延长(4.4月vs. 2.1月,P=0.019);既往接受放疗的患者OS也显著延长(10.7月vs. 5.3月,P=0.026)。既往接受胸内病灶放疗的患者中3例(13%)发生了肺炎,无胸内放疗史的患者仅1例(1%)肺炎,但两组患者3级以上不良反应无明显差别。该研究初步证实了ICIs治疗前的任何部位放疗可以增强Pembrolizumab的疗效。
随着放疗技术的进步,SBRT在晚期肺癌的应用越来越多[29]。有研究者提出SBRT相比于常规分割放疗更能有效激活机体的抗肿瘤免疫,是NSCLC免疫治疗时代联合ICIs的最佳“伴侣”[30]。多中心、Ⅱ期临床研究PEMBRO-RT纳入了76例化疗失败的转移性NSCLC患者,研究组单个病灶(以肺转移灶为主)SBRT后7天内开始应用Pembrolizumab治疗,对照组仅使用Pembrolizumab治疗,结果表明研究组和对照组的12周ORR分别为36%和18%(P=0.07),12周DCR(disease control rate)分别为64%和40%(P=0.04);中位PFS分别为6.6月和1.9月(P=0.19),中位OS期分别为15.9月和7.6月(P=0.16);尽管可能是由于样本量较小未达统计学预期值,但研究组患者的生存获益趋势显著,且亚组分析中PD-L1表达阴性的患者总体有效率提高了5倍[31]。Theelen等[32]合并分析了PEMBRO-RT和MDACC临床研究中晚期NSCLC患者放疗联合Pembrolizumab治疗的数据,联合治疗组远隔效应的发生率高达41.7%(对比Pembrolizumab组19.7%,P=0.0039),中位PFS和OS分别为9.0月vs. 4.4月(P=0.045)和19.2月vs. 8.7月(P=0.0004),SBRT联合ICIs显著改善了晚期NSCLC患者的预后。Welsh等[33]的一项Ⅰ/Ⅱ期、随机对照研究证实了单个病灶SBRT(50 Gy/4 F)联合Pembrolizumab治疗转移性NSCLC患者放射野外ORR明显高于传统分割放疗(45 Gy/15 F)联合Pembrolizumab组(33% vs. 17%),并且在PD-L1低表达组患者中PFS显著延长(20.8月vs.4.6月,P=0.004)。
在安全性方面,回顾性分析3个评价胸部放疗(NSCLC均采用SBRT)联合Ipilimumab或Pembrolizumab治疗晚期实体瘤的安全性数据发现,60例患者中无4/5级不良反应的发生;15例患者出现3级不良反应,其中4例患者出现9次肺部特异性不良反应(呼吸困难、肺炎、肺部感染、乏氧),该研究者认为SBRT联合ICIs短期内安全、可耐受[34]。另一项多中心、回顾性研究纳入了117例既往接受肺部病灶SBRT的患者,其中54例患者接受了肺部病灶SBRT同步ICIs,虽然3级肺炎的发生率明显增加(10.7% vs. 0, P < 0.01),可能是由于部分患者接受过ICI/ICI的治疗,但任何级别的肺炎发生率相似,整体是安全的[35]。除此之外,有研究者评估颅内转移灶SRS-SRT(stereotactic radiosurgery-stereotactic radiation therapy)联合ICIs的安全性。Hubbeling等回顾性分析了163例NSCLC脑转移灶放疗的数据,其中50例患者接受了SRS联合ICIs治疗,并未增加放疗相关的不良反应[36]。
晚期患者常有多个病灶,如何选择放疗病灶?Chang认为多部位放疗联合ICIs可能获益更大[37],因为多部位放疗可能释放更多的肿瘤抗原和放射线本身对肿瘤的杀灭作用更强。一项标准治疗后进展的多种实体瘤患者接受2~4转移灶SBRT联合PD-1抑制剂Pembrolizumab的Ⅰ期临床研究初步分析表明,所有患者ORR(objective response rate)为13.2%,中位OS达9.6月,且不良反应可控[38];COSINR研究的初步结果表明多病灶SBRT联合Ipilimumab/Nivolumab序贯或同步治疗Ⅳ期NSCLC患者的ORR达68%[39],同步组患者剂量限制毒性的发生率较低,证明了该联合治疗模式安全有效。尽管初步探索性研究取得了令人鼓舞的疗效,但该联合治疗模式仍需要前瞻性Ⅲ期临床研究的证据支持[30]。
5 放疗联合ICIs治疗NSCLC亟待解决的问题KEYNOTE-001研究提示似乎颅外病灶放疗联合ICIs治疗时患者获益更加显著,McGee发现不同部位病灶SBRT对晚期NSCLC患者外周血免疫表型的调节存在显著差异性,与脑或骨转移灶相比,肺和肝转移灶接受SBRT能够显著改善外周血免疫表型[40]。这种器官特异性的治疗反应除了肿瘤本身的组织病理特异性,有学者认为生长在不同器官的肿瘤确实存在器官特异性的肿瘤免疫微环境[41-42],而这很大程度决定了放疗后患者机体抗肿瘤免疫活化的程度,进而会影响晚期NSCLC患者的预后[43]。此外,尚无有效的生物标志物能筛选出晚期NSCLC患者ICIs联合SBRT的优势病灶。虽然放疗联合ICIs的临床研究正在逐年递增[44],但哪些患者适合接受联合治疗仍缺乏评价标准。目前免疫治疗还不能像EGFR那样通过明确的作用靶点筛选敏感人群,而放疗后肿瘤细胞PD-L1的表达可能会发生变化,因此放疗联合ICIs可能会更加复杂化生物标志物的作用,我们还需要更多研究探讨更优的标志物用于预测放疗联合ICIs的疗效,提高治疗的性价比[43]。最后,从Ⅲ期NSCLC患者同步放化疗联合ICIs治疗到晚期NSCLC患者SBRT联合ICIs治疗,无论是同步还是序贯ICIs,放疗联合ICIs的时机及ICIs用药持续时间仍需要随机对照的临床研究进一步优化。
6 总结放疗联合ICIs无论是生物学机制还是临床实践上都具有互补的优势,现有的临床证据也证实了两者的协同作用,为NSCLC的综合治疗开创了新的篇章。然而新的治疗必然伴随着更多的困难和挑战,我们也期待着未来会有更多高质量的研究回答和解决上述问题,为NSCLC患者制定更优化的治疗策略。
作者贡献
朱魁魁:检索文献及撰写文章
伍钢:指导及修改文章
| [1] |
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI:10.3322/caac.21660 |
| [2] |
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer[J]. Nature, 2018, 553(7689): 446-454. DOI:10.1038/nature25183 |
| [3] |
Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system[J]. Cancer, 2014, 120(Suppl 23): 3781-3792. |
| [4] |
Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer[J]. J Thorac Oncol, 2016, 11(1): 39-51. DOI:10.1016/j.jtho.2015.09.009 |
| [5] |
Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS[J]. Ann Oncol, 2019, 30(2): 171-210. DOI:10.1093/annonc/mdy554 |
| [6] |
Park K, Vansteenkiste J, Lee KH, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS[J]. Ann Oncol, 2020, 31(2): 191-201. DOI:10.1016/j.annonc.2019.10.026 |
| [7] |
Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach?[J]. Nat Rev Clin Oncol, 2016, 13(8): 516-524. DOI:10.1038/nrclinonc.2016.30 |
| [8] |
Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant[J]. Cancer Treat Rev, 2015, 41(6): 503-510. DOI:10.1016/j.ctrv.2015.03.011 |
| [9] |
Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect[J]. Nat Rev Cancer, 2018, 18(5): 313-322. DOI:10.1038/nrc.2018.6 |
| [10] |
Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers[J]. Clin Cancer Res, 2018, 24(2): 259-265. DOI:10.1158/1078-0432.CCR-16-0037 |
| [11] |
Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory Effects of Stereotactic Body Radiation Therapy: Preclinical Insights and Clinical Opportunities[J]. Int J Radiat Oncol Biol Phys, 2021, 110(1): 35-52. DOI:10.1016/j.ijrobp.2019.02.046 |
| [12] |
Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline[J]. J Clin Oncol, 2018, 36(7): 710-719. DOI:10.1200/JCO.2017.74.9671 |
| [13] |
Senthi S, Lagerwaard FJ, Haasbeek CJA, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis[J]. Lancet Oncol, 2012, 13(8): 802-809. DOI:10.1016/S1470-2045(12)70242-5 |
| [14] |
Vansteenkiste J, Wauters E, Reymen B, et al. Current status of immune checkpoint inhibition in early-stage NSCLC[J]. Ann Oncol, 2019, 30(8): 1244-1253. DOI:10.1093/annonc/mdz175 |
| [15] |
Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial[J]. Lancet Oncol, 2021, 22(6): 824-835. DOI:10.1016/S1470-2045(21)00149-2 |
| [16] |
Hallqvist A, Koyi H, de Petris L, et al. 63MO Safety analysis of durvalumab following stereotactic body radiotherapy (SBRT) in early-stage non-small cell lung cancer (NSCLC) patients: A first report of a randomized phase Ⅱ trial (ASTEROID)[J]. J Thor Oncol, 2021, 16(4): S729-S730. |
| [17] |
Tan WL, Chua KLM, Lin C-C, et al. Asian Thoracic Oncology Research Group Expert Consensus Statement on Optimal Management of Stage Ⅲ NSCLC[J]. J Thorac Oncol, 2020, 15(3): 324-343. DOI:10.1016/j.jtho.2019.10.022 |
| [18] |
Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage Ⅲ Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2017, 377(20): 1919-1929. DOI:10.1056/NEJMoa1709937 |
| [19] |
Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage Ⅲ NSCLC—an Update From the PACIFIC Trial[J]. J Thorac Oncol, 2021, 16(5): 860-867. DOI:10.1016/j.jtho.2020.12.015 |
| [20] |
Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage Ⅲ NSCLC[J]. N Engl J Med, 2018, 379(24): 2342-2350. DOI:10.1056/NEJMoa1809697 |
| [21] |
Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage Ⅲ, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study[J]. Lancet Oncol, 2019, 20(12): 1670-1680. DOI:10.1016/S1470-2045(19)30519-4 |
| [22] |
Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage Ⅲ non-small-cell lung cancer in the PACIFIC trial[J]. Ann Oncol, 2020, 31(6): 798-806. DOI:10.1016/j.annonc.2020.03.287 |
| [23] |
Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage Ⅲ non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179[J]. Cancer, 2020, 126(19): 4353-4361. DOI:10.1002/cncr.33083 |
| [24] |
Yan M, Durm GA, Mamdani H, et al. Consolidation nivolumab/ipilimumab versus nivolumab following concurrent chemoradiation in patients with unresectable stage Ⅲ NSCLC: A planned interim safety analysis from the BTCRC LUN 16-081 trial[J]. J Clin Oncol, 2020, 38(15_suppl): 9010. DOI:10.1200/JCO.2020.38.15_suppl.9010 |
| [25] |
Peters S, Felip E, Dafni U, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage Ⅲ non-small cell lung cancer-The ETOP NICOLAS trial[J]. Lung Cancer, 2019, 133: 83-87. DOI:10.1016/j.lungcan.2019.05.001 |
| [26] |
Saito G, Oya Y, Taniguchi Y, et al. Real-world survey of pneumonitis/radiation pneumonitis among patients with locally advanced non-small cell lung cancer treated with chemoradiotherapy after durvalumab approval: A multicenter retrospective cohort study (HOPE-005/CRIMSON)[J]. J Clin Oncol, 2020, 38(15_suppl): 9039. DOI:10.1200/JCO.2020.38.15_suppl.9039 |
| [27] |
Hanna NH, Schneider BJ, Temin S, et al. Therapy for Stage ⅣNon-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update[J]. J Clin Oncol, 2020, 38(14): 1608-1632. DOI:10.1200/JCO.19.03022 |
| [28] |
Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase ⅠKEYNOTE-001 Study[J]. J Clin Oncol, 2019, 37(28): 2518-2527. DOI:10.1200/JCO.19.00934 |
| [29] |
Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase ⅡRandomized Trial[J]. J Clin Oncol, 2020, 38(25): 2830-2838. DOI:10.1200/JCO.20.00818 |
| [30] |
Chen Y, Gao M, Huang Z, et al. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges[J]. J Hematol Oncol, 2020, 13(1): 105. DOI:10.1186/s13045-020-00940-z |
| [31] |
Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs. Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial[J]. JAMA Oncol, 2019, 5(9): 1276-1282. DOI:10.1001/jamaoncol.2019.1478 |
| [32] |
Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials[J]. Lancet Respir Med, 2021, 9(5): 467-475. DOI:10.1016/S2213-2600(20)30391-X |
| [33] |
Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase Ⅰ/Ⅱ trial[J]. J Immunother Cancer, 2020, 8(2): e001001. DOI:10.1136/jitc-2020-001001 |
| [34] |
Verma V, Cushman TR, Selek U, et al. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase Ⅰ/Ⅱ Trials[J]. Int J Radiat Oncol Biol Phys, 2018, 101(5): 1141-1148. DOI:10.1016/j.ijrobp.2018.04.054 |
| [35] |
Tian S, Switchenko JM, Buchwald ZS, et al. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity Analysis[J]. Int J Radiat Oncol Biol Phys, 2020, 108(1): 304-313. DOI:10.1016/j.ijrobp.2019.12.030 |
| [36] |
Hubbeling HG, Schapira EF, Horick NK, et al. Safety of Combined PD-1 Pathway Inhibition and Intracranial Radiation Therapy in Non-Small Cell Lung Cancer[J]. J Thorac Oncol, 2018, 13(4): 550-558. DOI:10.1016/j.jtho.2018.01.012 |
| [37] |
Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects[J]. Nat Rev Clin Oncol, 2019, 16(2): 123-135. DOI:10.1038/s41571-018-0119-7 |
| [38] |
Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors[J]. J Clin Oncol, 2018, 36(16): 1611-1618. DOI:10.1200/JCO.2017.76.2229 |
| [39] |
Chmura SJ, Bestvina CM, Karrison TG, et al. Safety and Efficacy of a Randomized PhaseⅠTrial to Evaluate Concurrent or Sequential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients with Stage Ⅳ Non-small Cell Lung Cancer (COSINR Study)[J]. Int J Rad Oncol Biol Physics, 2020, 108(3): S72. |
| [40] |
McGee HM, Daly ME, Azghadi S, et al. Stereotactic Ablative Radiation Therapy Induces Systemic Differences in Peripheral Blood Immunophenotype Dependent on Irradiated Site[J]. Int J Radiat Oncol Biol Phys, 2018, 101(5): 1259-1270. DOI:10.1016/j.ijrobp.2018.04.038 |
| [41] |
Salmon H, Remark R, Gnjatic S, et al. Host tissue determinants of tumour immunity[J]. Nat Rev Cancer, 2019, 19(4): 215-227. |
| [42] |
Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis[J]. Nat Rev Cancer, 2019, 19(1): 9-31. DOI:10.1038/s41568-018-0081-9 |
| [43] |
Käsmann L, Eze C, Manapov F. Stereotactic Body Radiation Therapy (SBRT) Combined with Immune Check-Point Inhibition (ICI) in Advanced Lung Cancer: Which Metastatic Site Should Be Irradiated to Induce Immunogenic Cell Death?[J]. Int Rad Oncol Biol Physics, 2020, 108(1): 225-226. DOI:10.1016/j.ijrobp.2020.04.002 |
| [44] |
Boothe D, Clyde JW, Christensen M, et al. A comprehensive analysis of clinical trials including both immunotherapy and radiation therapy[J]. J Rad Oncol, 2018, 7(3): 223-232. DOI:10.1007/s13566-018-0351-x |
 2021, Vol. 48
2021, Vol. 48


