文章信息
- PD-1抑制剂联合安罗替尼治疗晚期神经内分泌癌的疗效及安全性
- Efficacy and Safety of PD-1 Inhibitor Combined with Anlotinib on Advanced Neuroendocrine Carcinoma
- 肿瘤防治研究, 2021, 48(10): 974-978
- Cancer Research on Prevention and Treatment, 2021, 48(10): 974-978
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2021.21.0271
- 收稿日期: 2021-03-15
- 修回日期: 2021-07-05
神经内分泌肿瘤是一组起源于肽能神经元和神经内分泌细胞、可分泌生物活性物质的罕见恶性肿瘤[1],可发生于体内任何部位,以胃肠胰及肺最为常见[2-3]。神经内分泌肿瘤可依据核分裂相和(或)Ki-67指数分为神经内分泌瘤和神经内分泌癌[4-5],神经内分泌癌是一种低分化、高度恶性的异质性肿瘤。晚期神经内分泌癌最常见的一线治疗手段是化疗[6],但二线及后线尚无标准方案。近年来,临床工作者尝试将免疫治疗及靶向治疗应用于各瘤种的治疗。本研究回顾性分析接受免疫检查点抑制剂联合安罗替尼治疗的45例晚期神经内分泌癌患者的临床资料,探讨免疫检查点抑制剂联合靶向治疗在晚期神经内分泌癌中的疗效及安全性。
1 资料与方法 1.1 研究对象回顾性分析2018年6月—2020年10月郑州大学第一附属医院经病理组织学确诊、晚期不可切除、标准一线方案治疗失败、存在可测量病灶且应用PD-1联合安罗替尼治疗的神经内分泌癌患者临床资料。相关资料均从郑州大学第一附属医院病例系统及病理科获取(伦理号:2019-KY-175)。
1.2 病理诊断标准肺神经内分泌肿瘤诊断采用2015年世界卫生组织肺、胸膜、胸腺和心脏肿瘤分类标准[7],将肺神经内分泌肿瘤分为低级别典型类癌、中级别非典型类癌及高级别的小细胞肺癌和大细胞神经内分泌癌。消化系统神经内分泌肿瘤诊断依据中国胃肠胰神经内分泌肿瘤病理诊断共识(2013版)[8],其中排除高增殖活性的神经内分泌瘤外,将核分裂相 > 20/10高倍镜视野和(或)Ki-67指数 > 20%分类为神经内分泌癌。
1.3 疗效及不良反应评价标准所有患者在接受PD-1联合安罗替尼治疗2~3周期后复查1次增强电子计算机断层扫描(computed tomography, CT)和(或)磁共振;根据实体肿瘤疗效评价RECIST 1.1标准[9]进行疗效评价,分为完全缓解(complete response, CR)、部分缓解(partial response, PR)、疾病稳定(stable disease, SD)和疾病进展(progress disease, PD),客观缓解率(objective response rate, ORR)=CR+PR,疾病控制率(disease control rate, DCR)=CR+PR+SD。无进展生存期(progression free survival,PFS)定义为接受本研究治疗方案开始至疾病进展或死亡的时间。治疗相关不良反应评价依据美国国立综合癌症研究不良反应分级标准PRO-CTACE进行分级。
1.4 随访所有病例通过电话或短信方式进行随访,随访时间从接受本研究方案至疾病进展或死亡日期,末次随访时间为2020年11月30日。
1.5 统计学方法采用SPSS 21.0统计软件进行数据分析。计数资料以例数和率(%)表示;符合正态分布的计量资料用(x±s)表示;不符合正态分布的计量资料用中位数表示。生存率应用Kaplan-Meier法生存分析计算并绘制生存曲线。
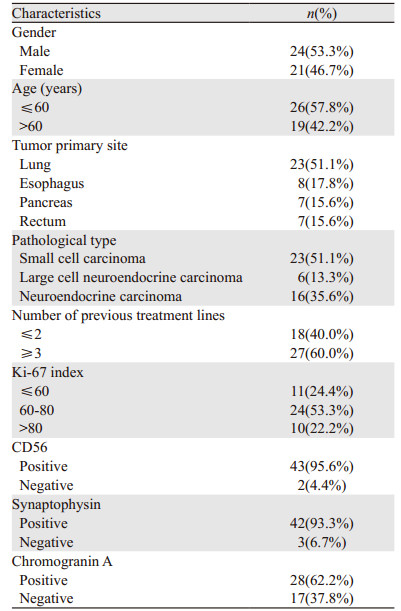
2 结果 2.1 临床资料共纳入符合标准的神经内分泌癌45例,基本特征见表 1。其中男性24例(53.3%),女性21例(46.7%),男女比8:7;年龄分布范围为42~82岁,中位年龄57岁。45例患者在应用免疫检查点抑制剂联合靶向治疗前均有淋巴结及远处转移,其中肝转移23例(51.1%),骨转移22例(48.9%),脑转移10例(22.2%),肺转移9例(20.0%),肾上腺转移6例(13.3%),其他部位转移8例(17.8%,其中胸膜2例、胰腺2例、乳腺1例、胸壁1例、肾脏1例、盆腔1例)。所有患者既往接受过标准一线方案治疗后失败。
|
截至2020年11月30日,所有患者共接受2~15周期的治疗,治疗周期中位数为7周期。其中PD-1抑制剂包括特瑞普利单抗(8例)、卡瑞利珠单抗(12例)、信迪利单抗(25例)。PD-1抑制剂用药剂量为特瑞普利单抗每次240 mg,3周1次,卡瑞利珠单抗和信迪利单抗每次200 mg,3周1次;安罗替尼用药剂量为每天12 mg,口服2周停1周,每3周为1周期。
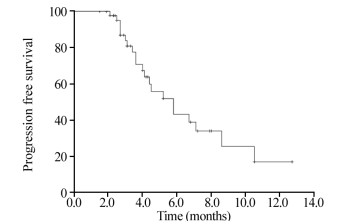
2.3 疗效截至2020年11月30日,随访2~13月,中位随访时间6.2月。45例患者中PR 5例(11.1%),SD 19例(42.2%),PD 18例(40.0%),3例(6.7%)因肿瘤进展死亡。ORR为11.1%(5/45),DCR为53.3%(24/45),见图 1。全组患者中位PFS为5.8月(95%CI: 3.9~7.7月);10月无进展生存率为25.5%。
|
| 图 1 45例晚期神经内分泌癌应用PD-1联合安罗替尼治疗无进展生存曲线图 Figure 1 Progression-free survival curve of 45 advanced neuroendocrine carcinoma patients treated with PD-1 combined with anlotinib |
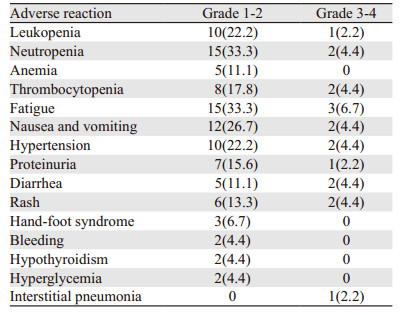
本研究主要不良反应包括骨髓抑制、消化道反应、乏力、手足综合征、皮疹等,与免疫治疗相关的不良反应包括甲状腺功能减退、血糖升高和间质性肺炎,见表 2。主要不良反应经暂停安罗替尼及给予对症支持治疗后好转。
|
神经内分泌肿瘤是一种少见病,但近40年间发病率增长6.4倍,已达到6.98/10万[10];这可能与人们健康体检意识的提高、CT等检查设备的普及和医务工作者对此类疾病认识的提高有关。既往晚期神经内分泌癌主要治疗手段以化疗为主,虽然有效率高,但极易发生耐药或复发,且方案均参照小细胞肺癌治疗。研究[11]显示:肺部、胃肠道及胰腺神经内分泌癌患者中位生存时间分别为7.6月、7.5月、5.7月。神经内分泌癌恶性程度高、治疗药物少,预后较差。有研究[12]显示,胃神经内分泌瘤患者辅助治疗可考虑顺铂联合依拍泊苷方案。免疫治疗的出现改变了肿瘤治疗的现状,为肿瘤治疗提供了更多手段。CheckMate 032[13]、KEYNOTE 028和KEYNOTE 158研究[14]的成功使免疫检查点抑制剂单药成功获批用于小细胞肺癌的二线、三线及后线治疗;IMpower 133研究[15]和CASPIAN研究[16]更是打开了晚期小细胞肺癌一线治疗的新局面。安罗替尼是国内自主研发的多靶点小分子酪氨酸激酶抑制剂,可靶向血管内皮生长因子受体、血小板衍生生长抑制受体、成纤维细胞生长因子受体、c-kit等靶点,具有抗肿瘤血管生成的作用,在中国不仅获批用于非小细胞肺癌[17]、肉瘤[18]、甲状腺髓样癌[19]的治疗,也获批用于小细胞肺癌的三线治疗[20]。既往研究证实抗血管生成药物可以改善肿瘤微环境,增强肿瘤的免疫应答,进而提高肿瘤免疫治疗疗效[21-22]。免疫单药后线治疗晚期小细胞肺癌中位PFS较短,而安罗替尼也仅有4.1月,两者联用是否能为晚期小细胞肺癌患者带来更多获益?而神经内分泌癌的治疗又是参照小细胞肺癌的治疗方法,同时我们在临床上观察到部分晚期神经内分泌癌患者接受了PD-1联合安罗替尼治疗的方案,故我们进行了该项回顾性研究。
本研究是首个真实世界回顾性分析PD-1单抗联合安罗替尼治疗晚期神经内分泌癌的研究,结果显示ORR为11.1%,疾病控制率达53.5%,mPFS为5.8月,10月PFS率为25.5%。本研究与CheckMate 032[13]研究结果ORR 11.9%相近,但mPFS较其mPFS 1.4月明显延长,这主要是因为抗血管生成药物对免疫治疗疗效的增强。KEYNOTE 028和KEYNOTE 158[14]研究ORR、mPFS较本研究高,分别为19.3%、7.7月;考虑与两项研究纳入的多为PD-L1表达阳性的患者有关;虽然目前尚无充分证据表明PD-L1表达水平与小细胞肺癌免疫治疗疗效具有相关性,但PD-L1表达水平被认为是许多肿瘤,特别是非小细胞肺癌免疫治疗疗效的重要预测指标[23-24]。安罗替尼三线及以上单药治疗复发小细胞肺癌的ORR仅为4.9%、mPFS为4.1月[25]。PD-1联合安罗替尼治疗晚期神经内分泌癌疗效优于免疫检查点抑制剂单药或安罗替尼单药治疗小细胞肺癌。
本研究中出现的不良反应主要为1~2级骨髓抑制和消化道反应,3~4级不良反应少,发生的不良反应主要考虑与安罗替尼相关;与既往有关安罗替尼的研究相比,未出现新的不良反应,暂停安罗替尼及对症治疗后缓解。本研究中出现的明确与免疫治疗相关的不良反应包括甲状腺功能减退(2例)、血糖升高(2例)及间质性肺炎(1例);其中甲减患者给予“优甲乐”、血糖患者升高给予“二甲双胍”后甲状腺功能和血糖得到控制,未因此中断免疫治疗;间质性肺炎患者经激素冲击后好转,但终止应用免疫抑制剂。既往一项系统综述和荟萃分析[26]显示接受PD-1联合治疗通常在14.5天发生致死性药物不良事件,死因多为肺炎、肝炎、心肌炎。考虑到部分患者随访时间短,在今后的治疗及随访过程中应注意可能出现的心、肝、肺等毒性。
本研究是一项小样本的单中心回顾性研究。在应用PD-1治疗前未对病理样本进行PD-1/程序性死亡配体-1(programmed death ligand-1, PD-L1)表达水平检测,亦未行肿瘤突变负荷(tumor mutation burden, TMB)及错配修复检测(mismatch repair, MMR)检测;此外,本研究PD-1抑制剂选择不一,可能影响研究结果。Bonanno等[27]研究显示25%的小细胞肺癌肿瘤细胞表达PD-L1,CheckMate 032[13]研究中仅有17%的小细胞肺癌患者PD-L1≥1,小细胞肺癌细胞中PD-L1阳性表达率低。KEYNOTE 028研究初步证实了帕博利珠单抗在PD-L1表达阳性小细胞肺癌患者中的抗肿瘤效应,KEYNOTE 158研究进一步证实了帕博利珠单抗在PD-L1表达阳性小细胞肺癌患者获益更多[14];但CheckMate 032[13]、IMpower 133[15]、CASPIAN[16]研究显示免疫治疗疗效与PD-L1表达无关。目前尚无充分证据表明PD-L1表达水平与小细胞肺癌免疫治疗疗效具有相关性。寻找一个有效预测小细胞肺癌免疫治疗疗效的指标是临床医务工作者面临的重要问题。目前FDA批准TMB作为泛瘤种免疫疗效预测指标[28]。Peifer等[29]研究显示小细胞肺癌较非小细胞肺癌突变负荷更高。Hellmann等[30]、Ricciuti等[31]研究显示TMB高的小细胞肺癌患者接受免疫治疗后获益更多。但目前有关TMB预测小细胞肺癌免疫治疗疗效研究较少,需要进一步的研究去确定TMB在小细胞肺癌免疫治疗中的预测价值。
综上,PD-1抑制剂联合安罗替尼治疗晚期神经内分泌癌显示出一定的疗效,耐受性好,可作为晚期神经内分泌癌标准方案治疗失败后的一种选择,但仍需进行随机对照研究进一步证实其疗效及安全性。
作者贡献
余旭旭:数据收集、随访、统计分析、论文撰写
李向柯、杨闽洁:论文修改
陈钟、毛迎港:数据收集
宋丽杰:课题设计、经费支持
| [1] |
Klöppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications[J]. Visc Med, 2017, 33(5): 324-330. DOI:10.1159/000481390 |
| [2] |
Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35, 825 cases in the United States[J]. J Clin Oncol, 2008, 26(18): 3063-3072. DOI:10.1200/JCO.2007.15.4377 |
| [3] |
翟雪佳, 于顺利, 马怡晖, 等. 神经内分泌瘤488例临床病理特征及预后分析[J]. 中华医学杂志, 2019, 99(32): 2527-2531. [Zhai XJ, Yu XL, Ma YH, et al. Clinicopathological features and prognosis of 488 patients with neuroendocrine tumors[J]. Zhonghua Yi Xue Za Zhi, 2019, 99(32): 2527-2531. DOI:10.3760/cma.j.issn.0376-2491.2019.32.012] |
| [4] |
Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems[J]. Pancreas, 2010, 39(6): 707-712. DOI:10.1097/MPA.0b013e3181ec124e |
| [5] |
Saeger W, Schnabel PA, Komminoth P. Grading of neuroendocrine tumors[J]. Pathologe, 2016, 37(4): 304-313. DOI:10.1007/s00292-016-0186-4 |
| [6] |
Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018[J]. J Natl Compr Canc Netw, 2018, 16(6): 693-702. DOI:10.6004/jnccn.2018.0056 |
| [7] |
Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart[J]. J Thorac Oncol, 2015, 10(9): 1240-1242. DOI:10.1097/JTO.0000000000000663 |
| [8] |
2013年中国胃肠胰神经内分泌肿瘤病理诊断共识专家组. 中国胃肠胰神经内分泌肿瘤病理诊断共识(2013版)[J]. 中华病理学杂志, 2013, 42(10): 691-694. [Chinese Expert Group on Pathological Diagnosis of Gastrointestinal Pancreatic Neuroendocrine Tumors. Chinese Consensus on Pathological Diagnosis of Gastrointestinal Pancreatic Neuroendocrine Tumors (2013 Edition)[J]. Zhonghua Bing Li Xue Za Zhi, 2013, 42(10): 691-694. DOI:10.3760/cma.j.issn.0529-5807.2013.10.011] |
| [9] |
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247. DOI:10.1016/j.ejca.2008.10.026 |
| [10] |
Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States[J]. JAMA Oncol, 2017, 3(10): 1335-1342. DOI:10.1001/jamaoncol.2017.0589 |
| [11] |
Dasari A, Mehta K, Byers LA, et al. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162, 983 cases[J]. Cancer, 2018, 124(4): 807-815. DOI:10.1002/cncr.31124 |
| [12] |
张剑, 臧凤琳, 张家丽, 等. 分化差的胃神经内分泌肿瘤预后分析[J]. 肿瘤防治研究, 2019, 46(5): 447-451. [Zhang J, Zang FL, Zhang J, et al. [ZHANG Prognosis of Poorly-differentiated Gastric Neuroendocrine Neoplasm[J]. Zhong Liu Fang Zhi Yan Jiu, 2019, 46(5): 447-451. DOI:10.3971/j.issn.1000-8578.2019.18.1770] |
| [13] |
Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial[J]. Lancet Oncol, 2016, 17(7): 883-895. DOI:10.1016/S1470-2045(16)30098-5 |
| [14] |
Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies[J]. J Thorac Oncol, 2020, 15(4): 618-627. DOI:10.1016/j.jtho.2019.12.109 |
| [15] |
Mansfield AS, Każarnowicz A, Karaseva N, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phaseⅠ/Ⅲ trial[J]. Ann Oncol, 2020, 31(2): 310-317. DOI:10.1016/j.annonc.2019.10.021 |
| [16] |
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial[J]. Lancet, 2019, 394(10212): 1929-1939. DOI:10.1016/S0140-6736(19)32222-6 |
| [17] |
Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial[J]. JAMA Oncol, 2018, 4(11): 1569-1575. DOI:10.1001/jamaoncol.2018.3039 |
| [18] |
Chi Y, Fang Z, Hong X, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma[J]. Clin Cancer Res, 2018, 24(21): 5233-5238. DOI:10.1158/1078-0432.CCR-17-3766 |
| [19] |
Sun Y, Du F, Gao M, et al. Anlotinib for the Treatment of Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer[J]. Thyroid, 2018, 28(11): 1455-1461. DOI:10.1089/thy.2018.0022 |
| [20] |
Syed YY. Anlotinib: First Global Approval[J]. Drugs, 2018, 78(10): 1057-1062. DOI:10.1007/s40265-018-0939-x |
| [21] |
Elamin YY, Rafee S, Toomey S, et al. Immune effects of bevacizumab: killing two birds with one stone[J]. Cancer Microenviron, 2015, 8(1): 15-21. DOI:10.1007/s12307-014-0160-8 |
| [22] |
Heine A, Held SA, Bringmann A, et al. Immunomodulatory effects of anti-angiogenic drugs[J]. Leukemia, 2011, 25(6): 899-905. DOI:10.1038/leu.2011.24 |
| [23] |
Kerr KM, Nicolson MC. Non-Small Cell Lung Cancer, PD-L1, and the Pathologist[J]. Arch Pathol Lab Med, 2016, 140(3): 249-254. DOI:10.5858/arpa.2015-0303-SA |
| [24] |
Kerr KM, Hirsch FR. Programmed Death Ligand-1 Immunohistochemistry: Friend or Foe?[J]. Arch Pathol Lab Med, 2016, 140(4): 326-331. DOI:10.5858/arpa.2015-0522-SA |
| [25] |
Cheng Y, Wang Q, Li K, et al. OA13.03 Anlotinib as Third-Line or Further-Line Treatment in Relapsed SCLC: A Multicentre, Randomized, Double-Blind Phase 2 Trial[J]. J Thorac Oncol, 2018, 13(10): S351-S352. |
| [26] |
Gao L, Yang X, Yi C, et al. Adverse Events of Concurrent Immune Checkpoint Inhibitors and Antiangiogenic Agents: A Systematic Review[J]. Front Pharmacol, 2019, 10: 1173. DOI:10.3389/fphar.2019.01173 |
| [27] |
Bonanno L, Pavan A, Dieci MV, et al. The role of immune microenvironment in small-cell lung cancer: Distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes[J]. Eur J Cancer, 2018, 101: 191-200. DOI:10.1016/j.ejca.2018.06.023 |
| [28] |
Subbiah V, Solit DB, Chan TA, et al. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: a decision centered on empowering patients and their physicians[J]. Ann Oncol, 2020, 31(9): 1115-1118. DOI:10.1016/j.annonc.2020.07.002 |
| [29] |
Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer[J]. Nat Genet, 2012, 44(10): 1104-1110. DOI:10.1038/ng.2396 |
| [30] |
Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer[J]. Cancer Cell, 2018, 33(5): 853-861. DOI:10.1016/j.ccell.2018.04.001 |
| [31] |
Ricciuti B, Kravets S, Dahlberg SE, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer[J]. J Immunother Cancer, 2019, 7(1): 87. DOI:10.1186/s40425-019-0572-6 |
 2021, Vol. 48
2021, Vol. 48


