文章信息
- 膀胱癌靶向分子载体的研究进展
- Research Progress of Targeted Molecular Carrier for Bladder Cancer
- 肿瘤防治研究, 2021, 48(3): 299-302
- Cancer Research on Prevention and Treatment, 2021, 48(3): 299-302
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2021.20.0681
- 收稿日期: 2020-06-15
- 修回日期: 2020-11-23
2. 030000 太原,山西医科大学第一医院泌尿外科
2. Department of Urology, The First Hospital of Shanxi Medical University, Taiyuan 030000, China
膀胱癌(bladder cancer, BC)是中国泌尿生殖系统常见的恶性肿瘤之一[1],约75%新诊断的BC为非肌层浸润性膀胱癌(non-muscle invasive bladder cancer, NMIBC),局限于膀胱壁黏膜或黏膜下层,主要治疗手段为经尿道膀胱肿瘤切除术(transurethral resection of the bladder tumor, TURBT)联合膀胱内灌注化疗药物。但TURBT术后1年肿瘤复发率为40%~80%,25%的患者进展为肌层浸润性膀胱癌(muscle invasive bladder cancer, MIBC)[2]。目前普通白光膀胱镜检查是BC诊断和术后随访的标准技术手段,其对微小肿瘤、原位癌、术中残留肿瘤的识别能力有限,导致肿瘤的漏诊,而常规膀胱内灌注药物的不良反应严重影响患者术后生活质量[3]。因此,如何精准地发现病灶及界定肿瘤的边界、既能彻底切除肿瘤又能减少正常组织细胞的损伤成为了BC临床诊疗亟需解决的问题。
随着BC靶向分子载体、靶向特异性作用机制、靶向光学分子影像和靶向光免疫疗法(photoimmunotherapy, PIT)研究的不断深入,BC的靶向诊疗有望取得新的进展。为此,本文结合国内外最新文献及本研究组的前期工作,将已发现的BC靶向分子载体的研究现状综述如下。
1 靶向基团的种类及特性靶向分子载体是指能够特异识别抗原蛋白并与之紧密结合的靶向基团,主要包括抗体、抗体片段、支架蛋白、肽和小分子等,见表 1[4]。抗体与抗原特异性结合以及成熟的抗体制备技术,使其成为临床应用最多的一类靶向基团,但单克隆抗体分子量较大(约150 kDa)、肾脏不能排泄、体内主要通过肝脏分解代谢,导致抗体具有血液内循环时间长、非特异性组织蓄积和组织穿透能力差等缺点[5-6]。而采用化学技术制备的抗体片段(微抗体、抗原结合片段、双抗体、单链可变片段等),分子量约为15~80 kDa,既保留了完整抗体特异性结合抗原的生物学特性,又具有组织渗透性强和血液滞留时间短的优点[7]。
另外,最新关注的靶向基团还有非免疫球蛋白类支架蛋白(scaffolds protein),如亲和体、结蛋白和Centyrin等[8-10],这些蛋白支架既有类似抗体的靶向功能,又具有相对分子量小、高亲和力、折叠速率快、理化性能稳定、能接受化学修饰等优点。
此外,肽类和小分子的分子量最小,能与肿瘤特异性结合,而不与或很少与正常组织、细胞结合的小分子肽被称为肿瘤导向肽(tumor homing peptide, THP)。THP为基础的载体系统能够有效穿透组织且几乎无免疫原性,其半衰期短、肾清除率快、组织蓄积少以及生产工艺便捷、成本更低等优势[11],具有良好的应用前景。
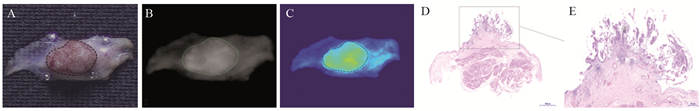
2 膀胱癌靶向分子载体 2.1 抗体及其衍生物CD47是一种普遍存在于人类细胞膜表面的跨膜糖蛋白,与巨噬细胞表面的信号调节蛋白α结合后能抑制其吞噬功能使肿瘤产生免疫逃逸[12]。CD47在80%以上的BC细胞膜上高表达,包括乳头状尿路上皮癌、鳞状细胞癌、微乳头状瘤及腺癌[13]。本项目组使用Alexa Fluor 790标记的抗-CD47孵育26例新鲜离体人BC组织进行近红外荧光成像,肿瘤组织的平均荧光强度明显高于临近正常组织(分别为132.31±6.67和52.27±12.09, P<0.001),见图 1[14]。Pan等[13]评价了抗-CD47-Qdot625介导的内镜分子成像检测BC的诊断准确性,21例新鲜完整膀胱标本经抗-CD47-Qdot625孵育后,在蓝光下检测到119个可疑区域,通过病理学验证,其诊断敏感度和特异性分别为82.9%和90.5%。
|
| A: The image of tumor specimen under white light; B: After anti-CD47-Alexa Fluor 790 incubating, the image of tumor specimen under NIR light; C: After anti-CD47-Alexa Fluor 790 incubating, the pseudo-color image of fresh integrated tumor specimen under NIR light; D: The HE staining image of fresh integrated tumor specimen; E: The HE staining representative image of tumor area; NIR: near-infrared. 图 1 膀胱癌组织图像 Figure 1 Images of bladder cancer tissues |
CAⅨ是碳酸酐酶家族中的一员,在缺氧条件下调节细胞内pH值,进而改变肿瘤细胞的黏附、增殖和进展的生物学特性[15]。免疫组织化学染色显示CAⅨ在BC组织中的阳性率高达67.1%,在正常的尿路上皮和膀胱慢性炎性病变组织中均呈阴性(P<0.01)[16]。Wang等[17]在8例新鲜完整膀胱标本内灌注抗-CAⅨ-Qdot625,白光膀胱镜诊断BC的敏感度为76.0%,特异性为90.5%,而抗-CAⅨ介导的蓝光内镜分子成像下的肿瘤检测获得了较高的诊断准确率,总体敏感度和特异性分别为88.00%和93.75%。
PIT是一种新型的分子靶向光动力治疗模式,亲水性酞菁染料IR700与单克隆抗体结合进行的靶向性PIT,有效减少了单纯IR700光动力疗法的不良反应[18]。Kiss等[19]研究发现,抗-CD47-IR700介导的PIT治疗,IR700使用剂量低,但显著增加了人膀胱肿瘤细胞系和原代膀胱肿瘤细胞的细胞毒作用。通过尾静脉注射抗-CD47-IR700对BC异种移植小鼠模型进行近红外光免疫治疗(NIR-PIT),单次治疗发现NIR-PIT组肿瘤生长明显减慢,在连续5周的治疗后,NIR-PIT能够有效抑制肿瘤生长(P=0.0104),明显延长治疗组小鼠的生存期(P=0.009)[20]。
单链可变片段(single-chain variable fragment, scFv)是抗体内部结合抗原的最小功能结构域,Rezaei等[20]开发了新型抗-CD47-scFv磁性纳米粒子(magnetic nanoparticles, MNP),体外研究表明抗-CD47-scFv-MNP对BC细胞系EJ138和5637具有高亲和力,经外磁场作用的靶向热疗后能显著降低肿瘤细胞存活率。
2.2 肿瘤导向肽(THP)目前,利用噬菌体展示肽库技术和组合化学方法,先后筛选出四种与BC结合的靶向配体,分别是九肽序列Bld-1[21]、环九肽序列PLZ4[22]、环七肽序列NYZL1[23]和PLSWT7[24]。其中前两种筛选自MIBC细胞系,后两种则来自NMIBC细胞系。研究显示,四种THP均能在离体细胞、组织和小鼠体内与BC特异性结合,但它们结合肿瘤细胞的分子位点目前还不清楚[21-24]。
PLSWT7是目前唯一运用于内镜下分子成像的多肽载体。Peng等[24]将PLSWT7-IRDye800CW分子探针灌注入8例离体人膀胱腔内,分析了40个膀胱内感兴趣区域,进行了近红外分子成像诊断与组织病理学比较研究,发现PLSWT7-IRDye800CW分子影像诊断BC的敏感度和特异性分别为84.00%和86.70%。
常规尿液脱落细胞检查是一种简单易行的非侵入性诊断方法,但检测的敏感度较低。Jia等[25]使用Bld-1-FITC探针结合80例血尿患者尿液中的肿瘤细胞,其诊断BC的敏感度和特异性分别为79.31%和100%,优于尿脱落细胞学检查和荧光原位杂交技术检查(敏感度分别为20.69%和72.41%)。另外一项包含66例BC的研究发现,NYZL1-FITC探针与尿液中肿瘤细胞的结合与肿瘤恶性程度成正比,平均阳性结合百分比在Ta、T1、T2和T3~T4期分别为30%、57%、73%和85%[26]。
在靶向化疗方面,Bld-1能够与凋亡肽KLA和阿霉素(DOX)构建靶向制剂,并内化进入人膀胱癌HT1376细胞[27-28]。Jung等[27]评价了Bld-1-KLA在小鼠体内的治疗效能,静脉注射给药4周后,Bld-1-KLA组小鼠的肿瘤体积明显小于对照组(P<0.001)。Wei等[28]使用Bld-1-DOX治疗荷瘤小鼠16天后,Bld-1-DOX组的肿瘤体积明显缩小,且小鼠无明显不良反应,而单用DOX治疗组出现明显的心脏和肝脏损害。Lin等[29]开发了PLZ4靶向纳米胶束,在靶向递送方面的效率是非靶向胶束的1.5倍(P<0.05)且是游离染料的14.3倍(P<0.01),靶向胶束不仅能黏附在肿瘤细胞表面,还能被靶细胞摄取。使用PLZ4纳米胶束介导的紫杉醇(PTX)进行靶向化疗,成功将荷瘤小鼠的中位存活期从单纯紫杉醇治疗组的55天提高到了靶向治疗组的69.5天(P=0.03)[30]。
术中光学分子影像引导的外科手术被认为是继开放手术、微创外科手术之后的第三代外科手术新模式。传统的依据白光内窥镜图像和操作者的经验确定肿瘤浸润深度和手术切缘的方式具有主观性。一项涉及8 490例经TURBT治疗BC患者的系统回顾显示,17%~67%的初发Ta期患者和20%~71%的初发T1期患者在复发时发现了残留肿瘤,36%~86%残留肿瘤位于初次切除部位[3]。Peng[24]等采用PLSWT7-IRDye800CW进行了分子成像引导BC手术治疗的临床前研究,将小鼠异种移植模型随机分为两组:对照组(n=20)和实验组(n=20)分别在自然光和光学分子成像下进行肿瘤切除术。1周后,对照组和实验组肿瘤复发率分别为95%和5%;术后30天两组的总存活率分别为0和90%。
3 膀胱癌的多载体靶向和多模态诊疗BC具有较强的异质性,单纯的一种靶向分子载体很难使全部患者受益,使用抗-CD47、抗-CAⅨ和无靶向性IgG4三种单抗对离体BC组织进行多路复合分子成像发现,诊断图像较单靶点成像噪声更低,分辨率更加精准(ROC AUC为0.93(0.73, 1.0))[31]。多模态分子探针能集成多种成像和(或)治疗模式,Lin等[32]开发的PLZ4-纳米卟啉平台,在进行BC光动力学诊断的同时实现BC的靶向光动力学治疗、靶向光热治疗和靶向化疗结合的三模态治疗,显著提升BC的临床诊断水平和治疗水平。
4 展望与挑战综上所述,靶向分子载体介导的靶向性定位功能在膀胱癌早期诊断、完整切除、靶向治疗等方面具有巨大的应用潜力,但仍有许多科学问题和技术难点需要解决,比如靶向载体、连接体、荧光染料等的安全性验证,临床有效性的标准化评价体系的建立等,需要临床医生、化学家、物理学家、药理学家和工程师之间的多学科交叉协作。
作者贡献
闫鹏宇:论文撰写
闫旭韬、杨勇军:资料获取与论文修改
杨晓峰:论文审校
| [1] |
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [2] |
Soukup V, Čapoun O, Cohen D, et al. Prognostic Performance and reproducibility of the 1973 and 2004/2016 World Health Organisation Grading Classification Systems in Non-muscle-invasive Bladder Cancer: A European Association of Urology Non Muscle invasive bladder cancer guidelines Panel Systematic Review[J]. Eur Urol, 2017, 72(5): 801-813. DOI:10.1016/j.eururo.2017.04.015 |
| [3] |
Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat Transurethral Resection in Non-muscle-invasive Bladder Cancer: A Systematic Review[J]. Eur Urol, 2018, 73(6): 925-933. DOI:10.1016/j.eururo.2018.02.014 |
| [4] |
Hernot S, van Manen L, Debie P, et al. Latest developments in molecular tracers for fluorescence image-guided cancer surgery[J]. Lancet Oncol, 2019, 20(7): e354-e367. DOI:10.1016/S1470-2045(19)30317-1 |
| [5] |
Ryman JT, Meibohm B. Pharmacokinetics of Monoclonal Antibodies[J]. CPT Pharmacometrics Syst Pharmacol, 2017, 6(9): 576-588. DOI:10.1002/psp4.12224 |
| [6] |
Xenaki KT, Oliveira S, van Bergen En Henegouwen PMP. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors[J]. Front Immunol, 2017, 8: 1287. DOI:10.3389/fimmu.2017.01287 |
| [7] |
Long NE, Sullivan BJ, Ding H, et al. Linker engineering in anti-TAG-72 antibody fragments optimizes biophysical properties, serum half-life, and high-specificity tumor imaging[J]. J Biol Chem, 2018, 293(23): 9030-9040. DOI:10.1074/jbc.RA118.002538 |
| [8] |
Samkoe KS, Gunn JR, Marra K, et al. Toxicity and Pharmacokinetic Profile for Single-Dose Injection of ABY-029: a Fluorescent Anti-EGFR Synthetic Affibody Molecule for Human Use[J]. Mol Imaging Biol, 2017, 19(4): 512-521. DOI:10.1007/s11307-016-1033-y |
| [9] |
Tummers WS, Kimura RH, Abou-Elkacem L, et al. Development and preclinical validation of a cysteine knottin peptide targeting Integrin αvβ6 for near-infrared fluorescent-guided surgery in pancreatic cancer[J]. Clin Cancer Res, 2018, 24(7): 1667-1676. DOI:10.1158/1078-0432.CCR-17-2491 |
| [10] |
Mahalingam SM, Dudkin VY, Goldberg S, et al. Evaluation of a centyrin-based near-infrared probe for fluorescence-guided surgery of epidermal growth factor receptor positive tumors[J]. Bioconjug Chem, 2017, 28(11): 2865-2873. DOI:10.1021/acs.bioconjchem.7b00566 |
| [11] |
苗婕, 张瑞, 杨晓峰. 肿瘤导向肽的研究进展[J]. 生命的化学, 2016, 36(2): 178-184. [Miao J, Zhang R, Yang XF. The research progress of tumor homing peptides[J]. Sheng Ming De Hua Xue, 2016, 36(2): 178-184.] |
| [12] |
Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors[J]. Proc Natl Acad Sci USA, 2012, 109(17): 6662-6667. DOI:10.1073/pnas.1121623109 |
| [13] |
Pan Y, Volkmer JP, Mach KE, et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody[J]. Sci Transl Med, 2014, 6(260): 260ra148. DOI:10.1126/scitranslmed.3009457 |
| [14] |
Yang Y, Yang X, Liu C, et al. Preliminary study on the application of en bloc resection combined with near-infrared molecular imaging technique in the diagnosis and treatment of bladder cancer[J]. World J Urol, 2020. Online ahead of print.
|
| [15] |
Mboge MY, Mahon BP, McKenna R, et al. Carbonic Anhydrases: Role in pH Control and Cancer[J]. Metabolites, 2018, 8(1): 19. DOI:10.3390/metabo8010019 |
| [16] |
章萍萍, 黄文斌, 王晓蕾, 等. 膀胱尿路上皮癌中碳酸酐酶Ⅸ的表达及意义[J]. 临床与实验病理学杂志, 2018, 34(3): 317-318. [Zhang PP, Huang WB, Wang XL, et al. Expression and Significance of Carbonic Anhydrase Ⅸ in Bladder Urothelial Carcinoma[J]. Lin Chuang Yu Shi Yan Bing Li Xue Za Zhi, 2018, 34(3): 317-318.] |
| [17] |
Wang J, Fang R, Wang L, et al. Identification of Carbonic Anhydrase IX as a Novel Target for Endoscopic Molecular Imaging of Human Bladder Cancer[J]. Cell Physiol Biochem, 2018, 47(4): 1565-1577. DOI:10.1159/000490875 |
| [18] |
Kobayashi H, Choyke PL. Near-Infrared Photoimmunotherapy of Cancer[J]. Acc Chem Res, 2019, 52(8): 2332-2339. DOI:10.1021/acs.accounts.9b00273 |
| [19] |
Kiss B, van den Berg NS, Ertsey R, et al. CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer[J]. Clin Cancer Res, 2019, 25(12): 3561-3571. DOI:10.1158/1078-0432.CCR-18-3267 |
| [20] |
Rezaei G, Habibi-Anbouhi M, Mahmoudi M, et al. Development of anti-CD47 single-chain variable fragment targeted magnetic nanoparticles for treatment of human bladder cancer[J]. Nanomedicine (Lond), 2017, 12(6): 597-613. DOI:10.2217/nnm-2016-0302 |
| [21] |
Lee SM, Lee EJ, Hong HY, et al. Targeting bladder tumor cells in vivo and in the urine with a peptide identified by phage display[J]. Mol Cancer Res, 2007, 5(1): 11-19. DOI:10.1158/1541-7786.MCR-06-0069 |
| [22] |
Zhang H, Aina OH, Lam KS, et al. Identification of a bladder cancer-specific ligand using a combinatorial chemistry approach[J]. Urol Oncol, 2012, 30(5): 635-645. DOI:10.1016/j.urolonc.2010.06.011 |
| [23] |
Yang X, Zhang F, Luo J, et al. A new non-muscle-invasive bladder tumor-homing peptide identified by phage display in vivo[J]. Oncol Rep, 2016, 36(1): 79-89. DOI:10.3892/or.2016.4829 |
| [24] |
Peng L, Shang W, Guo P, et al. Phage Display-Derived Peptide-Based Dual-Modality Imaging Probe for Bladder Cancer Diagnosis and Resection Postinstillation: A Preclinical Study[J]. Mol Cancer Ther, 2018, 17(10): 2100-2111. DOI:10.1158/1535-7163.MCT-18-0212 |
| [25] |
Jia XY, Yu Q, Zhang ZH, et al. Targeting bladder tumor cells in voided urine of Chinese patients with FITC-CSNRDARRC peptide ligand[J]. Onco Targets Ther, 2012, 5: 85-90. |
| [26] |
罗芳, 张帆, 罗俊茜, 等. FITC-NYZL1分子探针标记膀胱癌患者尿脱落细胞特异性的研究[J]. 现代泌尿外科杂志, 2015, 20(11): 775-778. [Luo F, Zhang F, Luo JQ, et al. The specificity of targeting urine exfoliated cells with FITC-NYZL1[J]. Xian Dai Mi Niao Wai Ke Za Zhi, 2015, 20(11): 775-778.] |
| [27] |
Jung HK, Kim S, Park RW, et al. Bladder tumor-targeted delivery of pro-apoptotic peptide for cancer therapy[J]. J Control Release, 2016, 235: 259-267. DOI:10.1016/j.jconrel.2016.06.008 |
| [28] |
Wei Y, Gao L, Wang L, et al. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy[J]. Drug Deliv, 2017, 24(1): 681-691. DOI:10.1080/10717544.2017.1309475 |
| [29] |
Lin TY, Zhang H, Luo J, et al. Multifunctional targeting micelle nanocarriers with both imaging and therapeutic potential for bladder cancer[J]. Int J Nanomedicine, 2012, 7: 2793-2804. |
| [30] |
Pan A, Zhang H, Li Y, et al. Disulfide-crosslinked nanomicelles confer cancer-specific drug delivery and improve efficacy of paclitaxel in bladder cancer[J]. Nanotechnology, 2016, 27(42): 425103. DOI:10.1088/0957-4484/27/42/425103 |
| [31] |
Davis RM, Kiss B, Trivedi DR, et al. Surface-Enhanced Raman Scattering Nanoparticles for Multiplexed Imaging of Bladder Cancer Tissue Permeability and Molecular Phenotype[J]. ACS Nano, 2018, 12(10): 9669-9679. DOI:10.1021/acsnano.8b03217 |
| [32] |
Lin TY, Li Y, Liu Q, et al. Novel theranostic nanoporphyrins for photodynamic diagnosis and trimodal therapy for bladder cancer[J]. Biomaterials, 2016, 104: 339-351. DOI:10.1016/j.biomaterials.2016.07.026 |
 2021, Vol. 48
2021, Vol. 48