文章信息
- miR-214通过PTEN调控AKT信号通路抑制鼻咽癌细胞凋亡
- miR-214 Inhibits Apoptosis of Nasopharyngeal Carcinoma Cells by Activating AKT Signaling Pathway Through Targeting PTEN
- 肿瘤防治研究, 2020, 47(10): 734-739
- Cancer Research on Prevention and Treatment, 2020, 47(10): 734-739
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2020.20.0149
- 收稿日期: 2020-03-01
- 修回日期: 2020-06-16
鼻咽癌(nasopharyngeal carcinoma, NPC)是一种来源于鼻咽上皮细胞的恶性肿瘤。大量研究证据表明EB病毒感染是鼻咽癌发病的重要原因[1]。早期鼻咽癌可通过临床同步放化疗得到较好的控制,但复发、转移的中晚期鼻咽癌患者预后仍有待提高[2]。因此,研究鼻咽癌新的治疗靶点及其潜在的作用机制,以更好地控制中晚期鼻咽癌的进展乃是当务之急。
MicroRNAs(miRNAs)是一类长度约为20 nt的非编码小RNA,可通过特异性结合于mRNA的3’-untranslation region(UTR)进行翻译抑制和降解,从而调控靶基因的表达[3]。越来越多的研究表明[4-6],miRNAs在多种人类癌症发病机制中起到重要调控作用, 涉及肿瘤细胞增殖、侵袭和凋亡等复杂的生物学调控过程。miR-214近期被研究发现可作为多种人类癌症治疗的潜在靶点以及预后判断的标志物[7-9]。根据生物信息学分析和既往研究发现,miR-214可以调节PTEN的表达水平[8]。PTEN是酪氨酸磷酸酶受体(receptor type tyrosine phosphatase, PTPR)的一员,可以使AKT去磷酸化,诱导AKT信号通路失活[10-11]。miR-214与PTEN/AKT信号调控通路在鼻咽癌发展中的作用机制尚未完全阐明。本研究拟验证miR-214是否通过抑制PTEN基因转录激活AKT信号通路,进而抑制鼻咽癌细胞凋亡,为中晚期鼻咽癌分子靶向治疗提供新的实验依据。
1 材料与方法 1.1 细胞培养鼻咽癌5-8F和6-10B细胞株源自中南大学湘雅中心实验室细胞库。两株鼻咽癌细胞均保存于RPMI 1640,并添加10%胎牛血清(Invitrogen, CA, USA)和100 u/ml青霉素-链霉素双抗(碧云天, 中国),在37℃、5%CO2培养箱培养。
1.2 细胞转染PTEN shRNA、miR-214 inhibitor及其阴性对照(NC)购自上海GenePharma公司。miR-214 inhibitor(hsa-miR-214-3pinhibitor)是一种经化学修饰的单链小RNA分子,特异性抑制成熟的miR-214-3p,可导致细胞转染后的miR-214敲低。鼻咽癌5-8F和6-10B细胞株接种于6孔板24 h,转染前达到80%融合。根据操作说明,应用Lipofectamine 2000(Invitrogen, CA, USA)将质粒转染到鼻咽癌细胞,转染48 h后进行后续实验。
1.3 MTT法检测细胞增殖将处理后的细胞以培养液重悬调整细胞密度为1×105/ml,接种于96孔板中,每孔加入180 μl(约1.8×104个细胞)培养液继续培养,不同时间点时每孔加入20 μl MTT(Sigma-Aldrich, CA, USA),继续于37℃培养箱中孵育4 h,弃上清液后每孔加入150 μl DMSO,Victor3 1420酶标仪(Perkin Elmer, CA, USA)检测490 nm吸光度值。
1.4 细胞凋亡检测鼻咽癌细胞转染48 h后,收集各组细胞转移至2 ml EP管,1 000 r/min离心5 min,弃上清液,后续按照Annexin V-FITC/PI凋亡试剂盒(碧云天, 中国)操作说明处理细胞,流式细胞术检测细胞凋亡。
1.5 荧光素酶实验扩增含有miR-214结合位点的PTEN mRNA野生型和突变型的3'-UTR片段,然后转染到pGL3-basic载体(Promega, WI, USA)。取1×106/ml 5-8F和6-10B鼻咽癌细胞1 ml接种于24孔板孵育24 h,使用Lipofectamine 2000共转染pGL3-basic载体和miR-214 inhibitor或miR-214 NC。转染48 h后裂解细胞,按照双荧光素酶检测试剂盒(Promega, WI, USA)操作说明处理样本,酶标仪检测荧光强度,计算Firefly/Renilla比值。通过荧光素酶活性的高低来判断抑制剂对目的基因的转录调控强度。
1.6 RNA提取和Real-time PCR检测用TRIzol试剂(Invitrogen, CA, USA)从细胞中提取总RNA。应用一步法PrimeScript miRNA cDNA Synthesis试剂盒(TaKaRa, Shiga, Japan)合成miRNA-214的互补DNA(cDNA),高通量cDNA反转录试剂盒(Applied Biosystems, Foster City, CA)用于PTEN基因的反转录合成。使用SYBR Green Real-time PCR Master Mix(TaKaRa, Shiga, Japan)试剂于Applied Biosystems 7500 Real-Time PCR系统(Thermo Fisher Scientific, MA, USA)上进行实时定量Real-time PCR检测。GAPDH和U6 snRNA分别作为内参,采用2-ΔΔCt法测定PTEN mRNA以及miRNA-214相对表达量。相关引物序列见表 1。
提取处理后的细胞总蛋白,采用BCA法测定蛋白浓度后配平,取蛋白质样品40 µl、Marker 5 µl加入泳道,用8%或10%SDS-PAGE分离胶进行电泳、转膜、封闭,洗涤后加入一抗PTEN(abcam, ab31392, 1:1 000)、p-AKTS473(abcam, ab81283, 1:1 000)、p-AKTT308(abcam, ab38449, 1:1 000)、AKT(abcam, ab8805, 1:1 000)、Bcl-2(abcam, ab196495, 1:1 000)、Bax(abcam, ab53154, 1:1 000)、Caspase-3(abcam, ab13847, 1:500)、Caspase-9(abcam, ab52298, 1:1 000)和GAPDH(abcam, ab181602, 1:5000)后4℃摇床孵育过夜,洗涤后加入荧光标记二抗(1:8 000),加入二抗后开始全程避光,室温孵育1 h,洗涤后用Odyssey双色红外激光成像系统(LI-COR, USA)进行扫描和分析。使用Quantity One软件对条带进行定量分析。
1.8 统计学方法所有数据以均数±标准差(x±s)表示。两组间的差异比较采用t检验(双尾检验),多组间的两两比较应用单因素方差分析时采用SNK法和Dunnett法比较。所有实验至少重复三次,统计分析采用Prism 6.0(GraphPad)软件,P < 0.05为差异有统计学意义。
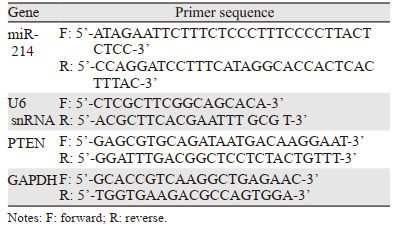
2 结果 2.1 miR-214抑制剂可以显著抑制鼻咽癌细胞的增殖miR-214 NC或miR-214 inhibitor转染5-8F和6-10B鼻咽癌细胞后,Real-time PCR检测结果显示,转染miR-214 inhibitor的两组细胞中miR-214的表达均明显低于对照组或NC组,见图 1A~B。MTT测定结果显示抑制miR-214表达可以明显减弱两种鼻咽癌细胞的增殖能力,见图 1C~D。
|
| A, B: relative miR-214 expression levels in 5-8F and 6-10B cells were measured using Real-time PCR; C, D: cell proliferation was measured using MTT assay after miR-214 inhibitor transfection; ***: P < 0.001, compared with NC or Control group. 图 1 miR-214抑制剂可以显著抑制鼻咽癌细胞的增殖 Figure 1 miR-214 inhibitor suppressed proliferation of nasopharyngeal carcinoma cell lines |
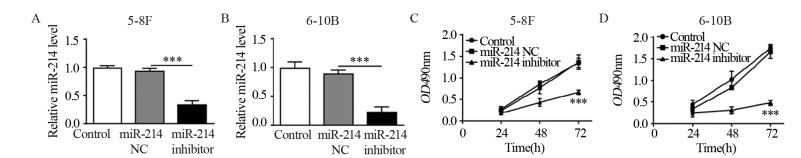
Annexin-V/PI双染法流式细胞仪检测不同处理条件下两种细胞的凋亡情况。转染miR-214 inhibitor的两株细胞凋亡率均显著高于转染miR-214 NC及空白对照组细胞,见图 2A。Western blot结果显示,在转染miR-214 inhibitor后,细胞凋亡相关蛋白Cleaved caspase-3、Cleaved caspase-9和Bax显著上调,而Bcl-2蛋白表达显著降低,见图 2B。结果表明,抑制miR-214的表达可以诱导鼻咽癌细胞凋亡。
|
| A: the apoptosis of 5-8F and 6-10B cells were determined by Annexin-V/PI assay using flow cytometry; B: the expression of apoptosis-related proteins in 5-8F and 6-10B cells after miR-214 inhibitor transfection; ***: P < 0.001, **: P < 0.01. 图 2 抑制miR-214表达可诱导鼻咽癌细胞凋亡 Figure 2 Inhibition of miR-214 induced apoptosis of nasopharyngeal carcinoma cell lines |
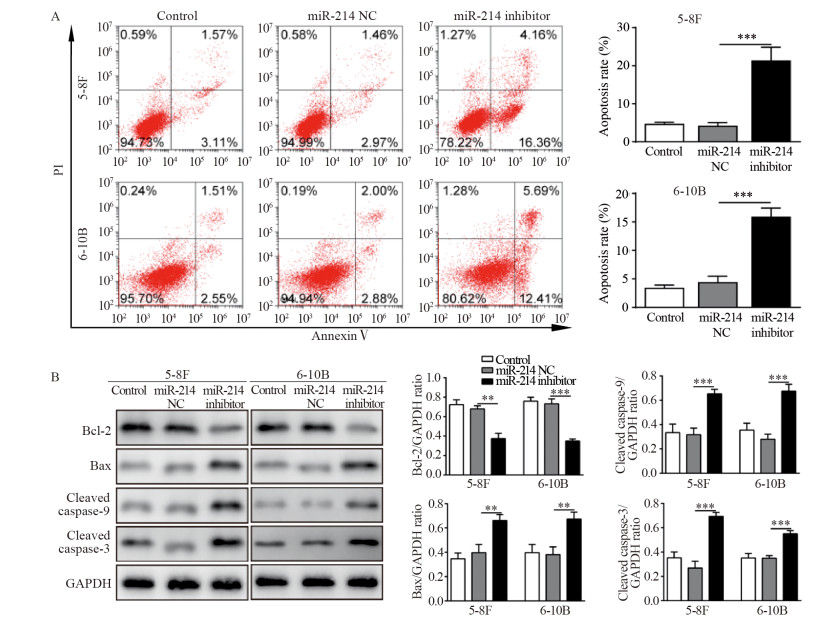
在5-8F细胞中沉默PTEN基因表达,对miR-214的表达没有显著影响,而miR-214 inhibitor+shPTEN共转组细胞中PTEN的mRNA水平明显低于miR-214 inhibitor转染组,抑制miR-214可以上调PTEN mRNA表达,见图 3A。miR-214 inhibitor对鼻咽癌细胞的凋亡促进作用也因转染shPTEN而受到抑制,见图 3B。结果表明,PTEN基因沉默显著逆转miR-214 inhibitor对鼻咽癌5-8F细胞的促凋亡作用,提示PTEN可能在miR-214鼻咽癌凋亡调控中起到主要作用。
|
| A: PTEN gene silencing had no effect on the expression of miR-214, while miR-214 inhibited the transcription of PTEN gene; B: shPTEN reversed the effect of miR-214 inhibitor on the apoptosis of 5-8F cells; ***: P < 0.001. 图 3 沉默PTEN基因表达逆转miR-214抑制剂对5-8F细胞凋亡的影响 Figure 3 Knockdown of PTEN reversed the effects of miR-214 inhibitor on apoptosis of 5-8F cell line |
TargetScan网站预测miR-214与PTEN的3’-UTR区域调控结合位点,进一步验证结果表明,转染miR-214 inhibitor后,鼻咽癌PTEN 3’-UTR的相对荧光素酶活性显著升高,见图 4A。Western blot结果显示,转染miR-214 inhibitor的5-8F和6-10B细胞中PTEN蛋白水平显著上调,且p-AKT(S473和T308)表达显著下降,这提示AKT磷酸化水平降低,AKT信号活性受到抑制,见图 4B。
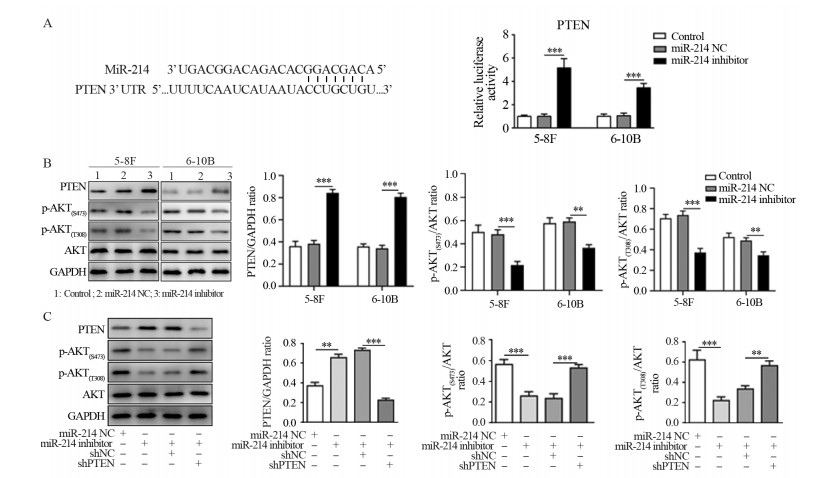
|
| A: the binding sites between miR-214 and PTEN 3'-UTR and relative luciferase activity of PTEN 3'-UTR after miR-214 inhibitor treatment; B: expression levels of PTEN and AKT signaling-associated proteins after miR-214 inhibitor treatment; C: Expression levels of PTEN and AKT signaling-associated proteins in 5-8F cells after transfection of miR-214 inhibitor and/or shPTEN; ***: P < 0.001, **: P < 0.01. 图 4 miR-214通过抑制PTEN转录激活下游AKT信号通路、沉默PTEN表达逆转miR-214 inhibitor对AKT信号通路的抑制作用 Figure 4 miR-214 directly inhibited PTEN expression to activate AKT signaling pathway, and knockdown of PTEN attenuated the effects of miR-214 inhibitor on AKT signaling pathway |
Western blot对5-8F细胞中AKT信号通路活性进行检测,转染miR-214 inhibitor的细胞中PTEN蛋白水平升高,而shPTEN和miR-214 inhibitor共转染的细胞中PTEN蛋白水平下降。对比miR-214 inhibitor转染组,共转染组细胞AKT(S473和T308)磷酸化水平明显升高,AKT信号通路受到激活,见图 4C。PTEN基因沉默可以逆转miR-214 inhibitor对AKT信号通路活性的抑制作用。以上结果提示,miR-214 inhibitor通过直接上调鼻咽癌细胞中PTEN蛋白表达来抑制AKT信号通路活性。
3 讨论在临床实践中,由于鼻咽癌发病部位隐匿,发病症状与其他良性疾病难以鉴别,大部分鼻咽癌患者确诊时已处于中晚期,转移和复发风险升高,这一部分患者治疗仍是具有挑战的工作[12]。因此,寻找更多鼻咽癌治疗的潜在靶点具有重要的临床意义。miR-214位于Dynamin-3基因的14号内含子中,位于人染色体1q24.3区域中。miR-214被发现在甲状腺乳头状癌、淋巴瘤等多种人类恶性肿瘤中表达,并通过调控与肿瘤侵袭转移相关基因的表达而发挥促肿瘤作用[13-14]。
有研究证实,miR-214在鼻咽癌中的表达明显升高,抑制miR-214表达可以促进细胞凋亡,抑制细胞增殖以及肿瘤在体生长[15]。鼻咽癌细胞中miR-214的高表达可能源于诸如转录因子Twist1或长链非编码RNA如lncRNA HOXA11-AS等异常表达所导致[16]。Caspase-3是细胞凋亡启动相关的主要标志性蛋白,其激活是细胞凋亡过程的核心事件,受到AKT信号通路活性的调控[17]。AKT是一种丝氨酸/苏氨酸蛋白激酶,在调控肿瘤细胞信号通路中起关键作用,其磷酸化与肿瘤细胞抗凋亡相关蛋白上调相关[18]。本研究在细胞水平证实,miR-214抑制剂可以抑制AKT磷酸化,降低Bcl-2表达,上调Bax、Cleaved caspase-9、Cleaved caspase-3水平,促进鼻咽癌细胞凋亡。
PTEN被证实为抑癌基因,抑制其表达可以促进鼻咽细胞侵袭转移[19]。既往也有研究报道[20-21],miR-214可以通过直接靶向骨肉瘤和肝癌中PTEN的表达来调控肿瘤的进展,但在鼻咽癌细胞中是否存在同样的机制尚未被阐明。本研究发现,miR-214通过与PTEN的3’-UTR区域结合,直接抑制PTEN的转录。此外,miR-214抑制剂可以显著提高PTEN蛋白表达水平,提示miR-214高表达对鼻咽癌细胞中抑癌基因PTEN表达具有抑制作用。本研究首次发现鼻咽癌细胞中miR-214通过下调PTEN表达,在细胞水平激活AKT信号通路,促进肿瘤的发展。miR-214 inhibitor的存在可以显著降低AKT的磷酸化水平,抑制细胞增殖,促进细胞凋亡。在本研究中,PTEN的敲除并未引起鼻咽癌细胞中miR-214表达的显著改变,因此PTEN被证实是miR-214的下游效应因子。shPTEN细胞转染可以逆转miR-214抑制剂对鼻咽癌细胞的凋亡促进作用。综上研究提示,miR-214可通过直接抑制鼻咽癌PTEN表达,促进AKT信号通路激活进而促进细胞增殖和抑制其凋亡。这一结果为靶向miR-214治疗鼻咽癌提供了理论支持。
作者贡献
韩继波:课题设计、实验实施、数据分析及论文撰写
黄茂凌:细胞及基因实验研究
申丽君:进行细胞实验
陈始明:数据分析及论文撰写指导
陶泽璋:课题设计及论文撰写指导
| [1] |
Bruce JP, Yip K, Bratman SV, et al. Nasopharyngeal Cancer: Molecular Landscape[J]. J Clin Oncol, 2015, 33(29): 3346-3355. DOI:10.1200/JCO.2015.60.7846 |
| [2] |
陆海军, 刘霁, 丁晓. 鼻咽癌的综合治疗研究进展[J]. 山东大学耳鼻喉眼学报, 2019, 33(2): 26-30. [Lu HJ, Liu J, Ding X. Progress in the comprehensive treatment for nasopharyngeal Carcinoma[J]. Shandong Da Xue Er Bi Hou Yan Xue Bao, 2019, 33(2): 26-30.] |
| [3] |
Durgesh T, Katrina P, Christina G. MicroRNA-induced silencing in epilepsy: opportunities and challenges for clinical application[J]. Dev Dyn, 2018, 247(1): 94-110. DOI:10.1002/dvdy.24582 |
| [4] |
AdamsBD, Kasinski AL, Slack FJ. Aberrant Regulation and Function of MicroRNAs in Cancer[J]. Curr Biol, 2014, 24(16): R762-R776. DOI:10.1016/j.cub.2014.06.043 |
| [5] |
He Y, Hu JL, Zhou L, et al. The FOXD3/miR-214/MED19 axis suppresses tumour growth and metastasis in human colorectal cancer[J]. Br J Cancer, 2016, 115(11): 1367-1378. DOI:10.1038/bjc.2016.362 |
| [6] |
Loh HY, Norman BP, Lai KS, et al. The Regulatory Role of MicroRNAs in Breast Cancer[J]. Int J Mol Sci, 2019, 20(19): 4940. DOI:10.3390/ijms20194940 |
| [7] |
Dettori D, Orso F, Penna E, et al. Therapeutic Silencing of miR-214 Inhibits Tumor Progression in Multiple Mouse Models[J]. Mol Ther, 2018, 26(8): 2008-2018. |
| [8] |
Fang Y, Qiu J, Jiang ZB, et al. Increased serum levels of miR-214 in patients with PCa with bone metastasis may serve as a potential biomarker by targeting PTEN[J]. Oncol Lett, 2019, 17(1): 398-405. |
| [9] |
Heishima K, Meuten T, Yoshida K, et al. Prognostic significance of circulating microRNA-214 and -126 in dogs with appendicular osteosarcoma receiving amputation and chemotherapy[J]. BMC Vet Res, 2019, 15(1): 39. DOI:10.1186/s12917-019-1776-1 |
| [10] |
Ebrahimi S, Hosseini M, Shahidsales S, et al. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer[J]. Curr Med Chem, 2017, 24(13): 1321-1331. |
| [11] |
Pérez-Ramírez C, Cañadas-Garre M, Molina MÁ, et al. PTEN and PI3K/AKT in non-small-cell lung cancer[J]. Pharmacogenomics, 2015, 16(16): 1843-1862. DOI:10.2217/pgs.15.122 |
| [12] |
Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma[J]. Lancet, 2019, 394(10192): 64-80. DOI:10.1016/S0140-6736(19)30956-0 |
| [13] |
Sun JR, Zhang X, Zhang Y. miR-214 prevents the progression of diffuse large B-cell lymphoma by targeting PD-L1[J]. Cell Mol Biol Lett, 2019, 24: 68. DOI:10.1186/s11658-019-0190-9 |
| [14] |
Liu F, Lou K, Zhao X, et al. miR-214 regulates papillary thyroid carcinoma cell proliferation and metastasis by targeting PSMD10[J]. Int J Mol Med, 2018, 42(6): 3027-3036. |
| [15] |
Zhang ZC, Li YY, Wang HY, et al. Knockdown of miR-214 promotes apoptosis and inhibits cell proliferation in nasopharyngeal carcinoma[J]. PLoS One, 2014, 9(1): e86149. DOI:10.1371/journal.pone.0086149 |
| [16] |
Wang XY, Li H, Shi J. LncRNA HOXA11-AS Promotes Proliferation and Cisplatin Resistance of Oral Squamous Cell Carcinoma by Suppression of miR-214-3p Expression[J]. Biomed Res Int, 2019, 2019: e8645153. |
| [17] |
Jeong CH, Chun KS, Kundu J, et al. Phosphorylation of Smac by Akt promotes the caspase-3 activation during etoposide-induced apoptosis in HeLa cells[J]. Mol Carcinog, 2015, 54(2): 83-92. DOI:10.1002/mc.22075 |
| [18] |
Zhang X, Tang N, Hadden TJ, et al. Akt, FoxO and regulation of apoptosis[J]. Biochim Biophys Acta, 2011, 1813(11): 1978-1986. DOI:10.1016/j.bbamcr.2011.03.010 |
| [19] |
Gao Q, Tang L, Wu L, et al. LASP1 promotes nasopharyngeal carcinoma progression through negatively regulation of the tumor suppressor PTEN[J]. Cell Death Dis, 2018, 9(3): 393. DOI:10.1038/s41419-018-0443-y |
| [20] |
Xin R, Bai F, Feng Y, et al. MicroRNA-214 promotes peritoneal metastasis through regulating PTEN negatively in gastric cancer[J]. Clin Res Hepatol Gastroenterol, 2016, 40(6): 748-754. DOI:10.1016/j.clinre.2016.05.006 |
| [21] |
Liu CJ, Yu KL, Liu GL, et al. MiR-214 promotes osteosarcoma tumor growth and metastasis by decreasing the expression of PTEN[J]. Mol Med Rep, 2015, 12(4): 6261-6266. DOI:10.3892/mmr.2015.4197 |
 2020, Vol. 47
2020, Vol. 47