文章信息
- 食管鳞癌脑转移患者的临床特征和影响因素及其对生存的影响
- Clinical Characteristics and Influencing Factors of Esophageal Squamous Cell Carcinoma Patients with Brain Metastases and Their Influence on Survival
- 肿瘤防治研究, 2020, 47(6): 437-440
- Cancer Research on Prevention and Treatment, 2020, 47(6): 437-440
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2020.19.0829
- 收稿日期: 2019-06-24
- 修回日期: 2020-04-02
食管癌是消化系统中最常见的恶性肿瘤之一,发病率在中国呈现升高趋势[1-2]。食管癌脑转移(brain metastases from esophageal squamous cell carcinoma, BM-ESCC)的发病率比其他肿瘤低[3-4],其治疗方案的选择尚存在争议[3]。对于预期生存期短的肿瘤患者,治疗方案主要以缓解症状和提高生活质量为主[5],对于预期生存期长的肿瘤患者则主要关注肿瘤控制率和远期不良反应。如何有效预测食管癌脑转移患者的预后及制定合理的治疗方案是此类患者获得更大生存获益的关键[6]。本研究探讨食管鳞癌脑转移患者的临床特征、影响因素及其对生存的影响,以期为指导生存和预后评估提供依据。
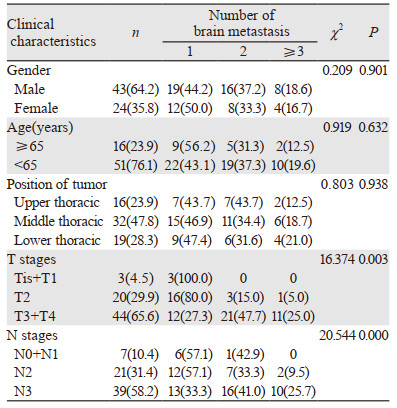
1 资料与方法 1.1 研究对象2000年12月—2015年12月我院共收集初治的食管癌患者8 673例。纳入标准:(1)经组织病理学确诊为鳞状细胞癌;(2)磁共振确诊颅内存在转移灶。排除标准:(1)病理信息不完整;(2)伴有原发第二肿瘤。最终共67例食管鳞癌伴脑转移患者纳入研究,其中31例颅内转移灶1个、24例颅内转移灶2个、12例颅内转移灶≥3个;单一颅内转移22例,同时伴有其他重要脏器转移45例;TNM分期采用国际抗癌联盟(UICC)食管癌分期标准。T分期:由于Tis和T4病例数过少,将Tis和T1的患者例数合并,T3和T4的患者例数合并;N分期:N0与N1合并,其余一般资料见表 1。本研究中所有研究对象均签署知情同意书,并经医院医学伦理委员会批准。
|
单一颅内转移灶患者中,有3例接受手术切除,并于术后补充全脑放射治疗,照射剂量40 Gy/20 f;其余19例患者接受单纯放射治疗,颅内转移灶总剂量56~60 Gy。伴有其他重要脏器转移患者中,17例未行手术或放射治疗,22例接受全脑放疗,6例接受全脑放疗后颅内转移灶局部推量放疗(剂量同前)。
1.3 随访截至2017年6月31日,中位随访时间22月,67例食管鳞癌脑转移患者随访率100%,中位生存时间为9.65月(0~30月)。采用门诊或者电话随访方式,每3月随访一次,详细记录患者的临床资料和生存状况。
1.4 统计学方法采用SPSS13.0软件进行统计学数据分析,计数资料用构成比和率来描述,组间比较采用卡方检验,食管鳞癌脑转移的主要危险因素采用Logistic回归分析,生存曲线分析采用Kaplan-Meier法和Log rank法,Cox回归模型进行多因素预后分析。P < 0.05为差异有统计学意义。
2 结果 2.1 脑转移灶数量与食管鳞癌脑转移患者临床病理特征的相关性结果显示:不同T、N分期患者的脑转移发生率差异有统计学意义(P=0.003、0.000)。T、N分期越晚,脑转移发生率越高;不同性别、年龄、食管肿瘤部位的脑转移发生率差异无统计学意义(P > 0.05),见表 1。
2.2 食管鳞癌脑转移的主要危险因素Logistic回归分析结果显示:N2、N3是食管鳞癌患者脑转移的独立危险因素,N0+N1的脑转移风险较小(P < 0.05);随着T分期的增加,脑转移风险的趋势增加,但差异无统计学意义(P > 0.05),见表 2。

|
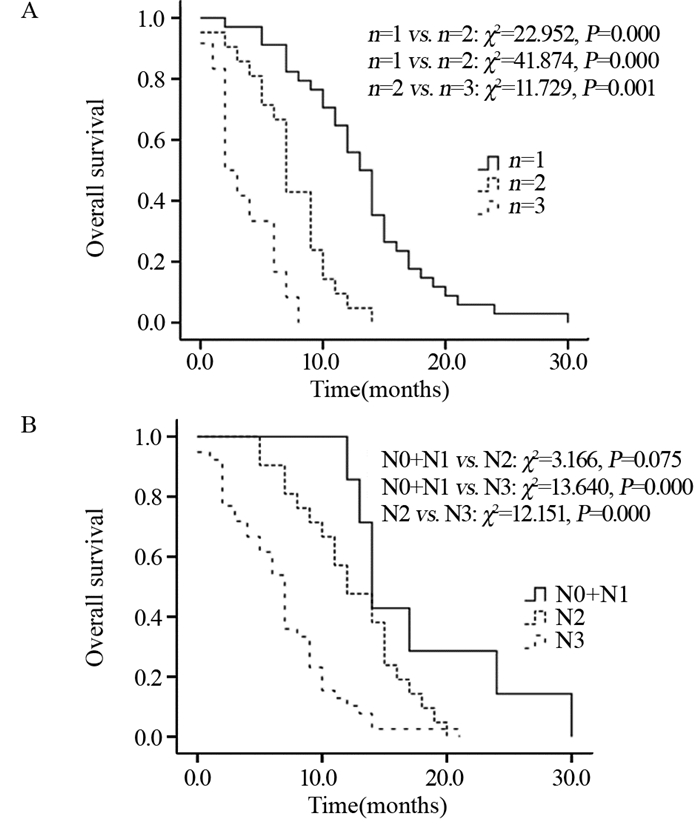
Kaplan-Meier法和Log rank法分析结果显示:脑转移瘤数量为1的患者生存期显著优于脑转移瘤数量为2和3的患者,见图 1A。将N分期作为独立危险因素进行分层分析,结果显示N0+N1患者的生存优于N2患者,但组间比较差异无统计学意义(P > 0.05);N0+N1患者和N2患者的生存优于N3患者,且差异均有统计学意义(P < 0.05),见图 1B。
|
| 图 1 不同脑转移数量(A)和N分期(B)的食管鳞癌患者生存分析 Figure 1 Survival analysis of esophageal squamous cell carcinoma patients with different number of brain metastases(A) and N stages(B) |
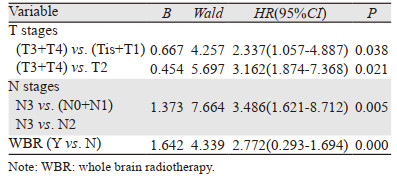
Cox回归模型多因素预后分析显示:T3+T4、N3、是否做全脑放疗是影响食管鳞癌脑转移患者的独立预后因素(P < 0.05),见表 3。
|
食管癌脑转移的发生率低[7-8]。本研究中同期食管癌脑转移发生率为0.77%,高于Cavanna等[9]报道的胃癌脑转移发生率0.16%~0.69%,病理类型、种族和饮食习惯是主要原因[10-11],但不同病理类型发生脑转移的病理机制、不同种族食管癌患者是否存在不同脑转移相关驱动基因,尚需进一步研究。在有脑转移的患者中,约67.2%的患者同时伴有多发脏器转移,这与既往的研究结果[12]类似。本研究结果显示食管鳞癌脑转移患者T分期、N分期与脑转移瘤数量呈正比,说明临床分期晚更易发生脑转移。一项有关术后病理完全缓解率与预后的关系研究证实,T分期越高,临床病理缓解率越低,且发生脑转移的风险也越高[13]。因此,也进一步证实T分期是评价是否出现脑转移的预后影响因素之一,但其敏感度和特异性尚需进一步研究。不同性别和不同肿瘤位置之间无明显差异,考虑食管鳞癌脑转移仍以肿瘤细胞的生物学行为为主,与患者的生理特征无相关性。针对食管癌患者,脑转移多发生于食管癌确诊后1年,且根据神经症状及相关体征进行影像学评估[14]。
本研究中T3以上、N3以上发生脑转移瘤数量≥2分别占72.7%和61.5%,因研究样本量的原因,将T3+T4进行合并统计,可能会对研究结果造成一定偏倚。按照Ⅰ~Ⅳ期的评估方法是否更准确,尚需扩大样本量进一步研究。T分期是食管鳞癌进展期的重要标志,T分期越晚,发生转移的概率越大[15]。本研究中Logistic回归分析结果显示食管鳞癌脑转移患者T分期越晚,脑转移风险有增加的趋势,但并未出现统计学差异,可能与样本量偏少有关。N分期对食管癌的治疗方案选择具有重要意义,是评价包括紫杉醇为主要化疗方案等治疗方法预后的独立危险因素[16],本研究也得到类似结果。N0+N1的脑转移风险显著低于N2和N3患者,说明N分期较T分期更能有效评价预后,与Sepesi等[17]的研究结论一致,对N分期进行分层的预后分析也进一步证实了该结论。本研究中食管鳞癌脑转移患者的中位生存时间为9.65月,高于Kothari等[18]报道的5月,与宋欣等[19]的结论类似,考虑与纳入研究对象的病理类型相关,欧美主要以腺癌为主,亚洲主要以鳞癌为主[20]。另外,本研究发现脑转移瘤个数与远期生存呈正比,既往研究结论中并未考虑脑转移瘤数量的因素[18-19],这可能会对结果造成一定偏倚。
包括食管癌在内的实体瘤脑转移的治疗方式主要有全脑放疗、立体定向放射治疗和手术等,不同的治疗方式对1~4个转移灶的脑转移瘤中并不造成生存差异[21]。本研究发现全脑放疗可明显降低食管鳞癌脑转移患者的死亡风险,考虑全脑放疗可以提高转移瘤的局控率、抑制颅内微小转移灶和降低神经死亡有关[22],与既往的研究结论[23-24]类似。原发食管肿瘤的T分期和N分期与死亡风险呈正比,说明消化道肿瘤的肿瘤体积负荷与预后密切相关[25-26]。因食管鳞癌脑转移发病率低,纳入研究对象少,且未进一步研究包括HER-2等基因过表达与食管癌脑转移的相关性[27],这是本研究的主要缺陷。
综上,食管鳞癌脑转移患者的脑转移瘤数量与T分期和N分期密切相关,T分期与N分期越晚,发生脑转移的风险越大,尤其应关注N分期。脑转移瘤数量越少,预后越好;同时,食管肿瘤的N分期越早,预后越好。食管鳞癌脑转移患者行全脑放疗可明显降低死亡风险。
作者贡献
骆华春:文章构思和撰写
傅志超:课题管理和科研思路构思
冯静、程惠华:患者随访
沈志勇:数据收集
应文敏、蔡履娟:数据统计
| [1] |
Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. DOI:10.3322/caac.21551 |
| [2] |
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [3] |
Schmidt Jensen J, Grønhøj C, Mirian C, et al. Incidence and survival of head and neck squamous cell carcinoma in children and young adults in Denmark: a nationwide study from 1980 to 2014[J]. Acta Oncol, 2018, 57(10): 1410-1413. DOI:10.1080/0284186X.2018.1473639 |
| [4] |
Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018, 68(1): 7-30. DOI:10.3322/caac.21442 |
| [5] |
Shaikt T, Meyer JE, Horwitz EM. Optimal use of combined modality therapy in the treatment of esophageal cancer[J]. Surg Oncol Clin N Am, 2017, 26(3): 405-429. |
| [6] |
Moaven O, Wang TN. Combined modality therapy for management of esophageal cancer: current approach based on experiences from east and west[J]. Surg Clin North Am, 2019, 99(3): 479-499. DOI:10.1016/j.suc.2019.02.004 |
| [7] |
Chukwueke UN, Brastianos PK. Precision medical approaches to the diagnoses and management of brain metastases[J]. Curr Treat Options Oncol, 2019, 20(6): 49. DOI:10.1007/s11864-019-0649-y |
| [8] |
Feng W, Zhang P, Zheng X, et al. Neuroimaging and clinical characteristics of brain metastases from esophageal carcinoma in Chinese patients[J]. J Cancer Res Ther, 2014, 10(Suppl): 296-303. |
| [9] |
Cavanna L, Seghini P, Di Nunzio C, et al. Gastric cancer with brain metastasis and the role of human epidermal growth factor 2 status[J]. Oncol Lett, 2018, 15(4): 5787-5791. |
| [10] |
Onal C, Akkus Yildirim B, Guler OC. Outcomes of aggressive treatment in esophageal cancer patients with synchronous solitary brain metastasis[J]. Mol Clin Oncol, 2017, 7(1): 107-112. |
| [11] |
Rades D, Dziggel L, Bartscht T, et al. Predicting overall survival in patients with brain metastases from esophageal cancer[J]. Anticancer Res, 2014, 34(11): 6763-6765. |
| [12] |
Liu M, Wang C, Gao L, et al. A nomogram to predict long-time survival for patients with M1 diseases of esophageal cancer[J]. J Cancer, 2018, 9(21): 3986-3990. DOI:10.7150/jca.27579 |
| [13] |
Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and surival-The university of Texas MD Anderson Cancer Center experience[J]. Cancer, 2017, 123(21): 4106-4113. DOI:10.1002/cncr.30953 |
| [14] |
Nobel TB, Dave N, Eljalby M, et al. Incidence and risk factors for isolated esophageal cancer recurrence to the brain[J]. Ann Thorac Surg, 2020, 109(2): 329-336. DOI:10.1016/j.athoracsur.2019.09.028 |
| [15] |
Chen X, Chen J, Zheng X, et al. Prognostic factors in patients with thoracic esophageal carcinoma staged pT1-4aN0M0 undergone esophagectomy with three-field lymphadenectomy[J]. Ann Transl Med, 2015, 3(19): 282. |
| [16] |
Nakajima M, Muroi H, Kikuchi M, et al. Adverse prognostic factors of advanced esophageal cancer in patients undergoing induction therapy with docetaxel, cisplatin and 5-fluorouracil[J]. Anticancer Res, 2018, 38(2): 911-918. |
| [17] |
Sepesi B, Schmidt HE, Lada M, et al. Survival in patients with esophageal adenocarcinoma undergoing trimodality therapy is independent of regional lymph node location[J]. Ann Thorac Surg, 2016, 101(3): 1075-1080. DOI:10.1016/j.athoracsur.2015.09.063 |
| [18] |
Kothari N, Mellon E, Hoffe SE, et al. Outcomes in patient with brain metastasis from esophageal carcinoma[J]. J Gastrointest Oncol, 2016, 7(4): 562-569. DOI:10.21037/jgo.2016.03.12 |
| [19] |
宋欣, 李红卫, 宋晖. 食管鳞状细胞癌脑转移73例的预后分析[J]. 中华消化杂志, 2017, 37(2): 106-109. [Song X, Li HW, Song H. Prognostic analysis of 73 cases of brain metastases from esophageal squamous cell carcinoma[J]. Zhonghua Xiao Hua Za Zhi, 2017, 37(2): 106-109. DOI:10.3760/cma.j.issn.0254-1432.2017.02.007] |
| [20] |
He L, Chapple A, Liao Z, et al. Bayesian regression analyses of radiation modality effects on pericardial and pleural effusion and survival in esophageal cancer[J]. Radiother Oncol, 2016, 121(1): 70-74. |
| [21] |
Churilla TM, Handorf E, Collette S, et al. Whole brain radiotherapy after stereotactic radiosurgery or surgical resection among patients with one to three brain metastases and favorable prognoses: a secondary analysisi of EORTV 22952-26001[J]. Ann Oncol, 2017, 28(10): 2588-2594. DOI:10.1093/annonc/mdx332 |
| [22] |
Rodrigues G, Zindler J, Warner A, et al. Propensity-score matched pair comparison of whole brain with simultaneous in-field boost radiotherapy and stereotactic radiosurgery[J]. Radiother Oncol, 2013, 106(2): 206-209. DOI:10.1016/j.radonc.2012.10.014 |
| [23] |
Kocher M, Soffieetti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study[J]. J Clin Oncol, 2011, 29(2): 134-141. DOI:10.1200/JCO.2010.30.1655 |
| [24] |
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases[J]. JAMA, 2006, 295(21): 2483-2491. DOI:10.1001/jama.295.21.2483 |
| [25] |
Huang CM, Xu M, Wang JB, et al. Is tumor size a predictor of preoperative N staging in T2-T4a stage advanced gastric cancer?[J]. Surg Oncol, 2014, 23(1): 5-10. DOI:10.1016/j.suronc.2014.01.003 |
| [26] |
Zu H, Wang F, Ma Y, et al. Stage-stratified analysis of prognostic significance of tumor size in patients with gastric cancer[J]. PLoS One, 2013, 8(1): e54502. DOI:10.1371/journal.pone.0054502 |
| [27] |
Ebbing EA, Steins A, Fessler E, et al. Esophageal adenocarcinoma cells and xenograft tumors exposed to Erb-b2 receptor tyrosine kinase 2 and 3 inhibitors activate transforming growth factor beta signaling, which induces epithelial to mesenchymal transition[J]. Gastroenterology, 2017, 153(1): 63-76. DOI:10.1053/j.gastro.2017.03.004 |
 2020, Vol. 47
2020, Vol. 47


