文章信息
- 腺病毒介导的Hes1基因抑制肝细胞癌细胞的增殖与迁移
- Adenovirus-mediated Hes1 Expression Inhibits Proliferation and Migration of Hepatocellular Carcinoma Cells
- 肿瘤防治研究, 2020, 47(3): 164-169
- Cancer Research on Prevention and Treatment, 2020, 47(3): 164-169
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2020.19.0756
- 收稿日期: 2019-06-10
- 修回日期: 2019-12-16
肝细胞癌(hepatocellular carcinoma, HCC)是世界上最常见的恶性肿瘤之一,其致死率占恶性肿瘤的第五位。我国是HCC高发地区,临床上HCC患者病情进展迅速、生存期短,是我国癌症致死的第三大原因[1-2]。目前HCC发生、发展的分子机制尚不明确,但研究表明Notch、Hedgehog和Wnt等信号通路在HCC的起始、浸润与转移等过程中起着重要作用[3]。其中Notch信号通路是进化过程中高度保守的一条信号通路,通过相邻细胞间受体与配体的相互作用转导信号,参与调控细胞的增殖、分化与凋亡等过程[4]。现已发现Notch信号通路在不同肿瘤中有着不同的生物学作用。多项研究表明在HCC中Notch信号通路的活化可抑制HCC细胞的增殖并促进其凋亡[5-7],因此,Notch信号通路可能是治疗HCC的潜在靶点之一。
Notch信号通路由Notch受体、Notch配体、DNA结合蛋白、效应分子和调节分子等组成[8]。转录因子Hes1(hairy and enhancer of split homolog-1)是Notch信号通路下游主要的效应因子之一,其属于碱性螺旋-环-螺旋(the basic helix-loop-helix)家族的转录调节因子[9]。Hes1通过结合在目标基因启动子区域的特定位点,如class C位点和N box结构域,从而抑制靶基因的转录及其自身的转录[10]。Hes1参与了肿瘤干细胞的自我更新、肿瘤细胞的转移、凋亡和耐药等过程[11],如在HCC细胞系中降低Hes1的表达量,抑制MHCC-97L细胞的凋亡,并促进其发生肺转移[12]。为了探讨Hes1对HCC细胞系Hep1-6的作用,本实验构建了表达Hes1基因的重组腺病毒载体并研究了Hes1对Hep1-6细胞增殖、迁移及侵袭的影响。
1 材料与方法 1.1 细胞与试剂Hep1-6和293A细胞(海军军医大学细胞生物学教研室保存)置于含10%胎牛血清的DMEM培养液,37℃、5%CO2条件下培养。以0.25%胰酶、0.02%乙二胺四乙酸消化传代。pAd-Track系统购自Addgene公司(美国),Match T1购自北京全式金生物技术有限公司(中国)。T4-DNA连接酶、限制性内切酶、DNA Marker、蛋白Marker、1%青霉素/链霉素、DMEM培养液、Lipofectamine 2000转染试剂和RNA抽取试剂TRIzol购自Thermo Fisher Scientific公司(美国),PrimeSTAR HS DNA Polymerase购自TAKARA公司(中国),2×T5 Fast qPCR Mix(SYBRGreenI)购自擎科新业生物技术有限公司(中国),Transwell小室及细胞培养耗材购自Corning公司(美国)、质粒提取试剂盒、DNA胶回收试剂盒购自生工生物公司(中国);M-MLV反转录酶购自Promega公司(美国);胎牛血清购自Hyclone公司(美国);抗Hes1多克隆抗体购自Santa Cruz公司(美国)。
1.2 重组腺病毒载体的构建以小鼠胆囊壁组织cDNA为模板,PCR扩增Hes1基因,上下游引物分别为5’-GCCGATATCGCCACCATGCCAGCTGATATAATGGA-3’和5’-TAACTCGAGGCTCTCAGTTCCGCCACGGTCT-3’,目的片段大约861 bp。将穿梭载体pAd-Track用EcoRⅤ和XhoⅠ双酶切后获得的骨架片段与EcoRⅤ和XhoⅠ酶切后的Hes1基因片段连接,构建pAd-Track-Hes1载体,并测序验证。将PmeI线性化后的pAd-Track-Hes1载体转化入经氯化钙制备的BJ5183感受态细胞,获得重组后的腺病毒载体pAd-Hes1,见图 1。
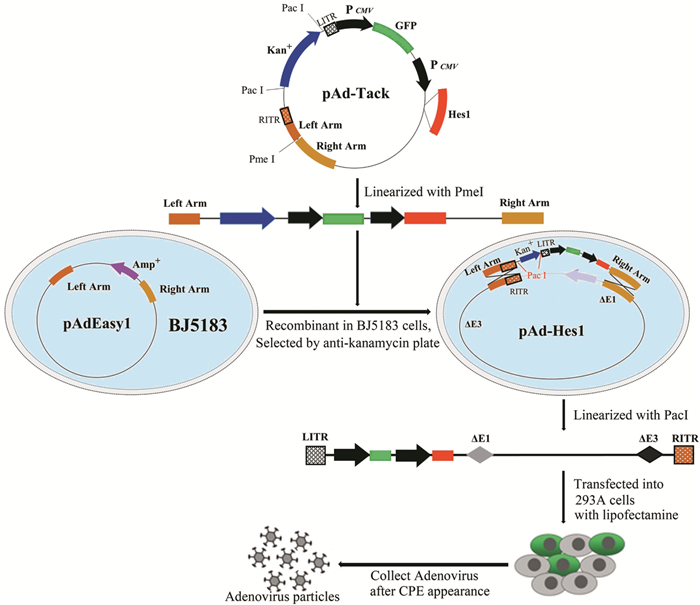
|
| 图 1 Hes1重组腺病毒载体构建、病毒包装示意图 Figure 1 Schema of construction of Hes1 recombinant adenovirus vector and generation of adenovirus in 293A packaging cells |
用Lipofectamine 2000转染试剂将PacⅠ酶切后的重组腺病毒载体pAd-Hes1导入大约50%融合生长的293A细胞。当出现完全细胞病变效应(cytopathic effect, CPE)时,收集细胞,-80℃、37℃反复冻融3次,释放Ad-Hes1病毒颗粒,将病毒悬液置于-80℃保存,见图 1。取适量的上述病毒悬液再次感染293A细胞,在293A细胞中大量扩增重组腺病毒颗粒,扩增后的腺病毒分装保存于-80℃。由于pAd-Track载体自身携带GFP的报告基因,细胞感染病毒24 h后,通过流式细胞仪测定表达GFP的细胞数来测定病毒滴度。病毒滴度(GFU/ml)=(GFP阳性细胞百分比×细胞总数)/[感染病毒的体积(ml)×病毒稀释倍数]。
1.4 细胞Hes1基因表达水平的检测感染Ad-Hes1病毒的Hep1-6细胞为实验组(Hep1-6-Hes1),感染Ad-GFP病毒的Hep1-6细胞为阴性对照组(Hep1-6-GFP),未感染病毒Hep1-6细胞为空白对照组(Hep1-6-BLK)。采用Real-time PCR和Western blot分别检测Hes1 mRNA和蛋白的表达。Real-time PCR检测Hes1 mRNA时,PBS洗涤细胞后加入TRIzol裂解液提取总RNA,用M-MLV将RNA反转录为第一链cDNA,再进行Real-time PCR检测Hes1基因的表达,GAPDH作为内参基因,Hes1相对表达量的变化采用2-ΔΔCt法计算,Real-time PCR所用引物序列为:Hes1正向引物:5’-ACACCGGACAAACCAAAGACGGCCT-3’,Hes1反向引物:5’-CCCGCTGCAGGTTCCGGAGGTGCTTC-3’,GAPDH正向引物:5’-GGTGAAGGTCGGTGTGAACG-3’,GAPDH反向引物:5’-CTCGCTCCTGGAAGATGGTG-3’。Western blot检测蛋白表达时,用RIPA缓冲液提取细胞总蛋白,BCA法测定总蛋白浓度,每样本取50 μg蛋白进行SDS-PAGE电泳分离,随后将蛋白转移至PVDF膜。用5%脱脂牛奶室温封闭1 h后,兔抗小鼠Hes1多克隆抗体(1:2000)4℃孵育过夜,PBS漂洗3次,每次10 min;加辣根过氧化物酶标记的二抗(1:500)37℃孵育30 min,PBS漂洗3次。然后,与ECL试剂反应2 min,在暗室中进行X线胶片曝光、显影和定影。
1.5 细胞增殖和克隆形成实验取生长良好的Hep1-6细胞,以5×103/孔接种于96孔板,培养24 h,每孔加入复合感染指数(MOI)=10的病毒悬液25 μl。当测定生长速率时,每孔加入10%体积的CCK-8溶液37℃培养2 h,用酶标仪测450 nm的吸光度。每组实验设置三个复孔,计算平均值,并绘制生长曲线。将500个感染病毒24 h后的细胞接种于3.5 cm培养皿,37℃、5%CO2培养2周,用4%多聚甲醛溶液固定10 min,以锥虫蓝染色,计数克隆数。
1.6 划痕愈合实验在6孔板中接种约5×105个细胞,待细胞长满后,用200 μl吸头垂直于培养板底划一直线,用PBS洗3次去除划痕时脱落的细胞,加入无血清培养基放入37℃、5%CO2培养箱中培养。按0、24、48 h拍照测量划痕的宽度。创面愈合率=(0 h划痕的宽度-指定测量时间划痕的宽度)/0 h划痕的宽度。
1.7 Transwell迁移与迁袭实验在24孔板中,每孔加入500 μl含20%FBS DMEM培养基,然后在孔中放置Transwell小室(迁袭实验时,在Transwell小室中应提前铺入Matrigel基质胶)。胰酶消化处于对数生长期的细胞,并用10%FBS DMEM培养基重悬细胞并调整细胞密度至5×104/ml,接种200 μl细胞悬液于Transwell小室中,置于37℃、5%CO2培养箱继续培养48 h,去除Transwell小室并吸弃24孔板中的培养液,PBS洗3次,4%多聚甲醛固定10 min,用PBS洗3次,结晶紫染色10 min,用PBS洗3次,显微镜下观察,并对迁移至小室底部24孔板内的细胞进行拍照和计数,每个孔均随机选取10个视野计数,求平均值,每组实验重复3次。
1.8 统计学方法使用GraphPad Prism6.0软件分析数据。数据结果以均数±标准差(x±s)表示,组间比较采用单因素方差分析,多个样本均数间的多重比较采用LSD-t检验,配对样本采用双尾Student's检验。P < 0.05为差异有统计学意义。
2 结果 2.1 腺病毒载体的鉴定构建的穿梭载体pAd-Track-Hes1经EcoRⅠ和XhoⅠ双酶切鉴定,酶切片段为3 720 bp和6 182 bp;重组后的腺病毒载体Ad-Hes1经PacⅠ酶切鉴定,酶切片段为4 500 bp和23 000 bp,见图 2,与预期相符,测序结果证实Hes1未发生突变。
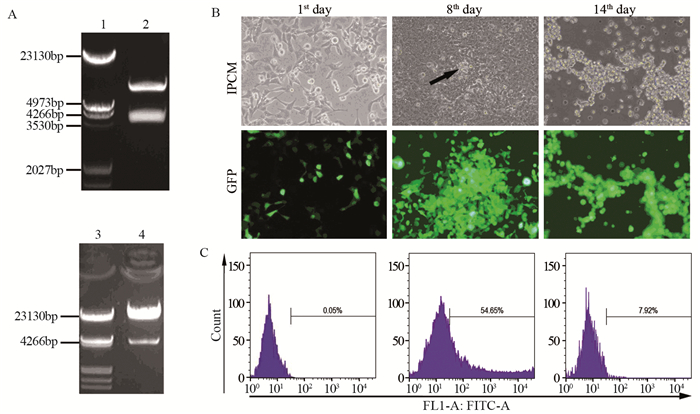
|
| A: pAd-Track-Hes1 shuttle vector were digested by EcoRⅠ and XhoⅠ (up), the recombinant pAd-Hes1 adenovirus vector were digested by PacⅠ (down), the lane of 1, 3 were loaded with λ-HindⅢ DNA Marker, the lane of 2 were loaded with digested pAd-Track-Hes1 vector, the lane of 4 were loaded with digested pAd-Hes1 vector; B: The linearized adenovirus vector Ad-Hes1 was transfected into 293A cells. The morphology and green fluorescence expression of 293A cells were observed on the 1st, 8th and 14th day after transfection. The CPE effect began on the 8th day after transfection (arrow); C: The ratio of green fluorescence-positive cells in 293A cells infected with 1×104 and 1×105 times diluted Ad-Hes1 adenovirus was detected by flow cytometry 24h after transfection. 图 2 Hes1腺病毒载体的酶切鉴定、病毒包装和滴度测定 Figure 2 Identification of Hes1 adenovirus vectors by enzyme digestion and the package and titer determination of adenovirus |
通过Lipofectamine 2000将PacⅠ线性化后的腺病毒载体PAd-Hes1转染至50%融合的293A细胞中,转染1天后在荧光显微镜下可见GFP阳性的细胞约占50%;细胞继续培养到8天时,GFP阳性的细胞约为90%,同时293A细胞开始出现CPE效应;培养到14天时,293A细胞出现完全CPE效应,细胞漂浮于培养液中呈现“葡萄串样”,见图 2B。此时,离心收集细胞,反复冻融释放病毒颗粒。将扩增后的病毒悬液稀释1×104和1×105倍后,分别以500 μl感染6孔板中293A细胞24 h后,胰酶消化收集细胞并计数每孔中的细胞总数,其数量分别为9.6×105和8.2×105个,流式细胞仪检测绿色荧光阳性细胞的阳性率分别为54.65%和7.92%,见图 2C,病毒滴度为1.04×1010和1.29×1010,病毒滴度平均为1.17×1010 GFU/ml。
2.3 腺病毒介导Hes1基因在Hep1-6细胞中的表达在Hep1-6细胞感染病毒24 h后报告基因GFP开始表达,见图 3A~B,36 h后收集细胞提取总RNA和蛋白。Real-time PCR检测结果显示,感染Ad-Hes1病毒的Hep1-6细胞的Hes1 mRNA表达量显著上调,约为阴性对照Hep1-6-GFP和空白对照Hep1-6-BLK细胞的95倍,见图 3C。同时Western blot检测结果表明感染Ad-Hes1病毒的Hep1-6细胞Hes1蛋白表达量强于阴性对照Hep1-6-GFP和空白对照Hep1-6-BLK细胞,见图 3D。
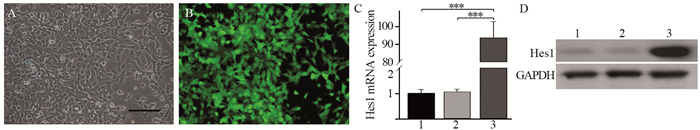
|
| 1: Hep1-6-BLK; 2: Hep1-6-GFP; 3: Hep1-6-Hes1; A: the morphology of Hep1-6 cells after Ad-Hes1 adenovirus infection for 24 hours under inverted phase contrast microscope, Scale bar: 100 μm; B: Hep1-6 cells after Ad-Hes1 adenovirus infection for 24 hours expressed the reporter gene GFP; C: Real-time PCR detected the expression of Hes1 gene in Hep1-6 cells after adenovirus infection for 36 hours. The expression of Hes1 gene was significantly higher than those in negative control (Hep1-6-GFP) and blank control (Hep1-6-BLK) groups. One way ANOVA analysis, ***: P < 0.001; D: the protein expression of Hes1 in Hep1-6-Hes1 cells was higher than those in negative control (Hep1-6-GFP) and blank control (Hep1-6-BLK) groups detected by Western blot. Hep1-6-Hes1 cells were infected with Ad-Hes1 adenovirus. Hep1-6-GFP cells were infected with Ad-GFP control adenovirus. Hep1-6-BLK cells were not infected with adenovirus. 图 3 腺病毒介导Hes1基因在Hep1-6细胞中的表达 Figure 3 Adenovirus-mediated Hes1 gene expression in Hep1-6 cells |
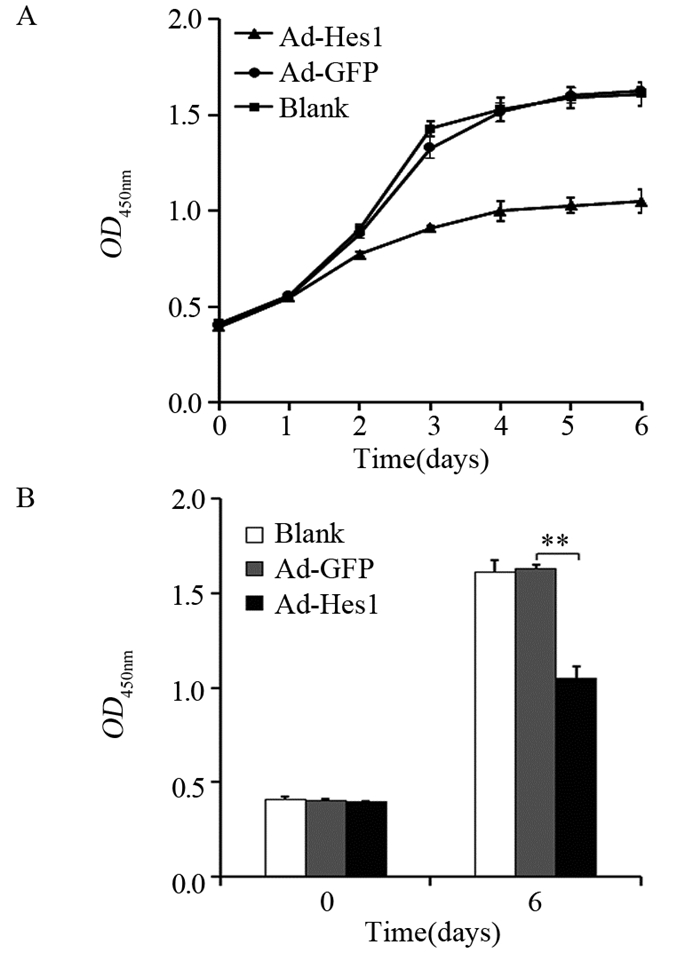
克隆形成实验表明,与感染Ad-GFP的对照组比较,感染Ad-hes1的细胞形成的克隆较小,且克隆数量明显减少,见图 4A,感染Ad-Hes1的Hep1-6克隆形成数为(42±5)个,感染Ad-GFP的对照组为(185±35)个(P < 0.001),见图 4B。在外源过表达Hes1第3天,Hep1-6细胞增殖开始受到抑制,见图 5A,而第5天时细胞活性明显下降,仅为对照细胞的55%(P < 0.01),见图 5B。
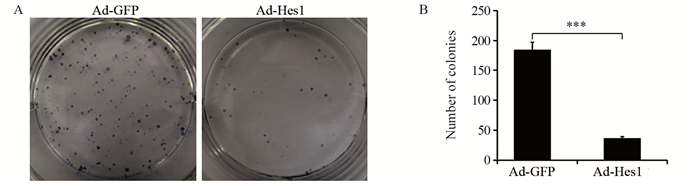
|
| A: Hep1-6-Hes1 cells and negative control Hep1-6-GFP cells were cultured for two weeks respectively to form clones; B: the numbers of colonies formed by Hep1-6-Hes1 cells and Hep1-6-GFP cells, Student's t test; ***: P < 0.001. 图 4 Hes1抑制Hep1-6细胞的克隆形成能力 Figure 4 Hes1 inhibited clonogenic capacity of Hep1-6 cells |
|
| A: Growth curves of Hep1-6-Hes1 cells, negative control Hep1-6-GFP cells and blank control Hep1-6 cells were measured by CCK-8 assay; B: OD values on day 0 and day 6 in three groups of cells, LSD-t test; **: P < 0.01. 图 5 Hes1抑制Hep1-6细胞的增殖能力 Figure 5 Hes1 inhibited proliferation of Hep1-6 cells |
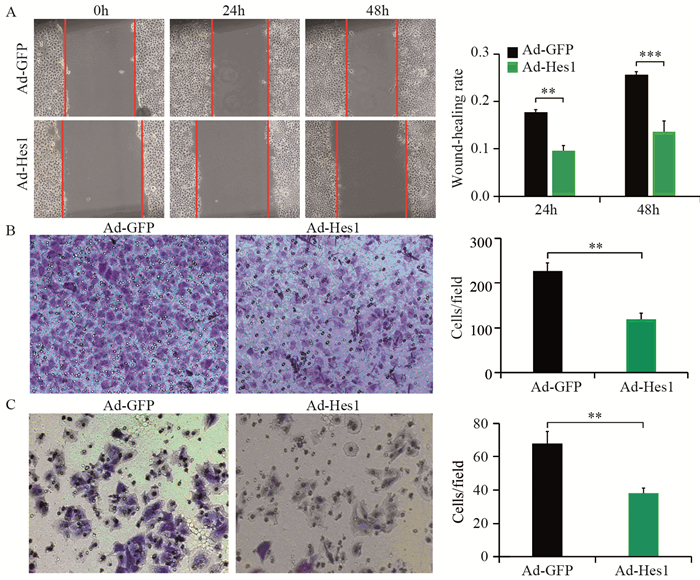
细胞划痕实验显示,感染Ad-Hes1的Hep1-6细胞在24、48 h划痕愈合率分别为0.1和0.12,显著低于感染Ad-GFP的对照组(0.18和0.26),见图 6A;Transwell迁移实验结果显示,感染Ad-Hes1的Hep1-6细胞接种入Transwell小室48 h后迁移进入Transwell下室的细胞数量为102个,显著低于感染Ad-GFP的对照组(222个),见图 6B;Transwell侵袭实验结果显示,感染Ad-Hes1的Hep1-6细胞接种入铺有Matrigel基质胶的Transwell小室48 h后,侵袭突破基质胶进入Transwell下室的细胞数目为38个,显著低于感染Ad-GFP的对照组(65个),见图 6C。划痕愈合实验和Transwell迁移与侵袭实验结果表明Hes1能明显降低Hep1-6细胞的体外迁移和侵袭能力。
|
| A: Wound healing assay showed that cell motilities of Hep1-6 infected with Ad-Hes1 virus at 24 and 48h were significantly lower than that of the control group, **: P < 0.01, ***: P < 0.001; B: Transwell migration experiment showed that the number of Hep1-6 infected with Ad-Hes1 virus migrated into the lower chamber of Transwell after 48h of culture was significantly less than that of the control group, **: P < 0.01; C: Transwell invasion test showed that the number of Hep1-6 cells infected with Ad-Hes1 virus penetrating into the lower chamber of Transwell coated with Matrigel after 48h of culture was significantly lower than that of the control group, **: P < 0.01. 图 6 Hes1抑制Hep1-6细胞的迁移和侵袭能力 Figure 6 Hes1 inhibited migration and invasion of Hep1-6 cells |
Notch信号通路是进化上高度保守的细胞间信号通路,在调节细胞分化、增殖和凋亡及一系列生理、病理过程中起着重要作用[13-14]。目前已有许多研究表明,Notch受体的异常表达和激活与多种肿瘤的发生相关[15-16]。在大多数HCC中Notch信号通路的重要因子Notch1和Jagged1的表达发生明显异常[17]。其中Hes1作为Notch通路下游重要的效应因子越来越受到人们的关注。
Hes1作为Notch的信号通路的效应因子之一,Hes1可由典型和非典型Notch途径激活,但Hes1也可被hedgehog信号途径和其他不同的途径所激活,因此,Hes1位于多种信号通路的交叉点上,被认为是治疗多种疾病的一个极好的中心靶点[18]。Hes1蛋白包含3个保守结构域:bHLH、Orange和Trp-Arg-Pro-Trp(WRPW)结构域。Hes1通过bHLH结构域介导N box(CACNAG)和WRPW结构域与分裂蛋白转导素样增强子(transducin-like enhancer of split, TLE)相互作用而发挥转录抑制作用,Hes1主要通过抑制p21kip1、p57kip2、PCNA、E2F1和CDKN1A等基因的表达来抑制细胞周期的进展[19]。
Hes1在不同类型肿瘤中的功能和其作用机制截然不同,对某些类型的肿瘤来说,Hes1可能有抑制增殖或诱导分化的作用,如在急性髓性白血病(acute myeloid leukemia, AML)细胞中,Hes1的表达通过促进p21的表达,进而引起AML细胞的细胞周期阻滞和细胞凋亡[20],而在小鼠畸胎瘤细胞P19干细胞中,维甲酸(Retinoic Acid, RA)诱导Hes1表达上调后则诱导P19细胞向神经细胞分化[21]。而在人肝癌细胞系HepG2和SNU398中,Hes1通过负向调节CDKN1C/P57促进肝癌细胞的衰老[22]。因此,Hes1基因在不同肿瘤中独特的生物学特性使其有可能成为肿瘤预警或治疗的突破点。
本研究利用腺病毒系统在HCC细胞系Hep1-6中成功介导了外源Hes1基因的表达。Hes1过表达后明显抑制了Hep1-6细胞的增殖并使其克隆形成能力下降,同时,Hes1也使Hep1-6细胞在体外的迁移与侵袭能力明显下降。因此,进一步深入研究Hes1在肝细胞癌发生中的作用及其作用的机制,可以为肝细胞癌的预防或治疗提供新的干预靶点。
作者贡献
于兵:设计研究方案、实施研究过程、撰写论文
尹刚:分析实验数据、修改论文
胡以平:提出研究思路、审核论文
| [1] |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. DOI:10.3322/caac.21551 |
| [2] |
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [3] |
Giakoustidis A, Giakoustidis D, Mudan S, et al. Molecular signalling in hepatocellular carcinoma: Role of and crosstalk among WNT/?-catenin, Sonic Hedgehog, Notch and Dickkopf-1[J]. Can J Gastroenterol Hepatol, 2015, 29(4): 209-217. DOI:10.1155/2015/172356 |
| [4] |
Nowell CS, Radtke F. Notch as a tumour suppressor[J]. Nat Rev Cancer, 2017, 17(3): 145-159. DOI:10.1038/nrc.2016.145 |
| [5] |
Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis[J]. J Clin Invest, 2017, 127(1): 137-152. |
| [6] |
Qi R, An H, Yu Y, et al. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis[J]. Cancer Res, 2003, 63(23): 8323-8329. |
| [7] |
Viatour P, Ehmer U, Saddic LA, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway[J]. J Exp Med, 2011, 208(10): 1963-1976. DOI:10.1084/jem.20110198 |
| [8] |
Li L, Tang P, Li S, et al. Notch signaling pathway networks in cancer metastasis: a new target for cancer therapy[J]. Med Oncol, 2017, 34(10): 180. DOI:10.1007/s12032-017-1039-6 |
| [9] |
Rani A, Greenlaw R, Smith RA, et al. HES1 in immunity and cancer[J]. Cytokine Growth Factor Rev, 2016, 30: 113-117. DOI:10.1016/j.cytogfr.2016.03.010 |
| [10] |
Murata K, Hattori M, Hirai N, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1[J]. Mol Cell Biol, 2005, 25(10): 4262-4271. DOI:10.1128/MCB.25.10.4262-4271.2005 |
| [11] |
Liu ZH, Dai XM, Du B. Hes1: a key role in stemness, metastasis and multidrug resistance[J]. Cancer Biol Ther, 2015, 16(3): 353-359. DOI:10.1080/15384047.2015.1016662 |
| [12] |
Zou JH, Xue TC, Sun C, et al. Prognostic significance of Hes-1, a downstream target of notch signaling in hepatocellular carcinoma[J]. Asian Pac J Cancer Prev, 2015, 16(9): 3811-3816. DOI:10.7314/APJCP.2015.16.9.3811 |
| [13] |
Singh A, Paul MS, Dutta D, et al. Regulation of notch signaling by a chromatin modeling protein Hat-trick[J]. Development, 2019, 146(14). pii: dev170837. https://www.researchgate.net/publication/333465365_Regulation_of_notch_signaling_by_a_chromatin_modeling_protein_Hat-trick
|
| [14] |
Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease[J]. Physiol Rev, 2017, 97(4): 1235-1294. DOI:10.1152/physrev.00005.2017 |
| [15] |
Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation[J]. Int J Mol Med, 2020, 45(2): 279-297. |
| [16] |
Meurette O, Mehlen P. Notch Signaling in the Tumor Microenvironment[J]. Cancer Cell, 2018, 34(4): 536-548. |
| [17] |
Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease[J]. Hepatology, 2015, 61(1): 382-392. |
| [18] |
Li X, Cao Y, Li M, et al. Upregulation of HES1 Promotes Cell Proliferation and Invasion in Breast Cancer as a Prognosis Marker and Therapy Target via the AKT Pathway and EMT Process[J]. J Cancer, 2018, 9(4): 757-766. DOI:10.7150/jca.22319 |
| [19] |
Kamińska A, Pardyak L, Marek S, et al. Bisphenol A and dibutyl phthalate affect the expression of juxtacrine signaling factors in rat testis[J]. Chemosphere, 2018, 199: 182-190. DOI:10.1016/j.chemosphere.2018.02.011 |
| [20] |
Tian C, Yu Y, Jia Y, et al. HES1 activation suppresses proliferation of leukemia cells in acute myeloid leukemia[J]. Ann Hematol, 2015, 94(9): 1477-1483. DOI:10.1007/s00277-015-2413-0 |
| [21] |
Wakabayashi N, Kageyama R, Habu T, et al. A novel cis-acting element regulates Hes-1 gene expression in P19 embryonal carcinoma cells treated with retinoic acid[J]. J Biochem, 2000, 128(6): 1087-1095. DOI:10.1093/oxfordjournals.jbchem.a022837 |
| [22] |
Giovannini C, Gramantieri L, Minguzzi M, et al. CDKN1C/P57 is regulated by the Notch target gene Hes1 and induces senescence in human hepatocellular carcinoma[J]. Am J Pathol, 2012, 181(2): 413-422. DOI:10.1016/j.ajpath.2012.04.019 |
 2020, Vol. 47
2020, Vol. 47


