文章信息
- 胶质瘤组织中miR-218的表达及其对血管生成的作用
- MiR-218 Expression in Glioma Tissues and Its Effect on Angiogenesis
- 肿瘤防治研究, 2019, 46(8): 672-676
- Cancer Research on Prevention and Treatment, 2019, 46(8): 672-676
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.2017
- 收稿日期: 2018-12-28
- 修回日期: 2019-06-13
2. 200040 上海,复旦大学附属华山医院检验医学科
2. Department of Clinical Laboratory, Huashan Hospital of Fudan University, Shanghai 200040, China
胶质瘤(glioma)是中枢神经系统肿瘤中最常见的恶性肿瘤,约占全部颅内肿瘤的40%~50%。目前即使联合手术、放疗和化疗等多种治疗手段,恶性胶质瘤的中位生存期仍只有诊断后14.6月[1]。胶质瘤的总体治愈率近10年来没有得到显著提高,原因在于其富血管性和高侵袭性。微小RNAs(microRNAs, miRNAs)是一类长度约18~24个核苷的小的、非编码RNA分子,通过与靶基因mRNA 3’-UTR区域配对调控基因的表达,调节细胞的分化、凋亡、周期、增殖和分裂等生理病理过程[2-5],如今以miRNAs为基础治疗的方法已经得到越来越多的关注[6-7]。在胶质瘤中,已有多种miRNAs被证实异常表达并存在包括miR-26a、miR-769-3p、miR-218在内的miRNAs网络调控系统[8],其中miR-218也被报道在多种肿瘤中扮演抑癌基因的角色,如胃癌、肺癌等[9-10],但其在胶质瘤中的研究仍然相对较少,因此本实验检测miR-218在临床胶质瘤样本中的表达情况,并探究miR-218对胶质瘤血管生成的影响,进一步挖掘了其潜在分子机制,发现p70s6k1是miR-218影响胶质瘤进展的新靶点。P70s6k1是细胞生长、增殖及血管新生的关键性调节因子,主要促进蛋白质的翻译,受磷脂酰肌醇3激酶(PI3K)以及哺乳动物雷帕霉素靶标(mTOR)信号途径的调节,PI3K/Akt/mTOR/p70s6k1信号通路已经被公认在促进细胞生长、增殖、侵袭、抗凋亡及促进血管生成等细胞生命活动中发挥着关键作用[11]。本实验为今后临床研究和分子靶向治疗提供了新的理论基础。
1 材料与方法 1.1 细胞株和组织标本人胶质瘤细胞株U87、人脐静脉血管内皮细胞HUVEC购自美国ATCC公司;裸鼠购自上海实验动物中心;28例临床组织标本(依据WHO病理分级,癌旁组织7例及胶质瘤2/3/4级各7例)来自上海市静安区中心医院神经外科,病例组织样本均来自手术切除标本,并且通过医院伦理委员会审核(伦(2016-10)号)。
1.2 稳定细胞株构建通过慢病毒感染U87细胞构建稳定表达miR-NC和miR-218的细胞株(U87/miR-NC和U87/miR-218),并在此基础上对U87/miR-NC和U87/miR-218稳定细胞株分别转染Scramble和(或)p70s6k1质粒,构建三组不同表达的稳定细胞株(U87/miR-NC+Scramble、U87/miR-218+Scramble及U87/miR-218+p70s6k1),观察不同组产生的条件培养基在体外对微管形成的影响,同时裸鼠基质胶塞实验观察不同组细胞在体内对血管新生的影响。
1.3 小管形成实验人脐静脉内皮细胞(HUVEC)在含有0.2%FBS的EBM-2基础培养基中培养24 h后,用于小管形成测定。用含1%FBS的DMEM培养基培养U87/miR-218和U87/miR-NC细胞24 h后,收集条件培养基,并储存在-20℃以备用。将HUVEC细胞消化计数并重悬于EBM-2基础培养基中,将细胞与等体积的条件培养基混合,并以每孔2×104个细胞的密度接种在Matrigel预处理的96孔板上。12 h后在光学显微镜下观察小管形成。使用CellSens Standard软件测量每个孔管的总长度。另外三组不同表达状态的细胞(U87/miR-NC+Scramble, U87/miR-218+Scramble, U87/miR-218+p70s6k1)进行相同处理。
1.4 裸鼠基质胶塞实验收获稳定表达miR-218和miR-NC(阴性对照)的U87细胞并重悬浮在无血清培养基中。取将100 μl细胞悬液(约含细胞2×106个)与200 μl Matrigel混合后立即注射到裸鼠的两侧。植入后第11天,处死裸鼠并剥离基质胶栓塞,测量血红蛋白。另外三组不同表达状态的细胞(U87/miR-NC+Scramble, U87/miR-218+Scramble, U87/miR-218+p70s6k1)进行相同处理。
1.5 免疫印迹实验提取细胞或组织蛋白,取等量10~20 μg蛋白在80 V下电泳。冰浴条件下90 V电转110 min将分离胶上的条带蛋白转移至PVDF膜上,电泳结束后取下PVDF膜。将PVDF膜浸于封闭液中1 h,洗涤后一抗4℃(1:1 000)孵育过夜,洗涤后二抗(1:5 000)孵育2 h,暗室显影。以GAPDH为内参检测各组细胞中p70s6k1蛋白表达水平。
1.6 荧光实时定量PCR(qRT-PCR)用TRIzol试剂根据试剂盒操作方法提取组织或细胞RNA,转录全程cDNA或miR-218,采用GAPDH作为内参检测各组细胞中p70s6k1的mRNA水平,以U6基因为内参检测不同细胞与组织中miR-218的表达水平,按照各自程序进行扩增。
1.7 报告基因检测构建靶基因p70s6k1 3’-UTR的荧光素酶报告质粒(wt)及其核心序列突变的荧光素酶报告基因质粒(mut)。分别将pMIR-p70s6k1-WT、pMIR-p70s6k1-mut、海肾荧光基因质粒以及miR-NC、miR-218共同转染U87胶质瘤细胞,24 h后将培养基吸弃,每孔加入200 μl裂解缓冲液,振荡15 min,充分裂解细胞,取80 μl裂解液加入96孔板中,GloMax检测仪检测荧光信号强度。
1.8 统计学方法实验数据经过不少于3次的独立实验算得平均值并用GraphPad Prism 5进行相关分析,组间数据进行t检验,使用GraphPad Prism 5进行方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 MiR-218在临床胶质瘤组织中表达下调并与WHO分级相关qRT-PCR结果显示,相较于癌旁组织,胶质瘤组织中miR-218的表达量明显减少,见图 1A。此外,随着胶质瘤WHO病理级别的升高,miR-218的表达量逐渐降低,提示miR-218的表达水平与胶质瘤的恶性程度密切相关,见图 1B。
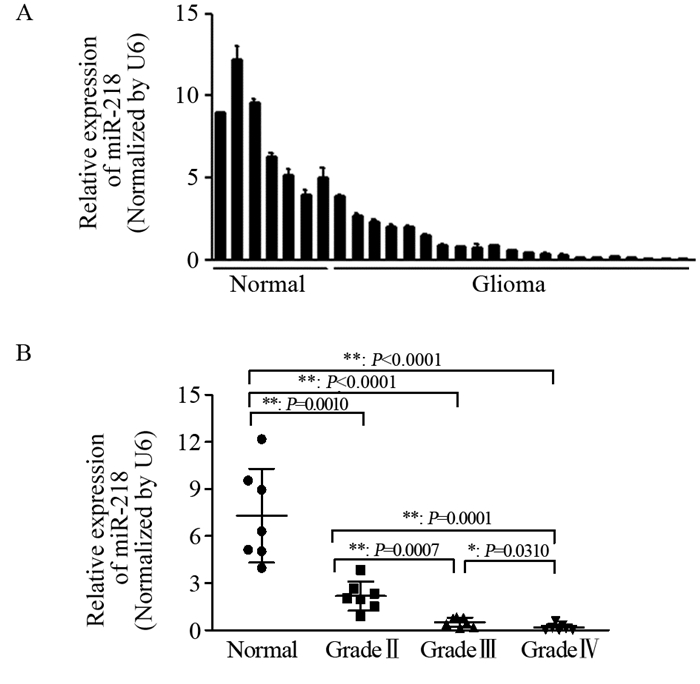
|
| 图 1 MiR-218在胶质瘤中的表达下降(A)且与胶质瘤WHO分级相关(B) Figure 1 MiR-218 expression was down-regulated in glioma tissues (A) and associated with WHO stage(B) |
小管形成实验结果显示,由过表达miR-218的U87细胞制备的条件培养基培养的HUVEC细胞微管形成数量明显低于对照组(P < 0.01),见图 2A。通过裸鼠基质胶塞实验发现,U87/miR-218组血管生成较U87/miR-NC组显著降低,见图 2B。
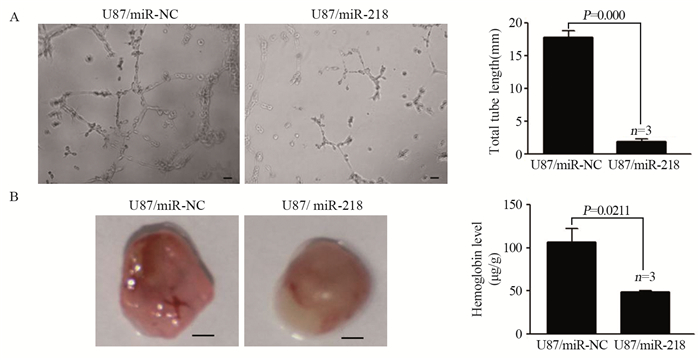
|
| A: MiR-218 inhibited tube formation in vitro; Bar: 50μm; B: MiR-218 inhibited glioma angiogenesis in vivo; Bar: 2mm 图 2 MiR-218抑制胶质瘤新血管生成的能力 Figure 2 MiR-218 inhibited glioma angiogenesis in vitro and vivo |
MiRNAs调控基因表达的主要方式是与目标基因mRNA3’-UTR区域配对结合从而诱导靶基因mRNA降解或者抑制其翻译。通过生物信息学软件发现miR-218可与p70s6k1的3’-UTR一段序列相匹配,见图 3A。此外,荧光素酶报告基因实验结果表明miR-218通过与靶基因p70s6k1 3’-UTR序列互补结合从而抑制野生型报告基因pMIR-p70s6k1-wt活性,但对其突变型报告基因pMIR-p70s6k1-mut却无明显作用,见图 3B。免疫印迹实验显示过表达miR-218显著降低p70s6k1的蛋白表达水平,见图 3C。qRT-PCR结果发现过表达miR-218的细胞株中p70s6k1的mRNA水平降低,证明miR-218可以在转录水平抑制p70s6k1,从而减少其蛋白水平的表达。
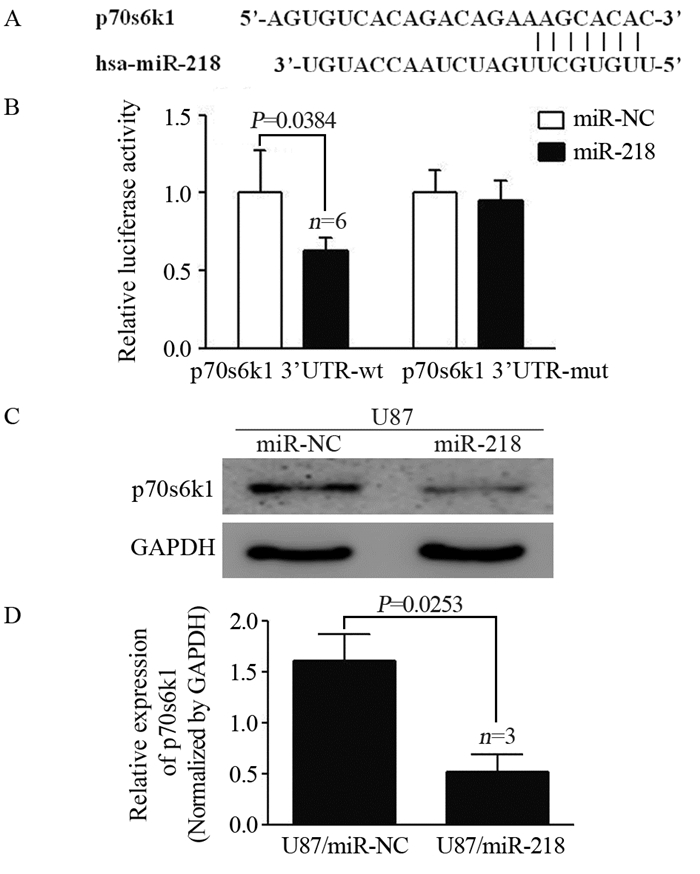
|
| A: The sequence of the relevant 3'-UTR region of p70s6k1 matched the miR-218 core sequence; B: Luciferase activity of the wild-type reporter gene pMIR-p70s6k1-wt and the mutant reporter gene pMIR-p70s6k1-mut in miR-218 and miR-NC; C: Western blot assay was used to detect the protein expression level of p70s6k1 in U87 cells; D: qRT-PCR was used to detect the expression level of p70s6k1 mRNA in U87 cells 图 3 MiR-218直接靶向p70s6k1 Figure 3 MiR-218 directly targeted p70s6k1 |
小管形成实验结果显示过表达miR-218组能在体内外显著抑制血管新生,见图 4A,而同时裸鼠基质胶塞实验也证明过表达p70s6k1的miR-218组血管新生作用被部分逆转,见图 4B。
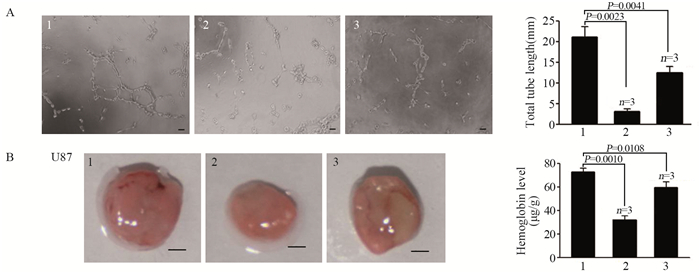
|
| 1: miR-NC+Scramble; 2: miR-218+Scramble; 3: miR-218+p70s6k1; A: p70s6k1 reversed the inhibition effect of miR-218 on tube formation, Bar: 50μm; B: p70s6k1 reversed the inhibition effect of miR-218 on glioma tumor angiogenesis in vivo, Bar: 2mm 图 4 过表达p70s6k1可部分逆转miR-218对胶质瘤血管新生的抑制作用 Figure 4 Overexpression of p70s6k1 could partially reverse inhibition effect of miR-218 on glioma angiogenesis |
目前国内外各项研究均表明miR-218在肿瘤的发生发展中扮演了抑癌基因的作用且与肿瘤的进展及预后密切相关[12-13],已有文献报道miR-218可以通过靶向E2F2、LHFPL3、HMGB1、Robo1等基因调控胶质瘤的发生发展并能通过靶点与其他miRNAs相互联系形成网络调控系统[8, 14-17]。此外,有学者统计了98例胶质瘤组织及癌旁组织中miR-218的表达情况并进行分析,发现miR-218的表达与神经胶质瘤患者的WHO分级和卡氏评分(Karnofsky performance score, KPS)密切相关,并与患者的无病生存期和总生存期独立相关[18]。本实验结果与前期文献报道结果吻合,通过对7例癌旁组织样本及21例不同级别胶质瘤组织样本进行检测,也发现在胶质瘤组织中miR-218表达降低,且随着胶质瘤WHO级别的增高,表达水平越低,但因样本量不多,可能存在个体差异性,实验存在局限性,后期实验将继续扩大样本量的收集,并进行miR-array芯片的分析,以明确胶质瘤中miR-218的表达情况及与胶质瘤级别的关系。
此外,本实验发现过表达miR-218对胶质瘤血管新生的生物学功能抑制作用较为明显,经过深入研究其调控机制发现,miR-218能直接靶向p70s6k1。PI3K/mTOR/p70s6k1在多项研究中均被证明在肿瘤血管生成等细胞生物学功能上发挥着至关重要的作用,且mTOR/p70s6k1已经被证明为癌症治疗过程中最重要的靶标之一[19-20]。本实验通过逆转p70s6k1后发现miR-218对血管新生的功能被部分逆转,因此确认miR-218确实可通过p70s6k1调控肿瘤血管生成,但本实验并未深入阐明其相关的下游信号转导机制,且细胞品种选择较为单一,计划后续在其他胶质瘤细胞系中进行功能及机制的验证。
综上,本实验确定了miR-218新的靶基因,从对miR-218的功能研究深入到机制研究,通过对临床组织样本的检测分析,并结合各类细胞实验及裸鼠基质胶塞实验,阐明miR-218/p70s6k1影响胶质瘤血管生成上的作用,为胶质瘤发生发展的分子机制提供理论依据,同时为应用miR-218/p70s6k1调控轴作为肿瘤治疗靶点进行胶质瘤治疗奠定理论基础。
作者贡献
陈秋丹:实验的整体设计、分子生物学实验、动物实验、统计学分析及撰稿
陈淑英:细胞生物学实验的实施
| [1] | Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma[J]. N Engl J Med, 2005, 352(10): 987–996. DOI:10.1056/NEJMoa043330 |
| [2] | Fesler A, Guo S, Liu H, et al. Overcoming chemoresistance in cancer stem cells with the help of microRNAs in colorectal cancer[J]. Epigenomics, 2017, 9(6): 793–796. DOI:10.2217/epi-2017-0041 |
| [3] | Blanca A, Cheng L, Montironi R, et al. Mirna Expression in Bladder Cancer and Their Potential Role in Clinical Practice[J]. Curr Drug Metab, 2017, 18(8): 712–722. |
| [4] | Singh A, Sen D. MicroRNAs in Parkinson's disease[J]. Exp Brain Res, 2017, 235(8): 2359–2374. DOI:10.1007/s00221-017-4989-1 |
| [5] | Khoshnam SE, Winlow W, Farzaneh M. The Interplay of MicroRNAs in the Inflammatory Mechanisms Following Ischemic Stroke[J]. J Neuropathol Exp Neurol, 2017, 76(7): 548–561. DOI:10.1093/jnen/nlx036 |
| [6] | Momtazi AA, Banach M, Pirro M, et al. MicroRNAs: New Therapeutic Targets for Familial Hypercholesterolemia?[J]. Clin Rev Allergy Immunol, 2018, 54(2): 224–233. DOI:10.1007/s12016-017-8611-x |
| [7] | Deng J, Xiao J, Ma P, et al. Manipulation of Viral MicroRNAs as a Potential Antiviral Strategy for the Treatment of Cytomegalovirus Infection[J]. Viruses, 2017, 9(5): pii: E118. DOI:10.3390/v9050118 |
| [8] | Liu F, Xiong Y, Zhao Y, et al. Identification of aberrant microRNA expression pattern in pediatric gliomas by microarray[J]. Diagn Pathol, 2013, 8: 158. |
| [9] | Zhang XL, Shi HJ, Wang JP, et al. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin[J]. World J Gastroenterol, 2014, 20(32): 11347–11355. DOI:10.3748/wjg.v20.i32.11347 |
| [10] | Shi ZM, Wang L, Shen H, et al. Downregulation of miR-218 contributes to epithelial-mesenchymal transition and tumor metastasis in lung cancer by targeting Slug/ZEB2 signaling[J]. Oncogene, 2017, 36(18): 2577–2588. DOI:10.1038/onc.2016.414 |
| [11] | Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives[J]. Cancer Treat Rev, 2019, 72: 45–55. DOI:10.1016/j.ctrv.2018.11.001 |
| [12] | Kumamoto T, Seki N, Mataki H, et al. Regulation of TPD52 by antitumor microRNA-218 suppresses cancer cell migration and invasion in lung squamous cell carcinoma[J]. Int J Oncol, 2016, 49(5): 1870–1880. DOI:10.3892/ijo.2016.3690 |
| [13] | Wang J, Zhou Y, Fei X, et al. Integrative bioinformatics analysis identifies ROBO1 as a potential therapeutic target modified by miR-218 in hepatocellular carcinoma[J]. Oncotarget, 2017, 8(37): 61327–61337. |
| [14] | Xuan C, Jin M, Gao Y, et al. miR-218 suppresses the proliferation of osteosarcoma through downregulation of E2F2[J]. Oncol Lett, 2019, 17(1): 571–577. |
| [15] | Li Z, Qian R, Zhang J, et al. MiR-218-5p targets LHFPL3 to regulate proliferation, migration and epithelial-mesenchymal transitions of human glioma cells[J]. Biosci Rep, 2018, 39(3): pii: BSR20180879. |
| [16] | Gu J, Xu R, Li Y, et al. MicroRNA-218 modulates activities of glioma cells by targeting HMGB1[J]. Am J Transl Res, 2016, 8(9): 3780–3790. |
| [17] | Gu JJ, Gao GZ, Zhang SM. MiR-218 inhibits the tumorgenesis and proliferation of glioma cells by targeting Robo1[J]. Cancer Biomark, 2016, 16(3): 309–317. DOI:10.3233/CBM-160568 |
| [18] | Cheng MW, Wang LL, Hu GY. Expression of microRNA-218 and its clinicopathological and prognostic significance in human glioma cases[J]. Asian Pac J Cancer Prev, 2015, 16(5): 1839–1843. DOI:10.7314/APJCP.2015.16.5.1839 |
| [19] | Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment[J]. Drug Resist Updat, 2008, 11(3): 63–76. DOI:10.1016/j.drup.2008.03.001 |
| [20] | Bahrami A, Hasanzadeh M, Hassanian SM, et al. The potential value of the PI3K/Akt/mTOR signaling pathway for assessing prognosis in cervical cancer and as a target for therapy[J]. J Cell Biochem, 2017, 118(12): 4163–4169. DOI:10.1002/jcb.26118 |
 2019, Vol. 46
2019, Vol. 46
