文章信息
- 胶质瘤中MKK7与c-Jun磷酸化的表达及其相关性
- Expression of MKK7 and Phospho-c-Jun in Glioma and Their Correlation
- 肿瘤防治研究, 2019, 46(9): 790-795
- Cancer Research on Prevention and Treatment, 2019, 46(9): 790-795
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.1780
- 收稿日期: 2018-11-21
- 修回日期: 2019-06-26
2. 510260 广州,广州医科大学附属第二医院神经外科,广州医科大学神经科学研究所
2. Department of Neurosurgery, The Second Affiliated Hospital and Institute of Neurosciences of Guangzhou Medical University, Guangzhou 510260, China
胶质瘤是中枢神经系统最常见的颅内原发恶性肿瘤,在所有颅内原发性肿瘤中占70%左右,其中成年人中最常见、恶性程度最高、预后最差的肿瘤是胶质母细胞瘤(glioblastoma, GBM, WHO Ⅳ)[1]。该肿瘤具有恶性增殖和高侵袭性的特点,手术及放化疗后肿瘤复发率高。
JNK/c-Jun信号通路在调控细胞增殖、分化、凋亡和存活等基本生物学效应中扮演重要角色。JNK磷酸化下游底物c-Jun并使其激活后,可增强肿瘤细胞的增殖、侵袭和迁移能力[2-4]。MKK4和MKK7作为丝裂原活化蛋白激酶激酶(mitogen-activated protein kinase kinase, MAPKK)家族的两个成员,均可以磷酸化并激活JNK[5-7]。有报道称MKK7通过调控JNK活性调控细胞凋亡[8-10]。本课题组前期研究也发现在神经胶质瘤细胞株U251中,小分子干扰沉默MKK7表达后,可以抑制JNK/c-Jun激活[11]。为进一步了解JNK信号通路的调控机制,本研究通过采用免疫组织化学法、小分子干扰、转染及Western blot等技术检测不同组织学类型胶质瘤样本及U87细胞株中MKK7、c-Jun及其磷酸化的表达情况,分析并验证MKK7是否为直接调控JNK/c-Jun活性的关键分子。
1 资料与方法 1.1 资料收集2015年11月—2017年11月广州医科大学附属第二医院病理科存档的胶质瘤石蜡包埋标本117例,其中92例胶质瘤和25例胶质母细胞瘤瘤旁正常脑组织。按2016年版“WHO中枢神经系统肿瘤的病理分类”,92例胶质瘤中:WHOⅡ:弥漫型星形细胞瘤15例,少突胶质细胞瘤5例;WHOⅢ:间变性星形细胞瘤11例,间变性少突胶质细胞瘤8例;WHOⅣ:胶质母细胞瘤53例(仅25例有相应的正常对照),患者临床病理特征见表 1。所有患者术前均未行放化疗,均知情同意。本研究得到医院医学伦理会审核批准。
人脑胶质瘤U87细胞株购自上海细胞生物学研究所中国科学院细胞库。胎牛血清、0.25%胰酶购于美国Gibco公司。培养基高糖DMEM、转染试剂RNAi MAX、opti-MEM reduced serum medium均购于美国Invitrogen公司。MKK7-1 siRNA、MKK7-2 siRNA、MKK4 siRNA、空白对照siRNA购自上海Gene Pharma公司。anti-MKK7(#4172)、anti-MKK4(#9152)、anti-p-c-Jun(#3270)、anti-GAPDH(#2118s)均购于美国Cell Signaling Technology公司。anti-c-Jun(sc-74543)购美国Santa Cruz Biotechnology公司。
MKK4-siRNA小分子片段序列正义链:5’-GCCUUACGAAGGAUGAAUCCATT-3’,反义链:5' -UGGAUUCAUCCUUCGUAAGGCTT-3’;MKK7-siRNA1小分子片段序列正义链:5’-CCAACACGGACGUCUUCAU-3’,反义链:5’-AUGAAGACGUCCGUGUUGG-3’;MKK7-siRNA2小分子片段序列正义链:5’-GCUGGCAACAGGACAGUUU-3’,反义链:5’-AAACUGUCCUGUUGCCAGG-3’。
1.2 方法 1.2.1 免疫组织化学染色及评判标准所有石蜡包埋病理标本均经10%中性福尔马林固定,常规HE染色组织学观察。采用Envision二步法进行免疫组织化学染色。检测标志物:MKK7、c-Jun和p-c-Jun。4 μm厚度切片经脱蜡水化和抗原修复后,滴加一抗,放入37℃水浴箱孵育60 min,PBS冲洗后按照采用Envision检测试剂盒(DAKO公司)说明书进行,室温孵育30 min,再次PBS冲洗,DAB显色,苏木精对比染色,然后脱水、透明、封片,每例均设阳性和阴性对照。按照抗体说明书分别用膀胱组织及肺癌组织作为阳性对照,另外用PBS代替一抗作为阴性对照。
免疫组织化学结果半定量判定:染色程度:基本不着色为0分;着色呈淡黄色为1分;着色呈黄色为2分;着色呈棕褐色为3分。染色阳性细胞百分比计数,计算阳性肿瘤细胞占总肿瘤细胞的比例,将其分为5个等级:着色阳性细胞占计数细胞≤5%为0分(-),5%~25%为1分(+),> 25%~50%为2分(++),> 50%~75%为3分(+++),> 75%为4分(++++)。将染色程度分级与染色细胞百分比相乘,乘积≥4分为高表达,< 4分为低表达[12-13]。
1.2.2 细胞培养、转染和Western blot检测将U87细胞株置于10%胎牛血清、高糖DMEM液体培养液,37℃、5%CO2及饱和湿度条件下培养,每3~5天用胰酶消化传代1次,取对数生长期细胞进行实验。
于6孔板内接种对数生长期U87细胞,细胞密度为2×105个每孔,培养液为不含抗生素的DMEM培养液。待细胞生长至60%~80%融合度时,更换为不含血清的DMEM培养液,12 h后按转染试剂RNAi MAX转染方法将MKK4-siRNA、MKK7-siRNA1、MKK7-siRNA2和空白对照siRNA分别转染入U87细胞。
U87转染细胞于37℃培养箱培养48 h后,每孔2 ml PBS漂洗2遍,每孔加入150 μl IP细胞裂解液(50 mmol/L Tris, HCl pH8.0, 150 mmol/L NaCl, 1%Triton×100, 100 μg/ml PMSF),10 min后收集各组细胞。按照IP裂解液法提取细胞总蛋白,并进行BCA法测定蛋白浓度。灌制4%集成胶和10%分离胶。200 V电压电泳45 min。100 V电压转膜60 min。5%脱脂奶粉室温封闭1 h。一抗(anti-MKK4 1:1 000,anti-MKK7 1:1 000,anti-c-Jun 1:1 000,anti-p-c-Jun 1:1 000,anti-GAPDH 1:5 000)4℃孵育过夜,HRP标记抗兔或抗鼠室温孵育1 h。ECL化学发光后曝光。
1.3 统计学方法用SPSS20.0统计分析软件包进行数据处理。多组间MKK7及p-c-Jun表达率的比较用多个样本率的χ2检验。数据以平均值±标准差(x±s)表示,多样本间比较采用单因素方差分析One-way ANOVA,两组间的比较采用独立样本t检验。相关性分析采用Spearman等级相关分析。P < 0.05为差异有统计学意义。
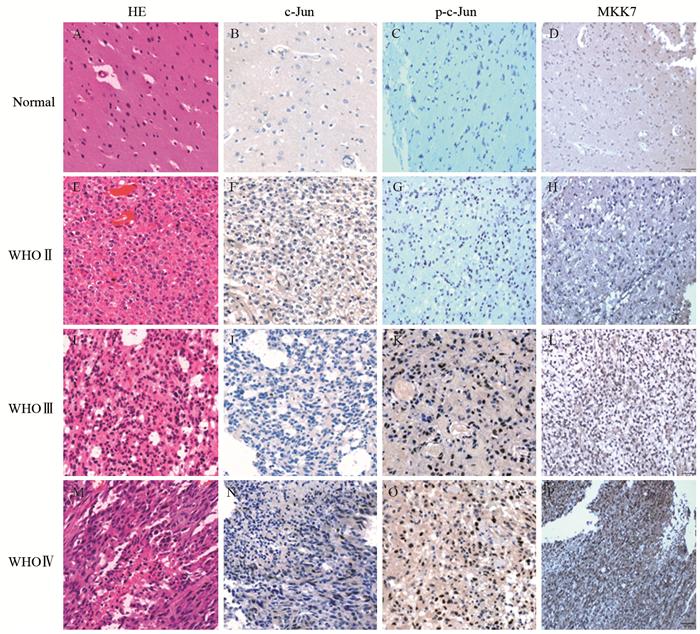
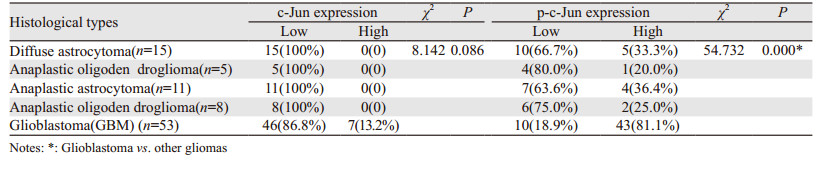
2 结果 2.1 c-Jun和p-c-Jun在胶质瘤及胶质母细胞瘤瘤旁正常脑组织中的表达c-Jun和p-c-Jun阳性表达表现为细胞核内见棕黄色颗粒,见图 1。25例胶质母细胞瘤中3例(12%)c-Jun高表达,其瘤旁正常脑组织中均低表达,两者间差异无统计学意义(χ2=3.128, P=0.077),而胶质母细胞瘤中p-c-Jun高表达19例(76%),明显高于瘤旁正常脑组织6例(4%),差异有统计学意义(χ2=27.000, P=0.000)。胶质母细胞瘤中c-Jun的表达与其他类型胶质瘤无明显差异(P=0.086),但p-c-Jun表达明显升高(P=0.000),见表 2。根据WHO分级,WHO Ⅳ级胶质瘤p-c-Jun高表达率明显高于WHO Ⅱ级及WHO Ⅲ级胶质瘤(P=0.000),见图 2A,且p-c-Jun表达强度与胶质瘤WHO分级呈明显正相关(r=0.494, P=0.000)。
|
| A-D: c-Jun, p-c-Jun and MKK7 expression in normal brain tissues adjacent to glioblastoma; E-H: oligodendroglioma(WHO Ⅱ); I-L: anaplastic astrocytoma(WHO Ⅲ); M-P: glioblastoma (WHO Ⅳ); A, E, I, M: HE×200; B-D, F-H, J-L, N-P: IHC ×200 图 1 不同WHO分级胶质瘤及胶质母细胞瘤瘤旁正常脑组织中c-Jun、p-c-Jun、MKK7的表达 Figure 1 Expression of c-Jun, p-c-Jun and MKK7 in gliomas with different WHO classification and normal brain tissues adjacent to glioblastoma |
|
|
| 1: normal brain tissue adjacent to GBM; 2: WHOⅡ; 3: WHOⅢ; 4: WHOⅣ 图 2 MKK7及p-c-Jun在胶质瘤中的表达相关性分析 Figure 2 Correlation of MKK7 and p-c-Jun expression in gliomas |
MKK7阳性表达表现为细胞核或质内见棕黄色颗粒,见图 1。胶质母细胞瘤中MKK7的表达高于其他类型胶质瘤及胶质母细胞瘤瘤旁正常脑组织(P=0.000),见表 3。且MKK7高表达率随着WHO分级的升高而增加(P=0.000),见图 2A。MKK7表达强度与胶质瘤WHO分级呈明显正相关(r=0.606, P=0.000)。
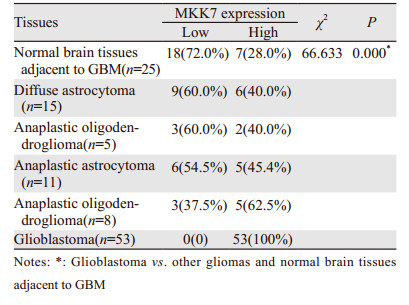
|
92例胶质瘤及25例胶质瘤母细胞瘤瘤旁正常脑组织中,48例MKK7和p-c-Jun均高表达,31例均低表达,30例MKK7高表达和p-c-Jun低表达,8例MKK7低表达和p-c-Jun高表达。根据Spearman相关分析检验,MKK7表达与c-Jun磷酸化水平正相关(r=0.387, P=0.000),见图 2B。
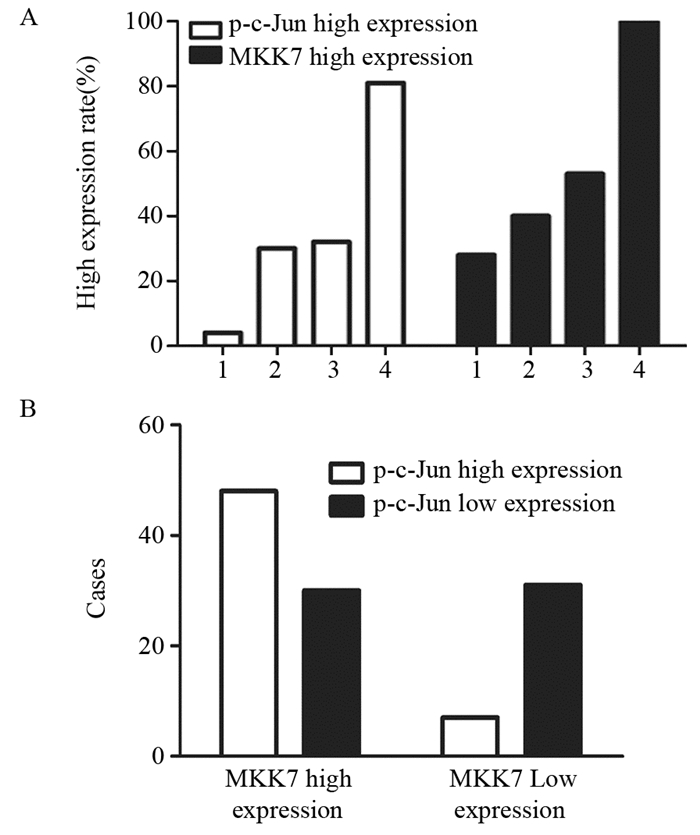
2.4 转染siRNA对MKK4、MKK7表达及c-Jun活性的影响与空白对照组siRNA相比,U87细胞转染MKK4-siRNA、MKK7-siRNA1和MKK7-siRNA2可分别显著抑制MKK4(P=0.003)和MKK7(P=0.009)表达,但只有转染MKK7-siRNA可同时下调c-Jun磷酸化水平(P=0.004),见图 3。
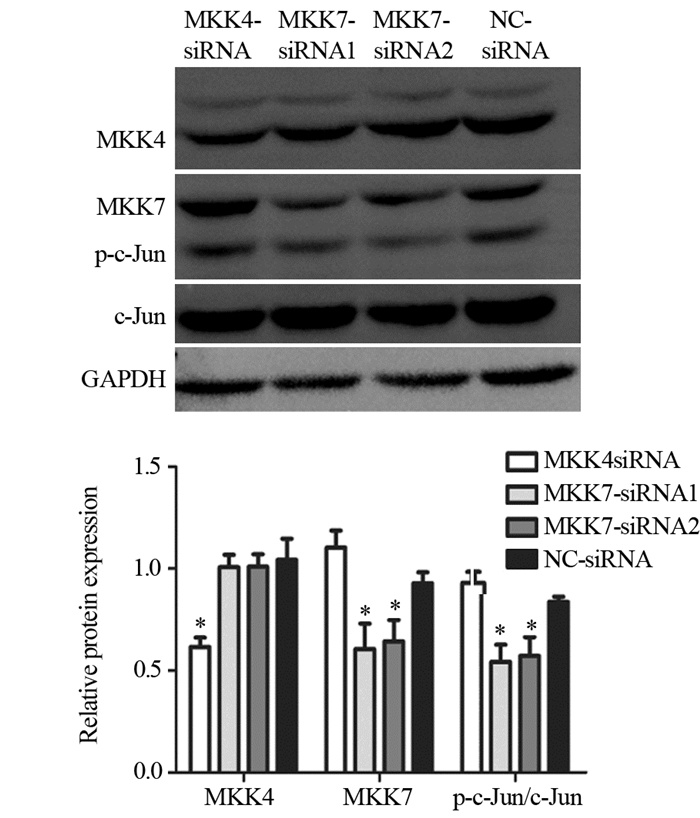
|
| *: P < 0.05, compared with NC-siRNA groups 图 3 U87细胞株小分子干扰对MKK4、MKK7的表达及对c-Jun活性的影响 Figure 3 Effects of siRNAs on MKK4, MKK7 expression and c-Jun activities in glioma U87 cell line analyzed by Western blot |
c-Jun N-末端激酶(C-Jun N-terminal kinase, JNK)属于丝裂原活化蛋白激酶家族,其家族包括JNK1、JNK2和JNK3[14],其中JNK1和JNK2广泛表达于各种组织中,而JNK3主要表达于脑、心脏及睾丸等组织中[15]。JNKs参与许多生理过程,如炎性反应、细胞增殖、分化及死亡,Nacken等[16]研究表明JNK活化可促进流感病毒A(IAV)的非结构蛋白(NS1)的表达,从而诱导细胞的凋亡。而且肿瘤的发生和进展也存在JNKs的持续激活,有研究通过体内或体外实验证明多种恶性肿瘤与JNK/c-Jun的激活密切相关,如胃癌、肝癌、胰腺癌、骨肉瘤、脑肿瘤及结直肠癌等中均存在不同JNK蛋白的高表达或突变[17-20]。本研究基于免疫组织化学方法,探讨胶质瘤中c-Jun的磷酸化水平的表达情况。与正常脑组织及非胶质母细胞瘤相比,胶质母细胞瘤中磷酸化c-Jun的表达明显升高,胶质母细胞瘤的高表达率为81.1%,表明磷酸化c-Jun过表达参与了胶质母细胞瘤的发生与发展。
替莫唑胺(TMZ)是目前公认的治疗脑胶质瘤效果较好的化疗药物,然而脑胶质瘤对TMZ产生耐药性是导致化疗失败的重要原因。因此,为胶质母细胞瘤的化疗寻找新的靶点及新的化疗药物成为了胶质瘤的研究热点。有研究证明在JNK/c-Jun激活后,可以增强替莫唑胺和尼莫司汀等烷化剂等药物对胶质母细胞瘤的促凋亡作用。Tomicic等[21]发现烷基化抗癌药物作用于胶质瘤细胞LN-229后,JNK/c-Jun激活参与了晚期促凋亡反应。Ueno等[22]通过建立耐TMZ细胞模型,与对照组相比,试验组c-Jun终末激酶(JNK)的磷酸化水平增加,其下游信号通路的活性增加。上调JNK表达或siRNA特异性干扰及JNK抑制剂抑制JNK表达可以促进或抑制试验组细胞迁移及侵袭。因此,表明JNK信号通路可能成为新型治疗TMZ耐药胶质瘤的靶点。本研究也进一步证明不同级别胶质瘤c-Jun的表达水平不同,为胶质瘤的化疗,特别是TMZ耐药的处理提供了证据。
JNK/c-Jun通路的激活在胶质母细胞瘤的发生发展过程中起重要作用,但其上游调控机制并不完全清楚。JNK上游有两个MAPKK,即MKK4和MKK7。我们通过小分子干扰的方法沉默胶质瘤U87细胞株中MKK4及MKK7,发现沉默MKK7后c-Jun磷酸化水平明显下调,而沉默MKK4后c-Jun磷酸化水平无明显变化,在细胞学水平证明了MKK7是调控JNK/c-Jun活性及胶质瘤细胞增殖的关键分子。为了进一步探讨MKK7和磷酸化c-Jun的表达在胶质瘤发生发展中的关系。本研究检测了胶质母细胞瘤瘤旁正常脑组织与不同组织学类型及WHO分级的胶质瘤中MKK7和磷酸化c-Jun的表达情况,发现随着胶质瘤WHO分级的增加,MKK7及磷酸化c-Jun的高表达率明显升高;且MKK7及磷酸化c-Jun的表达正相关,进一步验证了MKK7及JNK/c-Jun通路的信号在胶质母细胞瘤发生发展的中的重要作用,为寻找胶质瘤化疗的新靶点、新化疗药物提供重要的理论依据。
作者贡献
郅程:实验数据分析、全文统筹及文章撰写
赖妙玲、廖德贵:病理组织实验
郝卓芳:审核临床病理诊断准确性
王业忠:整体把握实验设计的合理性
吴力强、刘锶锶、曾淑莲、黄紫燕、陈丹敏:负责分子生物学实验
袁忠民:整体把握实验设计的合理性及文章修改
| [1] | Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011[J]. Neuro Oncol, 2014, 16 Suppl 4: ⅳ1–63. |
| [2] | Peng B, Chai Y, Li Y, et al. CIP2A overexpression induces autoimmune response and enhances JNK signaling pathway in human lung cancer[J]. BMC Cancer, 2015, 15: 895. DOI:10.1186/s12885-015-1899-0 |
| [3] | Shen KH, Li CF, Chien LH, et al. Role of galectin-1 in urinary bladder urothelial carcinoma cell invasion through the JNK pathway[J]. Cancer Sci, 2016, 107(10): 1390–1398. DOI:10.1111/cas.13016 |
| [4] | Sun W, Wu X, Gao H, et al. Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells[J]. Free Radic Biol Med, 2017, 108: 433–444. DOI:10.1016/j.freeradbiomed.2017.04.010 |
| [5] | Wolle P, Hardick J, Cronin SJF, et al. Targeting the MKK7-JNK (Mitogen-Activated Protein Kinase Kinase 7-c-Jun N-Terminal Kinase) Pathway with Covalent Inhibitors[J]. J Med Chem, 2019, 62(5): 2843–2848. DOI:10.1021/acs.jmedchem.9b00102 |
| [6] | Shraga A, Olshvang E, Davidzohn N, et al. Covalent Docking Identifies a Potent and Selective MKK7 Inhibitor[J]. Cell Chem Biol, 2019, 26(1): 98–108. DOI:10.1016/j.chembiol.2018.10.011 |
| [7] | Syc-Mazurek SB, Rausch RL, Fernandes KA, et al. Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death[J]. Cell Death Dis, 2018, 9(11): 1095. DOI:10.1038/s41419-018-1079-7 |
| [8] | Lin W, Wang S, Yang Z, et al. Heme Oxygenase-1 Inhibits Neuronal Apoptosis in Spinal Cord Injury through Down-Regulation of Cdc42-MLK3-MKK7-JNK3 Axis[J]. J Neurotrauma, 2017, 34(3): 695–706. DOI:10.1089/neu.2016.4608 |
| [9] | He P, Zhang B, Liu D, et al. Hepatitis B Virus X Protein Modulates Apoptosis in NRK-52E Cells and Activates Fas/FasL Through the MLK3-MKK7-JNK3 Signaling Pathway[J]. Cell Physiol Biochem, 2016, 39(4): 1433–1443. DOI:10.1159/000447846 |
| [10] | Wang S, Zhang T, Yang Z, et al. Heme oxygenase-1 protects spinal cord neurons from hydrogen peroxide-induced apoptosis via suppression of Cdc42/MLK3/MKK7/JNK3 signaling[J]. Apoptosis, 2017, 22(3): 449–462. DOI:10.1007/s10495-016-1329-z |
| [11] | 袁忠民, 曾敏灵, 唐晓梅, 等. 组蛋白去乙酰化酶抑制剂对胶质瘤细胞增殖及MKK7表达的影响[J]. 肿瘤防治研究, 2016, 43(1): 1–5. [ Yuan ZM, Zeng ML, Tang XM, et al. Effects for Histone Deacetylase Inhibitors on Proliferation and MKK7 Expression in Glioma Cells[J]. Zhong Liu Fang Zhi Yan Jiu, 2016, 43(1): 1–5. DOI:10.3971/j.issn.1000-8578.2016.01.001 ] |
| [12] | Puechl AM, Edwards J, Suri A, et al. The association between progesterone receptor expression and survival in women with adult granulosa cell tumors[J]. Gynecol Oncol, 2019, 153(1): 74–79. DOI:10.1016/j.ygyno.2019.01.016 |
| [13] | Girgis H, Masui O, White NM, et al. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma[J]. Mol Cancer, 2014, 13: 101. |
| [14] | Bubici C, Papa S. JNK signalling in cancer: in need of new, smarter therapeutic targets[J]. Br J Pharmacol, 2014, 171(1): 24–37. |
| [15] | Gehringer M, Muth F, Koch P, et al. c-Jun N-terminal kinase inhibitors: a patent review (2010-2014)[J]. Expert Opin Ther Pat, 2015, 25(8): 849–872. DOI:10.1517/13543776.2015.1039984 |
| [16] | Nacken W, Wixler V, Ehrhardt C, et al. Influenza A virus NS1 protein-induced JNK activation and apoptosis are not functionally linked[J]. Cell Microbiol, 2017, 19(7). |
| [17] | Fujita M, Hasegawa A, Yamamori M, et al. In vitro and in vivo cytotoxicity of troglitazone in pancreatic cancer[J]. J Exp Clin Cancer Res, 2017, 36(1): 91. DOI:10.1186/s13046-017-0557-6 |
| [18] | Pandey V, Bhaskara VK, Babu PP. Implications of mitogen-activated protein kinase signaling in glioma[J]. J Neurosci Res, 2016, 94(2): 114–127. DOI:10.1002/jnr.23687 |
| [19] | Gao GY, Ma J, Lu P, et al. Ophiopogonin B induces the autophagy and apoptosis of colon cancer cells by activating JNK/c-Jun signaling pathway[J]. Biomed Pharmacother, 2018, 108: 1208–1215. DOI:10.1016/j.biopha.2018.06.172 |
| [20] | 彭豆豆, 陈婧, 易永芬. 抑制JNK通路对胃癌细胞SGC7901增殖、凋亡、迁移和周期的影响[J]. 基因组学与应用生物学, 2018, 37(8): 3713–3718. [ Peng DD, Chen J, Yi YF. Effect of JNK Inhibitor SP600125 on Proliferation, Apoptosis, Cell Cycle and Migration in SGC7901 Cells[J]. Ji Yin Zu Xue Yu Ying Yong Sheng Wu Xue, 2018, 37(8): 3713–3718. ] |
| [21] | Tomicic MT, Meise R, Aasland D, et al. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM[J]. Oncotarget, 2015, 6(32): 33755–33768. |
| [22] | Ueno H, Tomiyama A, Yamaguchi H, et al. Augmentation of invadopodia formation in temozolomide-resistant or adopted glioma is regulated by c-Jun terminal kinase-paxillin axis[J]. Biochem Biophys Res Commun, 2015, 468(1-2): 240–247. DOI:10.1016/j.bbrc.2015.10.122 |
 2019, Vol. 46
2019, Vol. 46