文章信息
- NDUFA4过表达对人结直肠癌细胞体外生长的影响
- Effects of NDUFA4 Overexpression on Growth of Human Colon Cancer Cells in vitro
- 肿瘤防治研究, 2019, 46(5): 406-410
- Cancer Research on Prevention and Treatment, 2019, 46(5): 406-410
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.1693
- 收稿日期: 2018-11-09
- 修回日期: 2019-03-04
2. 563099 遵义,遵义医学院免疫学教研室
2. Department of Immunology, Zunyi Medical University, Zunyi 563099, China
结直肠癌是人类最常见的恶性肿瘤之一,其发生发展机制的阐明对于诊断、治疗及预后均有重要意义[1-5]。Ⅰ型辅酶脱氢酶1α亚复合物4(NADH dehydrogenase 1 alpha subcomplex 4, NDUFA4),是由定位于人7号染色体p21.3上的NDUFA4基因编码的线粒体呼吸链蛋白质,也是哺乳动物线粒体细胞色素C氧化酶(COX; Complex Ⅳ)中的重要亚基,能够将电子从NADH转移至呼吸链,参与能量代谢[6-8]。研究显示NDUFA4的异常表达与包括肿瘤在内的多种疾病发生发展密切相关[9-12]。我们课题组前期发现NDUFA4过表达可促进人肺癌细胞的增殖和侵袭[13]。然而,NDUFA4在人结直肠癌发生中的可能作用和研究机制尚未阐明。因此,本研究利用前期构建的p-NDUFA4真核表达载体,观察NDUFA4过表达对人结直肠癌细胞体外生长的可能影响并探讨其机制,以期为后续研究NDUFA4在人结直肠癌发生中的作用提供前期实验依据。
1 材料与方法 1.1 质粒、细胞株和主要试剂人结直肠癌HCT116细胞株购自中国生命科学院,质粒p-NDUFA4和p-Cont均由本实验室保存。McCoy' s 5A细胞培养基、胎牛血清购自美国Gibco公司;100×三抗(青霉素-链霉素-两性霉素B)购自上海第四制药股份有限公司;CCK-8试剂盒购自东仁化学科技(上海)有限公司;1%结晶紫染液购自北京索莱宝科技有限公司;Lipofectamine3000试剂购自赛默飞世尔科技公司; SYBR Premix Ex Taq,Premix Ex Taq Version2.0,以及RNAisoTM Plus均购自北京宝日医生物技术有限公司;RevertAidTM First Strand cDNA Synthesis Kit反转录合成试剂盒购自美国Fermentas公司;总RNA提取试剂盒购自北京天根科技有限公司,兔抗NDUFA4、β-actin、p-AKT、AKT一抗以及兔抗ERK1/2、p-ERK1/2一抗、HRP标记的羊抗兔二抗均购自Abcam公司(英国的剑桥科学园);全蛋白提取试剂盒、ECL显色液、RIPA裂解液均购自上海生工生物工程股份有限公司。
1.2 方法 1.2.1 重组质粒p-NDUFA4瞬时转染人结直肠癌HCT116细胞将人结直肠癌HCT116细胞株置于10%优质胎牛血清、100×三抗(青霉素-链霉素-两性霉素B)、McCoy' s 5A细胞培养液中培养,待HCT116细胞密度达到80%左右时,使用0.25%胰蛋白酶37℃消化2 min,离心收集细胞,使用PBS重复冲洗3次后备用。细胞实验分组:空载体对照组(p-Cont)、重组质粒实验组(p-NDUFA4)。以每孔1×105个细胞铺于6孔板中,置于培养箱中培养12 h后,按照Lipofectamine3000转染试剂盒说明书进行转染,并于荧光显微镜下观察转染细胞的形态学变化和转染效率。
1.2.2 实时荧光定量PCR和Western blot检测转染细胞中NDUFA4的表达总RNA的提取参考文献[13]。按照RevertAidTM First Strand cDNA合成试剂盒说明书的反应条件和反应体系进行RNA反转录。
1.2.2.1 Real-time PCR反应反应体系:TaKaRa PCR Mix 10 μl,cDNA 2 μl,NDUFA4 1 μl,上下游引物各0.5 μl,ddH2O 6 μl。反应条件:95℃预变性30 s;95℃ 15 s、60℃ 1 min,40个循环。以GADPH为内参照,NDUFA4基因的PCR引物序列:上游引物:5'-TCCCCCTCTTTGTATTTATTGG-3';下游引物:5'-GGGCTCTGGGTTATTTCTGTC-3' 。反应结束后,采用2-ΔΔCt法计算NDUFA4的相对表达量[13]。
1.2.2.2 Western blot检测转染细胞中NDUFA4的表达(1)制备浓缩胶和分离胶:10%分离胶,5%浓缩胶;(2)跑胶:SDS-PAGE电泳80 V跑浓缩胶,120 V跑分离胶;(3)上样:将蛋白样品上样到SDS-PAGE胶加样孔内;(4)电泳1 h;(5)转膜:将目的蛋白转至PVDF膜(30 min);(6)封闭:转膜完毕后立刻把蛋白置于含5%脱脂奶粉的TBST中,封闭1 h;(7)一抗孵育:将兔抗人的β-actin、NDUFA4(1:2 000)加入上述封闭液中孵育,4℃冰箱过夜;(8)洗膜:24 h后用TBST洗膜,10 min×3次;(9)二抗孵育、洗膜:将含有NDUFA4蛋白的HRP标记的羊抗兔二抗(1:2 000)加入含PBST的孵育膜,1 h;PBST洗膜,10 min×3次;(10)加入等量发光试剂,进行蛋白曝光。以β-actin作为内参分析结果。
1.2.3 CCK-8法检测HCT116细胞的增殖情况以每孔1×104个细胞接种至96孔板,并设置三个复孔,每孔加入细胞悬液和培养液的总量为200 μl,待细胞密度达90%,Lipofectamine3000瞬时将p-NDUFA4质粒和p-Cont质粒转染人结直肠癌HCT116细胞。在指定的时间分别向每孔加入10 μl CCK-8试剂,并置于37℃培养箱中继续培养3 h,在酶标仪主波长为450 nm处读取每孔吸光度(OD)值变化。
1.2.4 克隆形成实验检测HCT116细胞的克隆形成能力收集已转染的细胞,制成细胞悬液,细胞悬液反复吹打,使单个细胞百分率在95%以上,并进行细胞计数,调整细胞悬液密度为1×103个/毫升细胞,并以400个/孔和1 000个/孔接种于6孔板中,用于克隆形成实验,然后将细胞置于37℃、5%CO2细胞培养箱中静置培养2~3周。当出现肉眼可见克隆时,终止培养,弃去培养液,PBS液小心清洗3次,空气干燥。甲醇固定15 min,弃甲醇后空气干燥。1%结晶紫染液染色菌落,并对菌落直径和数量进行统计学分析。
1.2.5 实时荧光定量PCR检测HCT116细胞中与生长相关基因的表达细胞周期蛋白依赖性激酶(cyclin- dependent kinase, CDK)表达的检测同前期工作[13]。
1.2.6 Western blot检测HCT116细胞中AKT、ERK1/2、磷酸化AKT、磷酸化ERK1/2蛋白的表达情况细胞蛋白质样品的制备及BCA法定量蛋白的检测、Western blot检测AKT等相关信号通路的变化的方法参考文献[13]。
1.3 统计学方法GraphPad Prism 5.0软件统计分析数据。所有数据均独立重复3次,实验数值结果以(x±s)表示。其中计量资料组间比较采用Student t检验或单因素方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 瞬时转染p-NDUFA4重组质粒后HCT116细胞中NDUFA4的表达结果显示:与p-Cont组相比,p-NDUFA4组中NDUFA4 mRNA和蛋白表达水平均明显上调(Pm-RNA=0.0015,P蛋白=0.0024),见图 1。
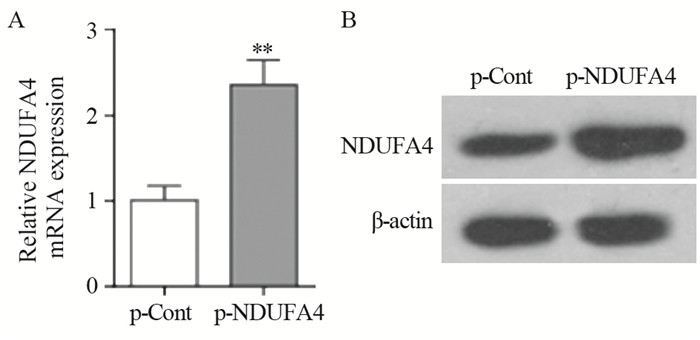
|
| **: P<0.01, compared with p-Cont group 图 1 p-NDUFA4转染HCT116细胞后NDUFA4 mRNA(A)和蛋白(B)的表达 Figure 1 Expression levels of NDUFA4 mRNA(A) and protein(B) in human colon cancer HCT116 cells transfected with p-NDUFA4 |
结果显示:与p-Cont组相比,转染24 h时,p-NDUFA4组细胞的增殖无明显差异(P=0.0546);但从48 h开始,p-NDUFA4组细胞的增殖均明显高于p-Cont组细胞(P48h=0.0077; P72h=0.0033; P96h=0.0442; P120h=0.0310),见图 2。

|
| *: P<0.05, **: P<0.01, compared with p-Cont group 图 2 NDUFA4过表达对HCT116细胞增殖的影响 Figure 2 Effects of NDUFA4 overexpression on proliferation of HCT116 cells |
结果显示:与p-Cont组相比,p-NDUFA4组HCT116细胞的克隆形成率较p-Cont组明显增加(P400/well=0.0015, P1000/well=0.0011),见图 3。

|
| **: P<0.01, compared with p-Cont group 图 3 NDUFA4过表达对HCT116细胞克隆形成的影响 Figure 3 Effects of NDUFA4 overexpression on clonal formation of HCT116 cells |
结果显示:与p-Cont组相比,p-NDUFA4组中肿瘤细胞生长周期相关的周期蛋白依赖性激酶CDK3和CDK4的表达水平均显著上调(PCDK2=0.0285, PCDK3=0.0025, PCDK4=0.0197, PCDK6=0.0046),见图 4。

|
| *: P<0.05, **: P<0.01, compared with p-Cont group 图 4 NDUFA4过表达对细胞中细胞生长周期相关的CDKs表达的影响 Figure 4 Effects of NDUFA4 overexpression on CDKs expression related with cell cycle of HCT116 cells |
结果显示:与p-Cont组相比,p-NDUFA4组中p-ERK1/2、p-AKT的蛋白表达水平明显增加(Pp-ERK1/2= 0.0075, Pp-AKT=0.0254),见图 5。

|
| *: P<0.05, **: P<0.01, compared with p-Cont group 图 5 NDUFA4过表达对HCT116细胞中AKT、ERK1/2和AKT、ERK1/2磷酸化水平的影响 Figure 5 Effects of NDUFA4 overexpression on expression levels of AKT, ERK1/2, p-AKT and p-ERK1/2 in HCT116 cells |
NDUFA分子参与了肿瘤发生发展过程,且不同的NDUFA分子在特定肿瘤中的表达功能存在差异[14]。例如,NDUFA13在人肺癌组织中表达量明显下降,与肿瘤增殖和转移等恶性生物学行为存在密切联系[15]。陆仁飞等[16]发现人结直肠癌患者标本中NDUFA13的表达显著下调,且高表达NDUFA13能够抑制结直肠癌细胞的增殖。此外,NDUFA4L2在不同癌中的表达量存在明显差异,其在肝细胞癌、神经母细胞瘤、肾透明细胞癌中表达量上调[17-18]。这些研究提示NDUFA家族分子参与肿瘤发生过程中的复杂性。因此,探讨不同NDUFA家族分子在特定肿瘤发生中的作用不仅有利于NDUFA家族分子生物学功能的解析,而且对于相关肿瘤发生机制认识和临床生物防治新策略开发均具有重要意义。
作为NDUFA家族成员之一,NDUFA4也参与了肿瘤的发生过程[12]。我们在前期研究中发现,肿瘤抑癌分子微小RNA-7可抑制肺癌中NDUFA4的表达,且过表达NDUFA4可促进人肺癌细胞的生长[13]。本研究,我们将前期构建的p-NDUFA4真核表达载体瞬时转染HCT116细胞,发现细胞中NDUFA4的表达水平明显增加,提示该载体可在人结直肠癌细胞中有效表达NDUFA4分子,这与我们前期工作一致。重要的是,CCK-8实验和克隆形成实验显示,上调NDUFA4表达后,HCT116细胞的增殖能力和克隆形成能力明显增强。为了进一步明确NDUFA4过表达对人结直肠癌细胞体外生长的效应,我们进一步检测了细胞中生长周期相关分子CDKs家族成员的表达,发现CDK2和CDK3等家族成员明显上调,提示NDUFA促进人结直肠癌细胞体外生长的效应与细胞周期变化有关。现已知,AKT和ERK信号途径与肿瘤细胞生长密切相关[19-21],且与细胞呼吸链能量传递存在关联[22-24]。因此,我们检测了细胞中p-AKT和p-ERK等蛋白的表达情况,且发现p-AKT和p-ERK等蛋白的表达水平均明显上调。这些研究结果提示,NDUFA4过表达促进人结直肠癌细胞体外生长的效应可能与相关信号途径传递变化有关。然而,鉴于NDUFA4分子在细胞能量代谢中的重要作用,以及AKT等信号途径与能量代谢的密切关系,因此,NDUFA4过表达对人结直肠癌细胞体外生长的作用机制,包括细胞能量代谢改变,仍有待后续研究阐明。
综上所述,本研究发现过表达NDUFA4可促进人结直肠癌细胞的体外生长,且AKT和ERK等信号途径传递发生变化,为后续深入NDUFA4在人结直肠癌发生中的作用及相关机制提供了重要的前期实验基础。
作者贡献
刘士明:大部分实验的实施及文稿初稿撰写
丁涛、雷良玉、陈超、郭萌萌:给予实验技术支持
胡琳、冒灵:数据的整理及帮助文章撰写
徐 林:实验构思及文稿的撰写与修改
| [1] | Kirana C, Ruszkiewicz A, Stubbs RS, et al. Soluble HLA-G is a differential prognostic marker in sequential colorectal cancer disease stages[J]. Int J Cancer, 2017, 140(11): 2577–86. DOI:10.1002/ijc.v140.11 |
| [2] | 孟庆彬, 吴彪, 肖新波, 等. 结直肠癌组织中Krüppel样因子17的表达及其与预后的关系[J]. 肿瘤防治研究, 2015, 42(3): 256–60. [ Meng QB, Wu B, Xiao XB, et al. Expression of KLF17 Protein in Human Colorectal Cancer Tissue and Its Correlation with Prognosis[J]. Zhong Liu Fang Zhi Yan Jiu, 2015, 42(3): 256–60. DOI:10.3971/j.issn.1000-8578.2015.03.010 ] |
| [3] | Wu J, Yang X, Lu H, et al. Inhibitory effect and mechanism of exogenous mammalian sterile 20-like kinase 1 on the growth of human colorectal cancer[J]. Oncol Lett, 2017, 13(4): 2656–64. DOI:10.3892/ol.2017.5786 |
| [4] | Liu T, Zhang X, Gao S, et al. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer[J]. Oncotarget, 2016, 7(51): 85551–63. |
| [5] | 武雪亮, 王立坤, 薛军, 等. 结直肠癌组织中DNA结合分化抑制蛋白1和MMP-9的表达及其与LMVD的相关性[J]. 肿瘤防治研究, 2016, 43(11): 959–63. [ Wu XL, Wang LK, Xue J, et al. Expression and Correlation of Id-1, MMP-9 and LMVD in Colorectal Adenocarcinoma tissues[J]. Zhong Liu Fang Zhi Yan Jiu, 2016, 43(11): 959–63. DOI:10.3971/j.issn.1000-8578.2016.11.008 ] |
| [6] | Balsa E, Marco R, Perales-Clemente E, et al. NDUFA4 Is a Subunit of Complex Ⅳ of the Mammalian Electron Transport Chain[J]. Cell Metab, 2012, 16(3): 378–86. DOI:10.1016/j.cmet.2012.07.015 |
| [7] | Hayashi T, Ishimori C, Takahashi-Niki K, et al. DJ-1 binds to mitochondrial complex Ⅰ and maintains its activity[J]. Biochem Biophys Res Commun, 2009, 390(3): 667–72. DOI:10.1016/j.bbrc.2009.10.025 |
| [8] | Zong NC, Li H, Li H, et al. Integration of cardiac proteome biology and medicine by a specialized knowledgebase[J]. Circ Res, 2013, 113(9): 1043–53. DOI:10.1161/CIRCRESAHA.113.301151 |
| [9] | Pitceathly RDS, Taanman JW. NDUFA4 (Renamed COXFA4) Is a Cytochrome-c, Oxidase Subunit[J]. Trends Endocrinol Metab, 2018, 29(7): 452–4. DOI:10.1016/j.tem.2018.03.009 |
| [10] | Fu F, Li Y, Li R, et al. NDUFA4 enhances neuron growth by triggering growth factors and inhibiting neuron apoptosis through Bcl-2 and cytochrome C mediated signaling pathway[J]. Am J Transl Res, 2018, 10(1): 164–74. |
| [11] | Kadenbach B. Regulation of Mammalian 13-Subunit Cytochrome c Oxidase and Binding of other Proteins: Role of NDUFA4[J]. Trends Endocrin Met, 2017, 28(11): 761–70. DOI:10.1016/j.tem.2017.09.003 |
| [12] | Müller FE, Braun M, Syring I, et al. NDUFA4 expression in clear cell renal cell carcinoma is predictive for cancer-specific survival[J]. Am J Cancer Res, 2015, 5(9): 2816–22. |
| [13] | Lei L, Chen C, Zhao J, et al. Targeted Expression of miR-7 Operated by TTF-1 Promoter Inhibited the Growth of Human Lung Cancer through the NDUFA4 Pathway[J]. Mol Ther Nucleic Acids, 2017, 6: 183–97. DOI:10.1016/j.omtn.2016.12.005 |
| [14] | 李雪峰, 陈临溪, 伍尤华. 生物信息学分析肾透明细胞癌相关分子标志物及其关键通路[J]. 中南医学科学杂志, 2016, 44(5): 481–5, 493. [ Li XF, Chen LX, Wu YH. Analysis of the Molecular Markers and the Critical Pathway in Clear Cell Renal Cell Carcinoma Through Bioinformatics[J]. Zhongnan Yi Xue Ke Xue Za Zhi, 2016, 44(5): 481–5, 493. ] |
| [15] | Chen Y, Lu H, Liu Q, et al. Function of GRIM-19, a mitochondrial respiratory chain complex Ⅰ protein, in innate immunity[J]. J Biol Chem, 2012, 287(32): 27227–35. DOI:10.1074/jbc.M112.340315 |
| [16] | 陆仁飞, 周敏, 肖峰, 等. 探讨NDUFA13抑制结肠癌细胞增殖的机制[J]. 江苏医药, 2012, 38(24): 2972–4. [ Lu RF, Zhou M, Xiao F, et al. An investigation on the mechanism for NDUFA13 to inhibit proliferation of human Colon cancer[J]. Jiangsu Yi Yao, 2012, 38(24): 2972–4. ] |
| [17] | Lv Y, Nie SL, Zhou JM, et al. Overexpression of NDUFA4L2 is associated with poor prognosis in patients with colorectal cancer[J]. ANZ J Surg, 2016, 87(12): E251–R5. |
| [18] | Liu L, Lan G, Peng L, et al. NDUFA4L2 expression predicts poor prognosis in clear cell renal cell carcinoma patients[J]. Ren Fail, 2016, 38(8): 1199–205. DOI:10.1080/0886022X.2016.1208517 |
| [19] | Zhang YJ, Xu ZG, Li SQ, et al. Benzimidazoisoquinoline derivatives inhibit glioblastoma cell proliferation through down-regulating Raf/MEK/ERK and PI3K/AKT pathways[J]. Cancer Cell Int, 2018, 18: 90. DOI:10.1186/s12935-018-0588-x |
| [20] | Wang X, Zhu Y, Zhu L, et al. Eupatilin inhibits the proliferation of human esophageal cancer TE1 cells by targeting the AKT-GSK3β and MAPK/ERK signaling cascades[J]. Oncol Rep, 2018, 39(6): 2942–50. |
| [21] | Zhu J, Yao J, Huang R, et al. Ghrelin promotes human non-small cell lung cancer A549 cell proliferation through PI3K/AKT/mTOR/P70S6K and ERK signaling pathways[J]. Biochem Biophy Res Commun, 2018, 498(3): 616–20. DOI:10.1016/j.bbrc.2018.03.031 |
| [22] | Trotta AP, Gelles JD, Serasinghe MN, et al. Disruption of mitochondrial electron transport chain function potentiates the pro-apoptotic effects of MAPK inhibition[J]. J Biol Chem, 2017, 292(28): 11727–39. DOI:10.1074/jbc.M117.786442 |
| [23] | Song HP, Chu ZG, Zhang DX, et al. PI3K-AKT Pathway Protects Cardiomyocytes Against Hypoxia-Induced Apoptosis by MitoKATP-Mediated Mitochondrial Translocation of pAKT[J]. Cell Physiol Biochem, 2018, 49(2): 717–27. DOI:10.1159/000493037 |
| [24] | Fang R, Zhang LL, Zhang LZ, et al. Sphingosine 1-Phosphate Postconditioning Protects Against Myocardial Ischemia/reperfusion Injury in Rats via Mitochondrial Signaling and AKT-Gsk3β Phosphorylation[J]. Arch Med Res, 2017, 48(2): 147–55. DOI:10.1016/j.arcmed.2017.03.013 |
 2019, Vol. 46
2019, Vol. 46


