文章信息
- 反义寡核苷酸下调miRNA-21表达对人结肠癌模型体内生长的作用
- Effects of Antisense Oligonucleotides Against miRNA-21 on Growth of Human Colon Cancer Cells in vivo
- 肿瘤防治研究, 2019, 46(6): 504-508
- Cancer Research on Prevention and Treatment, 2019, 46(6): 504-508
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.1435
- 收稿日期: 2018-11-29
- 修回日期: 2019-02-14
2. 563099 遵义,遵义医科大学免疫学教研室
2. Department of Immunology, Zunyi Medical University, Zunyi 563099, China
反义寡核苷酸技术(antisense oligonucleotides, ASOs)又称反义技术,是指一种利用一段人工合成或表达的能与RNA或DNA互补结合的寡核苷酸链,抑制目的基因表达的技术[1-2],也可用于干扰肿瘤细胞中特定基因的表达,从而探讨肿瘤发生的机制和开发基因的治疗策略。近来研究显示,微小RNA-21(miR-21)与包括结直肠癌在内的多种肿瘤的发生密切相关[3-8],可能是肿瘤基因治疗的潜在新靶标[9-10]。我们在前期研究中发现,通过ASOs可显著下调人结肠癌SW620细胞中miR-21的表达,并抑制肿瘤的生长[11]。本研究中进一步利用人结肠癌HCT116细胞建立人结肠癌裸鼠实验动物模型,观察ASOs下调miR-21的效果及对人结肠癌细胞体内生长的效应,为开发基于ASOs技术的人结肠癌基因治疗策略提供实验基础。
1 材料与方法 1.1 实验动物、细胞株和主要试剂12只雌性BALB/c裸鼠,4~5周龄,体质量11~14 g左右,购自北京维通利华实验动物技术有限公司[实验动物许可证编号:SCXK(京)2011-0011],于遵义医学院SPF级免疫学实验室人工饲养。人结肠癌HCT116细胞株购自中国生命科学院,重组质粒p-miR-21-ASOs和对照质粒p-Cont均由本实验室保存。McCoy's 5A培养基、磷酸盐缓冲液(PBS)均购自北京索莱宝科技有限公司,优质胎牛血清购自美国Gibco公司,100×三抗(青霉素-链霉素-两性霉素B)购自上海第四制药股份有限公司;SYBR Premix Ex Taq,Premix Ex Taq Version2.0以及RNAisoTM Plus均购自北京宝日医生物技术有限公司;miR-21探针试剂盒购自美国ABI公司;RevertAidTM First Strand cDNA Synthesis Kit反转录合成试剂盒购自美国Fermentas公司;总RNA提取试剂盒购自北京天根科技有限公司,0.5%Triton-X-100(聚乙二醇辛基苯基醚)购自北京索莱宝科技有限公司;APC标记的兔抗小鼠Ki-67单克隆抗体、DAPI染液均购自美国ThermoFisher公司;兔抗p-Akt、Akt一抗以及兔抗ERK1/2、p-ERK1/2一抗、HRP标记的羊抗兔二抗均购自英国Abcam公司;全蛋白提取试剂盒购自江苏凯基生物公司;ECL显色液、RIPA裂解液、PVDF膜购自中国联科生物技术公司。
1.2 方法 1.2.1 细胞培养按如下条件培养人结肠癌HCT116细胞株:人结肠癌HCT116细胞株在10%优质胎牛血清、100 u/ml青霉素、100 μg/ml链霉素、0.25 μg/ml两性霉素B及11.9 g/L McCoy's 5A细胞培养液中培养,将人结肠癌HCT116细胞株置于37℃、5%CO2的条件下培养,待HCT116细胞处于对数生长期时,使用0.25%胰蛋白酶37℃消化2 min,离心收集细胞,使用PBS重复清洗3次,计算细胞总数,利用PBS调整HCT116细胞密度为4.5×107个/毫升备用。
1.2.2 建立人结肠癌裸鼠皮下移植瘤模型4~5周龄裸鼠购回后先于本实验室SPF级环境下观察并饲养5~7 d。5~7 d天后小鼠饮食精神状况良好,随后接种100 μl密度为4.5×107个/毫升的人结肠癌HCT116细胞于裸鼠右侧腋窝外侧中部皮下,荷瘤后继续饲养并随时观察注射部位有无红肿溃烂、裸鼠的饮食、精神情况以及每只裸鼠荷瘤成功后的成瘤时间。接种后5~7 d出现肉眼可见的肿瘤包块时,即为荷瘤裸鼠模型构建成功。将实验荷瘤小鼠随机分为实验组(p-miR-21-ASOs组)和对照组(p-Cont组),每组6只,随后每隔4 d分别向两组荷瘤小鼠的肿瘤组织中注射100 μg p-Cont质粒和p-miR-21-ASOs质粒,共注射4次,并每隔3 d测量一次裸鼠皮下肿瘤体积。
1.2.3 观察荷瘤裸鼠体内肿瘤生长情况观察方法同前期工作[11]。
1.2.4 免疫组织荧光法检测肿瘤组织中Ki-67蛋白的表达从-80℃超低温冰箱取出用75%冰乙醇固定好的冰冻组织切片,室温(15℃~25℃)平衡10 min,PBS缓冲液漂洗3次,每次5 min;山羊血清封闭液封闭1.5 h,PBS漂洗3次,每次10 min;加0.5%Triton-X-100,冰上破膜2 min,PBS漂洗3次,每次10 min;吸弃封闭液,滴加APC标记的兔抗小鼠单克隆Ki-67抗体后置于湿盒内,4℃冰箱避光过夜孵育,次日,取出冰冻切片肿瘤组织用PBS缓冲液漂洗3次,每次10 min;滴加DAPI染液,染色3 min,PBS漂洗3次,每次10 min;滴加含有DAPI染液的封片剂,封片并固定24 h后观察;激光共聚焦显微镜观察细胞染色情况,拍片。
1.2.5 实时荧光定量PCR检测肿瘤组织中miR-21和肿瘤生长相关因子的表达肿瘤组织中miR-21、细胞周期蛋白依赖性激酶(cyclin- dependent kinase, CDK)表达的检测同前期工作[11]。
1.2.6 Western blot检测Akt、ERK1/2、磷酸化Akt、磷酸化ERK1/2蛋白的表达蛋白样品的制备及BCA法定量蛋白的检测同前期工作[11]。Western blot检测相关信号通路的变化:(1)电泳:分离胶10%,积层胶5%,80 V跑积层胶,120 V跑分离胶;(2)上样:第一泳道Marker,其余泳道加提取的总蛋白;(3)转膜:PVD膜,36 V,180 min电转;(4)洗膜:用TBS或者PBS洗膜;5 min一次;(5)封闭:用含5%脱脂奶粉的TBST或者PBST封闭1 h;(6)加一抗:加入相关目的蛋白的一抗(稀释比例均为1:2 000),4℃过夜;(7)洗膜:次日用TBST或者PBST洗膜,10 min三次;(8)加二抗:加入HRP标记的相关目的蛋白的二抗(稀释比例均为1:2 000),室温孵育1 h;(9)用PBST洗膜,10 min×3次;(10)用发光试剂(ECl)对膜进行处理,于发光仪上进行蛋白曝光检测。
1.3 统计学方法实验数据使用GraphPad Prism 5软件进行统计学分析。本研究中各实验数据均独立重复3次,结果以(x±s)表示。其中组间比较采用t检验或单因素方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 p-miR-21-ASOs重组质粒注射后各组裸鼠荷瘤模型中肿瘤的生长变化结果显示,与p-Cont组相比,p-miR-21-ASOs组皮下肿瘤生长显著延缓,且肿瘤组织的体积、大小、重量都明显减小,差异有统计学意义,见图 1。
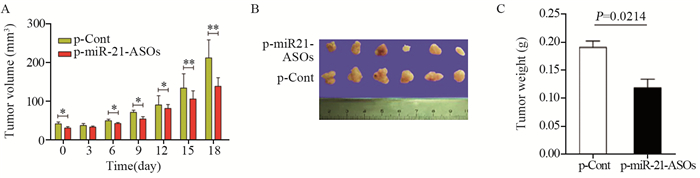
|
| *: P < 0.05 (P0 day=0.0341, P6 day=0.0202, P9 day=0.0177, P12 day=0.0101); **: P < 0.01(P15 day=0.0024, P18 day=0.0010) 图 1 重组质粒注射后裸鼠皮下种植瘤的肿瘤体积(A)、大小(B)和重量(C)变化 Figure 1 Tumor volume(A), size(B) and weight(C) in nude mice after subcutaneous injection of recombinant plasmid |
HE染色显示:p-Cont组肿瘤细胞形态不一,细胞核深染且核呈多形性,核质比加大;而p-miR-21-ASOs组则可见肿瘤组织大面积坏死,见图 2。

|
| 图 2 重组质粒局部注射荷瘤裸鼠后肿瘤组织HE染色结果(×400) Figure 2 HE staining results of tumor tissues after local injection of recombinant plasmid into xenograft tumor in nude mice (×400) |
结果显示:与p-Cont组相比,p-miR-21-ASOs组肿瘤组织中miR-21的表达水平显著下调,且差异有统计学意义(P < 0.05),见图 3。
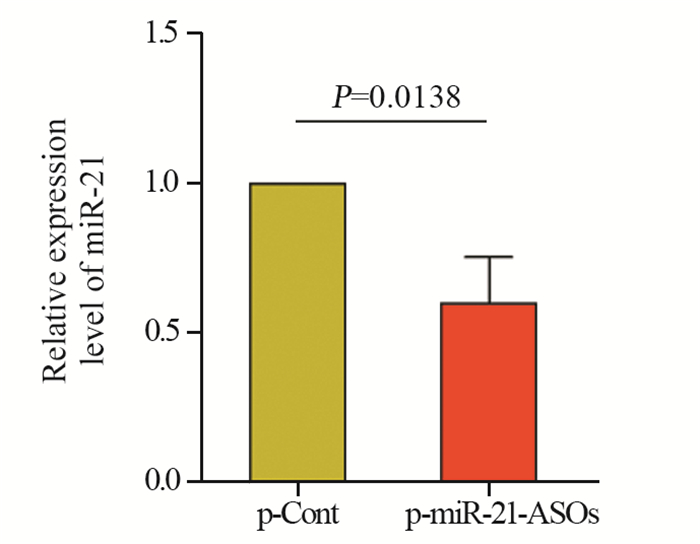
|
| 图 3 Real-time PCR探针法检测肿瘤组织中miR-21的表达 Figure 3 Expression of miR-21 in tumor tissues detected by real-time PCR |
免疫荧光共聚焦观察发现:相比于p-Cont组,p-miR-21-ASOs组肿瘤组织中Ki-67的表达水平明显减少(P < 0.01),见图 4。

|
| 图 4 p-miR-21-ASOs重组质粒注射荷瘤裸鼠后激光共聚焦检测肿瘤组织中Ki-67的表达 Figure 4 Expression level of Ki-67 protein after injection of p-miR-21-ASOs recombinant plasmid into xenograft tumor in nude mice detected by immunofluorescence technique |
结果显示:与p-Cont组相比,p-miR-21-ASOs组肿瘤细胞生长周期相关的周期蛋白依赖性激酶CDK2、CDK3、CDK4以及CDK6的表达水平均明显下调,差异有统计学意义(P < 0.05, P < 0.001),见图 5。

|
| **: P < 0.05(PCDK3=0.0308, PCDK6=0.0252); ***: P < 0.001(PCDK2=0.0001, PCDK4=0.0003) 图 5 实时荧光定量PCR法检测肿瘤组织中CDKs的表达 Figure 5 Expression of CDKs in tumor tissues detected by real-time fluorescent quantitative PCR |
结果显示:与p-Cont组相比,p-miR-21-ASOs组中p-ERK1/2,p-Akt的蛋白表达水平明显降低(P < 0.05, P < 0.01),见图 6。
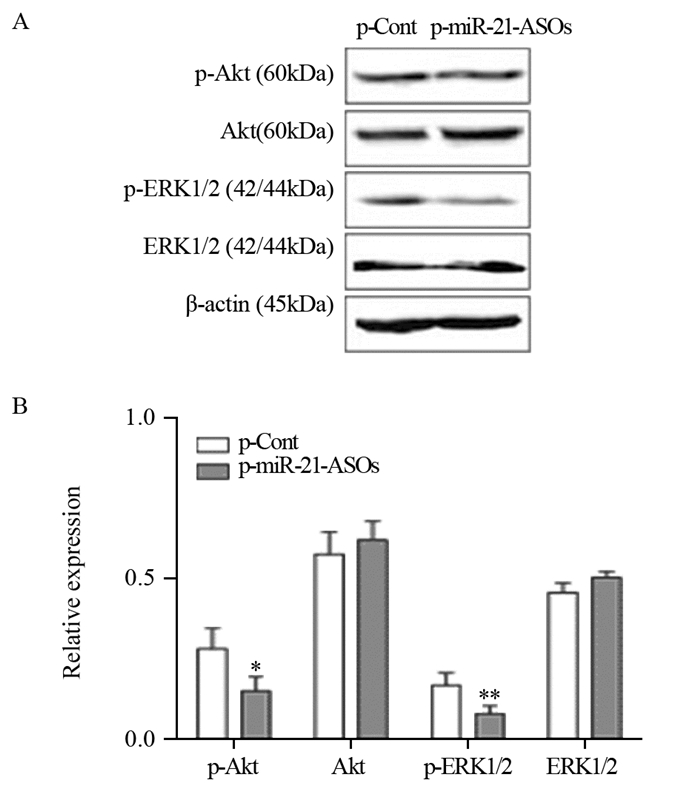
|
| *: Pp-Akt=0.0440, **: Pp-ERK1/2=0.0095, compared with p-Cont group 图 6 Western blot检测肿瘤组织中总Akt、ERK1/2和Akt、ERK1/2磷酸化的水平 Figure 6 Expression levels of total Akt, ERK1/2, p-Akt and p-ERK1/2 in tumor tissues analyzed by Western blot |
近来的研究发现,ASOs技术可广泛用于多种临床疾病发生机制探讨和治疗策略开发[12-18]。对于肿瘤基因治疗来说,利用ASOs技术干预特定基因表达来影响肿瘤生长方面的研究也取得了重要进展[19-20]。我们在新近工作中也发现利用真核载体过表达ASOs干预miR-21表达可显著抑制人结肠癌SW620细胞的体内生长[11]。本研究中,我们进一步发现,真核载体过表达ASOs也可有效抑制人结肠癌HCT116细胞中miR-21的表达,同时肿瘤细胞体内生长受到抑制。范钰等[21]用ASOs下调人喉癌Hep-2细胞中miR-21的表达,发现肿瘤细胞的生长、侵袭能力明显减弱。吴子晏等[22]研究发现ASOs干预miR-21联合顺铂在体内、外能显著抑制人骨肉瘤MG-63细胞生长,并促进细胞凋亡,显示了一定的治疗效应。这些研究结果提示,利用ASOs技术干预miR-21表达可能是一种能有效影响特定肿瘤生长的手段,为后续开发基于ASOs的人结肠癌基因治疗策略提供了重要工作基础。
研究显示,miR-21可通过对多种靶分子和信号途径传递的调节来调控人结肠癌的生长[23-28]。在前期工作中,我们也发现ASOs下调miR-21表达后,肿瘤细胞生长相关信号途径Akt和ERK1/2明显改变,与其靶分子PTEN的表达变化有关[4, 10]。本研究进一步发现ASOs下调miR-21表达后,肿瘤组织存在坏死,肿瘤细胞的增殖能力明显减弱,同时,肿瘤组织中p-Akt和p-ERK1/2等蛋白的表达水平均明显下调,进一步提示了miR-21对Akt和ERK1/2等信号途径的调控作用。现已知,细胞内CDKs家族分子的表达改变与肿瘤细胞生长相关。本研究也发现肿瘤细胞内CDKs家族成员表达水平显著减低。鉴于已有的研究表明,肿瘤细胞生长相关信号途径Akt和ERK1/2与细胞内CDKs家族成员表达间存在密切联系[29-31]。因此,推测ASOs干预miR-21表达后对人结肠癌细胞体内生长的抑制效应机制,包括肿瘤组织坏死的变化,与Akt和ERK1/2等信号途径传递改变,进而影响CDKs家族分子的表达有关。然而,鉴于不同实验条件的差异性,ASOs下调miR-21后抑制人结肠癌细胞体内生长的确切分子机制仍有待后续研究阐明。
总之,本研究发现ASOs可显著下调人结肠癌动物模型中肿瘤组织miR-21的表达,同时肿瘤体内生长受到明显抑制,该效应与Akt和ERK1/2等信号途径传递变化有关,为后续深入探讨miR-21调控肿瘤生长的分子机制,以及开发基于ASOs技术的人结肠癌基因治疗策略提供前期实验基础。
作者贡献
刘士明:承担实验、文章初稿撰写
崔盼盼、丁涛、陈超、郭萌萌:前期实验、文章撰写、数据整理和实验技术支持
徐林:本文的构思、撰写和修改
| [1] | Crooke ST. Molecular Mechanisms of Antisense Oligonucleo-tides[J]. Nucleic Acid Ther, 2017, 27(2): 70–7. DOI:10.1089/nat.2016.0656 |
| [2] | Ling LS, Leung HM, Tam DY, et al. α-l-Threose Nucleic Acids as Biocompatible Antisense Oligonucleotides for Suppressing Gene Expression in Living Cells[J]. ACS Appl Mater Interfaces, 2018, 10(11): 9736–43. DOI:10.1021/acsami.8b01180 |
| [3] | Zhang J, Yao T, Wang Y, et al. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21[J]. Cancer Biol Ther, 2016, 17(1): 104–113. DOI:10.1080/15384047.2015.1108496 |
| [4] | An F, Liu Y, Hu Y. miR-21 inhibition of LATS1 promotes proliferation and metastasis of renal cancer cells and tumor stem cell phenotype[J]. Oncol Lett, 2017, 14(4): 4684–8. DOI:10.3892/ol.2017.6746 |
| [5] | Shuo C, Xi C, Tao S, et al. MiR-21-mediated Metabolic Alteration of Cancer-associated Fibroblasts and Its Effect on Pancreatic Cancer Cell Behavior[J]. Int J of Biol Sci, 2018, 14(1): 100–10. DOI:10.7150/ijbs.22555 |
| [6] | Gu JB, Bao XB, Ma Z. Effects of miR-21 on proliferation and apoptosis in human gastric adenocarcinoma cells[J]. Oncol Lett, 2018, 15(1): 618–22. |
| [7] | Yang C, Wang G, Yang J, et al. Long noncoding RNA NBAT1 negatively modulates growth and metastasis of osteosarcoma cells through suppression of miR-21[J]. Am J Cancer Res, 2017, 7(10): 2009–19. |
| [8] | 罗彬瑞, 郭天虹, 黄远帅. 唾液中miRNA-21对早期喉鳞状细胞癌的诊断价值[J]. 肿瘤防治研究, 2018, 45(5): 320–5. [ Luo BR, Guo TH, Huang YS. Diagnostic values of salivary mirna-21 for early laryngeal squamous cell carcinoma[J]. Zhong Liu Fang Zhi Yan Jiu, 2018, 45(5): 320–5. DOI:10.3971/j.issn.1000-8578.2018.17.1137 ] |
| [9] | Wu QB, Sheng X, Zhang N, et al. Role of microRNAs in the resistance of colorectal cancer to chemoradiotherapy[J]. Mol Clin Oncol, 2018, 8(4): 528–32. |
| [10] | Falzone L, Scola L, Zanghì A, et al. Integrated analysis of colorectal cancer microRNA datasets: identification of microRNAs associated with tumor development[J]. Aging(Albany NY), 2018, 10(5): 1000–14. |
| [11] | 丁涛, 崔盼盼, 陶弋婧, 等. 微RNA-21表达下调可抑制人结肠癌SW620细胞在裸鼠体内的生长[J]. 肿瘤, 2017, 37(2): 117–25. [ Ding T, Cui PP, Tao YJ, et al. Down-regulation of microRNA-21 expression inhibits the growth of human colon carcinoma SW620 cells in nude mice[J]. Zhong Liu, 2017, 37(2): 117–25. ] |
| [12] | Schoch KM, Miller TM. Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases[J]. Neuron, 2017, 94(6): 1056–70. DOI:10.1016/j.neuron.2017.04.010 |
| [13] | Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides[J]. Nucleic Acids Res, 2016, 44(2): 863–77. DOI:10.1093/nar/gkv1206 |
| [14] | Billioud G, Kruse RL, Carrillo M, et al. In vivo Reduction of Hepatitis B Virus Antigenemia and Viremia by Antisense Oligonucleotides[J]. J Hepatol, 2016, 64(4): 781–9. DOI:10.1016/j.jhep.2015.11.032 |
| [15] | Li Y, Chen Y, Li J, et al. Co-delivery of microRNA-21 antisense oligonucleotides and Gemcitabine using nanomedicine for pancreatic cancer therapy[J]. Cancer Sci, 2017, 108(7): 1493–503. DOI:10.1111/cas.2017.108.issue-7 |
| [16] | Xue XY, Mao XG, Zhou Y, et al. Advances in the delivery of antisense oligonucleotides for combating bacterial infectious diseases[J]. Nanomedicine, 2018, 14(3): 745–58. DOI:10.1016/j.nano.2017.12.026 |
| [17] | Andreakos E, Rauchhaus U, Stavropoulos A, et al. Amphoteric liposomes enable systemic antigen-presenting cell-directed delivery of CD40 antisense and are therapeutically effective in experimental arthritis[J]. Arthritis Rheum, 2009, 60(4): 994–1005. DOI:10.1002/art.v60:4 |
| [18] | 赵娟娟, 徐华林, 郭萌萌, 等. microRNA-7敲减对内毒素诱导小鼠急性肺损伤的影响[J]. 中国免疫学杂志, 2016, 32(9): 1257–61. [ Zhao JJ, Xu HL, Guo MM, et al. Effect of microRNA-7 knockdown on patholoth of Enterotoxin-induced murine acute lung injury[J]. Zhongguo Mian Yi Xue Za Zhi, 2016, 32(9): 1257–61. DOI:10.3969/j.issn.1000-484X.2016.09.003 ] |
| [19] | 吕海丽, 严波, 张秋航. Stat3反义寡核苷酸对人喉癌Hep-2细胞BALB/c裸鼠皮下移植瘤生长的影响[J]. 临床耳鼻咽喉头颈外科杂志, 2018, 32(9): 661–4. [ Lyu HL, Yan B, Zhang QH. Effects of Stat3 antisense oligodeoxynucleotide on growth of subcutaneous transplanted tumors in human Hep-2 cell tumor-bearing BALB/c nude mouse[J]. Lin Chuang Er Bi Hou Tou Jing Wai Ke Za Zhi, 2018, 32(9): 661–4. ] |
| [20] | Zhou T, Kim Y, Macleod AR. Targeting Long Noncoding RNA with Antisense Oligonucleotide Technology as Cancer Therapeutics[J]. Methods Mol Biol, 2016, 1402: 199–213. DOI:10.1007/978-1-4939-3378-5 |
| [21] | 范钰, 蒋孟林, 沈荣, 等. 下调微小RNA-21对人肿瘤Hep-2细胞生长和侵袭的影响[J]. 中华实验外科杂志, 2016, 33(1): 198–201. [ Fan Y, Jiang ML, Shen R, et al. Effects of microRNA-21 on growth and invasion of laryngopharynx cancer Hep-2 cells[J]. Zhonghua Shi Yan Wai Ke Za Zhi, 2016, 33(1): 198–201. DOI:10.3760/cma.j.issn.1001-9030.2016.01.061 ] |
| [22] | 吴子晏, 张卫国. miR-21反义寡核苷酸联合顺铂对裸鼠骨肉瘤生长的抑制[J]. 华中科技大学学报(医学版), 2017, 46(5): 571–4. [ Wu ZY, Zhang WG. Effects of miR-21 Antisense Oligonucleotides and Cisplatin Combined on the Growth of Osteosarcoma Xenotransplants in Nude Mice[J]. Hua Zhong Ke Ji Da Xue Xue Bao(Yi Xue Ban), 2017, 46(5): 571–4. DOI:10.3870/j.issn.1672-0741.2017.05.015 ] |
| [23] | Song L, Liu S, Zhang L, et al. MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1α feedback loop and the Akt-mTOR signaling pathway[J]. Tumor Biol, 2016, 37(9): 12161–8. DOI:10.1007/s13277-016-5073-3 |
| [24] | Peralta-Zaragoza O, Deas J, Meneses-Acosta A, et al. Relevance of miR-21 in regulation of tumor suppressor gene PTEN in human cervical cancer cells[J]. BMC Cancer, 2016, 16: 215. DOI:10.1186/s12885-016-2231-3 |
| [25] | Wang CY, Guo ST, Wang JY, et al. Reactivation of ERK and Akt confers resistance of mutant BRAF colon cancer cells to the HSP90 inhibitor AUY922[J]. Oncotarget, 2016, 7(31): 49597–610. |
| [26] | Sun Y, Zhao Y, Wang X, et al. Wogonoside prevents colitis-associated colorectal carcinogenesis and colon cancer progression in inflammation-related microenvironment via inhibiting NF-κB activation through PI3K/Akt pathway[J]. Oncotarget, 2016, 7(23): 34300–15. |
| [27] | Gong B, Liu WW, Nie WJ, et al. MiR-21/RASA1 axis affects malignancy of colon cancer cells via RAS pathways[J]. World J of Gastroenterol, 2015, 21(5): 1488–97. DOI:10.3748/wjg.v21.i5.1488 |
| [28] | Mercado-Pimentel ME, Onyeagucha BC, Li Q, et al. The S100P/RAGE signaling pathway regulates expression of microRNA-21 in colon cancer cells[J]. FEBS Lett, 2016, 589(18): 2388–93. |
| [29] | Rostam MA, Kamato D, Piva TJ, et al. The role of specific Smad linker regionphosphorylation in TGF-β mediated expression of glycosaminoglycan synthesizing enzymesin vascular smooth muscle[J]. Cell Signal, 2016, 28(8): 956–66. DOI:10.1016/j.cellsig.2016.05.002 |
| [30] | Lai IC, Lai GM, Chow JM, et al. Active fraction (HS7) fromTaiwanofungus camphoratusinhibits Akt-mTOR, ERK and STAT3 pathways and induces CDK inhibitors in CL1-0 human lung cancer cells[J]. Chin Med, 2017, 12(1): 33–45. DOI:10.1186/s13020-017-0154-9 |
| [31] | Zhang JX, Yun M, Xu Y, et al. GNA13 as a prognostic factor and mediator of gastric cancer progression[J]. Oncotarget, 2016, 7(4): 4414–27. |
 2019, Vol. 46
2019, Vol. 46


