文章信息
- 敲低EN2诱导肝癌细胞凋亡并提高PTEN蛋白的表达
- Knockdown of EN2 Induces Apoptosis and Enhances PTEN Protein Expression in Hepatocellular Carcinoma Cells
- 肿瘤防治研究, 2019, 46(4): 316-321
- Cancer Research on Prevention and Treatment, 2019, 46(4): 316-321
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.1295
- 收稿日期: 2018-09-10
- 修回日期: 2018-10-24
肝癌作为一种常见的恶性肿瘤具有恶性程度高、进展迅猛等特点,同其他恶性肿瘤一样,肝癌的发病机制十分复杂,其涉及到一系列基因、信号转导过程,这些基因的异常表达通常与肿瘤细胞周期、凋亡等生物学特性有关[1-2]。Engrailed-2(EN2)属于非Ⅰ类同源性盒基因家族成员,其具有特异性结合DNA调控基因转录的作用,目前的研究表明,EN2参与肿瘤发生,在正常细胞中高表达EN2可以促进细胞恶性增殖,缩短细胞周期[3-5]。EN2在肝癌组织中高表达,对于其在肝癌细胞凋亡中的作用尚不明确[6]。本研究拟通过敲低肝癌细胞中EN2的表达水平,明确EN2在肝癌细胞凋亡中的作用,为以后靶向EN2治疗肝癌和研究肝癌的发病机制奠定基础。
1 材料与方法 1.1 材料HB-9018肝癌细胞HuH-7购自美国ATCC细胞库; EN2 siRNA和siRNA阴性对照慢病毒载体由广州辉骏生物科技有限公司构建; E092 SYBR Green qRT-PCR Super Mix、反转录试剂均购自德国Qiagen公司; ab28731EN2抗体购自美国Abcam公司; 12963细胞色素C(Cytochrome C)抗体、52873激活型Caspase-9(Cleaved Caspase-9)抗体、9559第10号染色体同源缺失性磷酸酶-张力蛋白(phosphatase and tensin homolog deleted on chromosome 10, PTEN)抗体、9661激活型Caspase-3(Cleaved Caspase-3)抗体、12231细胞周期素B 1(Cyclin B l)抗体购自美国Cell Signaling Technology公司; 70-MJ101JC-1线粒体膜电位检测试剂盒购自杭州联科生物技术股份有限公司; BC3740胞质蛋白提取试剂盒购自北京索莱宝科技有限公司。
1.2 慢病毒感染人肝癌细胞HuH-7接种到12孔细胞板中,观察细胞融合度为60%时进行感染,添加适量的慢病毒液,使MOI=10,同时在细胞中加入polybrene(浓度为5 μg/ml),培养12 h以后,将慢病毒培养液吸弃以后,更换新鲜的培养液,在感染后72 h观察荧光表达情况,感染率高于85%时可用于后续实验。取稳定感染EN2 siRNA和siRNA阴性对照(NC)慢病毒的HuH-7细胞标记为EN2 siRNA、siRNA-NC,未感染慢病毒的细胞作为空白对照(Control)。
1.3 qRT-PCR测定干扰效果RNA抽提:Control、EN2 siRNA、siRNA-NC细胞中添加TRIzol,静置5 min,添加1/5 TRIzol体积的氯仿,振荡混合30 s,将水相层和有机相混合以后,静置2 min分层,吸取上层水相层溶液与500 μl的异丙醇轻轻混合后,置于室温中结合10 min,此时RNA存在于底部沉淀中,用75%的乙醇将RNA洗涤,风干,溶解在DEPC水中。反转录:1.0 μg RNA85℃孵育5 min后,立即放于冰上冷却,PCR管中配制反转录体系(0.5 μl Oligo dT,0.5 μl Random primer,2.0 μl的10 mmol/L dNTP,4.0 μl 5×Buffer,0.5 μl M-MLV),30℃反应10 min,42℃反应60 min,85℃反应10 min。PCR反应:取5.0 μl cDNA,添加0.5 μl上下游引物、10 μl 2×SYBR Green qRT-PCR Super Mix、4.0 μl的dH2O,95℃变性5 min后,95℃反应15 s,60℃反应32 s,72℃反应30 s,循环40次。使用Ct值进行分析,内参为β-actin。EN2上游:5’-TCTTGGAGTGGCTGCTTCTG-3’,下游:5’-TCCTGGAGGATTCTGAGTTCTT -3’。β-actin上游:5’-AGAGCTACGAGCTGCCTGAC-3’,下游:5’-TCTGCGCAACTTAGGTTTTG-3’。
1.4 Western blot测定干扰效果细胞蛋白提取:Control、EN2 siRNA、siRNA-NC细胞中添加PIRA裂解液,冰上孵育30 min,4℃,12 000 g离心10 min后,吸取上清液,用Bradford法测定蛋白浓度。SDS-PAGE电泳:配制8%的分离胶和5%的浓缩胶,将蛋白同上样缓冲液混合煮沸5 min变性,用恒压80 V电泳约50 min以后,蛋白进入到分离胶,120 V恒压电泳2 h,关闭电泳,取出凝胶,100 V恒压转膜80 min,将NC膜在5%的牛血清白蛋白中室温孵育1 h,取出封闭后的NC膜,放在TBST中室温洗涤3次,每次5 min。同1:200稀释的EN2抗体在4℃结合过夜,TBST中洗涤3次,每次5 min。再与1:6 000稀释的HRP标记的二抗在室温结合2 h后,TBST中洗涤3次,每次5 min。ECL化学发光,使用Lab Works对电泳的条带分析灰度值,以β-actin作为参照。
1.5 细胞活力测定细胞活力检测用MTT法,步骤为:Control、EN2 siRNA、siRNA-NC细胞配制成4×104个/毫升的细胞悬浮液,分别种植到96孔板(每孔添加100 μl),分别在各个时间点(24、48、72和96 h)将培养板从培养箱内取出,加入MTT溶液,孵育4 h,弃上清液。继续添加150 μl的DMSO,用不含细胞的孔调零,在酶标仪上读板,测定490 nm波长处的A值。
1.6 PI单染法测定细胞周期检测Control、EN2 siRNA、siRNA-NC细胞配制成每毫升含有1×106个细胞的单细胞悬浮液,取1 ml至离心管中,2 000 g离心10 min,添加预冷后的PBS洗涤细胞3次,用预冷的75%甲醇悬浮后在4℃条件过夜,离心后,添加PI染液,4℃反应30 min,置于流式细胞仪上检测细胞周期。
1.7 Annexin V-FITC/PI双染法检测细胞凋亡Control、EN2 siRNA、siRNA-NC细胞配制成每毫升含有1×106个细胞的单细胞悬浮液,取1 ml至离心管中,2 000 g离心10 min,PBS洗涤3次,添加200 μl缓冲液,加入PI和Annexin V-FITC染液,再加入300 μl缓冲液,立即置于流式细胞仪上检测。
1.8 Western blot方法检测细胞中Cleaved Caspase-3、Cleaved Caspase-9、PTEN及Cyclin B1蛋白表达步骤同1.4,Cleaved Caspase-3抗体稀释倍数为1:200、Cleaved Caspase-9抗体稀释倍数为1:200、PTEN抗体稀释倍数为1:400及Cyclin B1抗体稀释倍数为1:400。
1.9 线粒体膜电位及胞质中Cytochrome C蛋白检测细胞线粒体膜电位检测用JC-1法,结果用红色荧光与绿色荧光的比值表示,具体的操作步骤同杭州联科生物技术股份有限公司70-MJ101线粒体膜电位检测试剂盒(JC-1法)说明书,细胞胞质中Cytochrome C蛋白检测方法用Western blot,步骤同1.4,用北京索莱宝科技有限公司BC3740胞质蛋白提取试剂盒提取胞质蛋白。
1.10 统计学方法实验数据经过SPSS21.0软件统计分析以后,以(x±s)表示,组间比较采用SNK-q检验,P < 0.05为差异有统计学意义。
2 结果 2.1 敲低EN2后的检测结果肝癌细胞感染EN2 siRNA慢病毒后,细胞中的EN2转录和表达水平均降低(mRNA: F=70.336, P=0.000;蛋白:F=29.404, P=0.001),见图 1。表明成功构建了稳定下调EN2表达的肝癌细胞株。

|
| *: P < 0.05, compared with siRNA-NC and Control group; EN2: engrailed-2 图 1 EN2 siRNA慢病毒感染后肝癌细胞中EN2 mRNA(A)和蛋白(B~C)表达变化 Figure 1 EN2 mRNA(A) and protein(B-C) expression in hepatocellular carcinoma cells after EN2 siRNA lentivirus infection determined by Western blot |
肝癌细胞经MTT检测后发现,与对照组比较,敲低EN2表达的细胞24、48、72和96 h的A值明显降低(24 h: F=8.690, P=0.017; 48 h: F=16.814, P=0.004;72 h: F=23.165, P=0.002; 96 h: F=35.707, P=0.001),细胞活力降低,见图 2。表明敲低EN2降低肝癌细胞活力。

|
| *: P < 0.05, compared with siRNA-NC and Control group 图 2 敲低EN2对肝癌细胞活力的影响 Figure 2 Effect of knock down EN2 on the viability of hepatocellular carcinoma cells |
与对照组相比,敲低EN2表达的肝癌细胞G2/M期比例明显升高(G0/G1期:F=4.95, P=0.054; S期:F=0.906, P=0.453;G2/M期:F=29.301, P=0.001),细胞周期受到阻滞,见图 3。
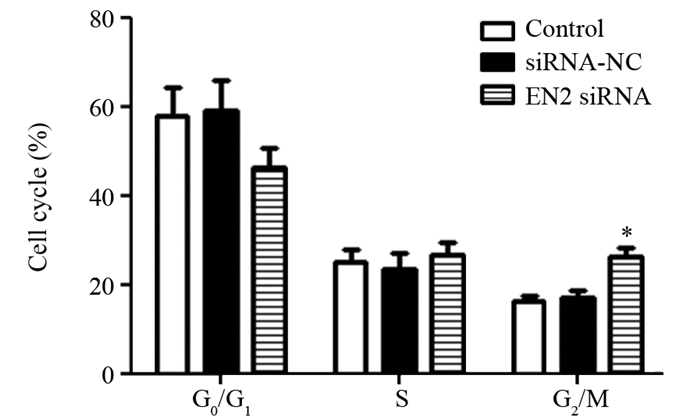
|
| *: P < 0.05, compared with siRNA-NC and Control group 图 3 各组肝癌细胞细胞周期分布变化 Figure 3 Cell cycle distribution of liver cancer cells in each group |
与对照组相比,敲低EN2表达的肝癌细胞凋亡率明显升高(F=221.807, P=0.000),见图 4。说明敲低EN2可以诱导肝癌细胞凋亡。
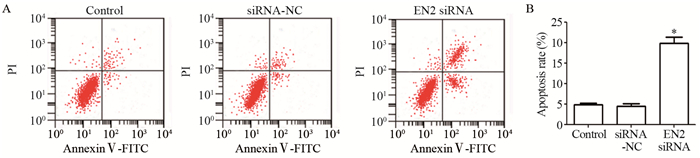
|
| *: P < 0.05, compared with siRNA-NC and Control group 图 4 敲低EN2后肝癌细胞凋亡的变化 Figure 4 Apoptosis of hepatocellular carcinoma cells after knocking down EN2 detected by flow cytometry |
与对照组相比,敲低EN2表达的肝癌细胞中Cleaved Caspase-3、Cleaved Caspase-9和PTEN蛋白水平明显升高,Cyclin B1蛋白水平明显降低(Cleaved Caspase-3: F=54.177, P=0.000; Cleaved Caspase-9: F=128.368, P=0.000; PTEN: F=17.299, P=0.003; Cyclin B1: F=19.500, P=0.002),见图 5。敲低EN2可以影响肝癌细胞中凋亡蛋白、周期蛋白及PTEN蛋白表达。
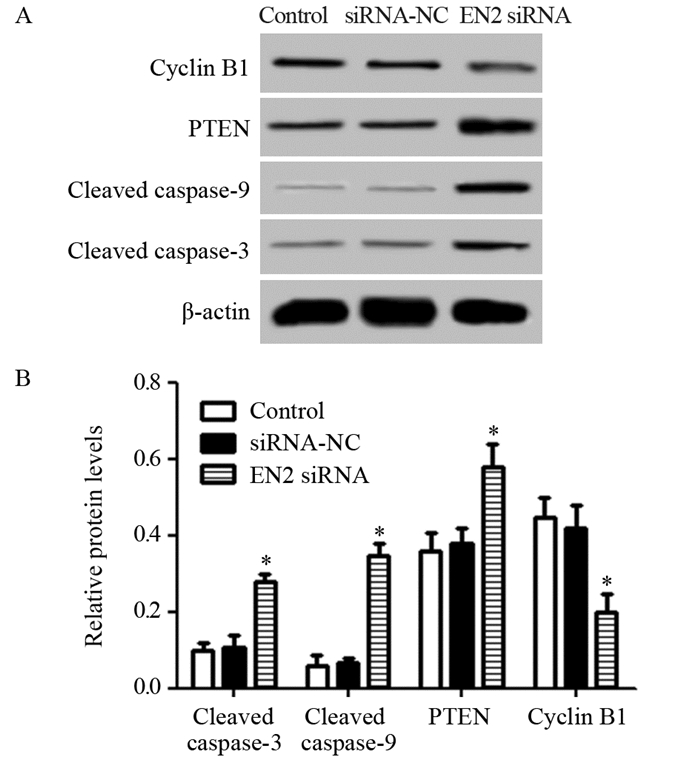
|
| *: P < 0.05, compared with siRNA-NC and Control group 图 5 敲低EN2对肝癌细胞中Cleaved Caspase-3、Cleaved Caspase-9、PTEN及Cyclin B1蛋白水平的影响 Figure 5 Effect of EN2 knockdown on Cleaved Caspase-3, Cleaved Caspase-9, PTEN and Cyclin B1 protein levels in hepatocellular carcinoma cells detected by Western blot |
敲低EN2后肝癌细胞胞质中Cytochrome C蛋白水平明显升高(F=31.371, P=0.001),线粒体膜电位明显降低(F=17.966, P=0.003),见图 6。敲低EN2可以降低肝癌细胞线粒体膜电位,提高胞质中Cytochrome C蛋白水平。

|
| *: P < 0.05, compared with siRNA-NC and Control group 图 6 敲低EN2对肝癌细胞胞质中Cytochrome C蛋白表达和线粒体膜电位的影响 Figure 6 Effect of EN2 knockdown on Cytochrome C protein expression and mitochondrial membrane potential in hepatocellular carcinoma cells |
人类的EN2基因可以编码333个氨基酸,其定位于7q36染色体上,EN2蛋白包括5个功能较为保守的亚区,按照N端到C端依次命名为EH1~EH5,EH4为同源结构区域,具有调控EN2蛋白的摄取及分泌作用,EH1、EH5能够抑制EN2蛋白的促转录作用,EH2、EH3调控EN2蛋白同DNA的亲和能力[7-9]。目前研究报道表明,EN2在胚胎发育、细胞恶性增殖等过程中具有重要作用,其表达水平的高低与肿瘤发生有关[10-11]。在人类膀胱癌中的研究表明,沉默EN2表达可以降低膀胱癌细胞的增殖能力,同样在胃癌、肾癌等肿瘤细胞中得到证实[9, 12-14]。本实验结果显示,敲低EN2的肝癌细胞活力降低,细胞凋亡增多,G2/M期细胞比例升高,敲低EN2具有抑制肝癌细胞增殖的作用,EN2在肝癌中可能发挥促进作用。
细胞周期进程是细胞增殖的基础,细胞周期进程与细胞内多种蛋白的表达调控有关,其中G2期是细胞周期进程中的检查点,Cyclin B1是调控G2期的周期相关蛋白,其可以通过促进周期相关蛋白复合物的形成促使细胞完成G2/M期的转变[15-16]。EN2具有调控细胞周期的作用,沉默EN2表达可以将肾小管上皮细胞周期阻滞在G2/M期,在正常乳腺细胞中的研究也显示,高表达EN2可以缩短细胞周期,促进细胞快速增殖[17-18]。本实验的结果表明,敲低EN2后的肝癌细胞G2/M期比例升高,细胞中Cyclin B1蛋白水平降低,敲低EN2可以通过调控周期蛋白的表达阻滞肝癌细胞周期。
肿瘤细胞恶性增殖的同时凋亡水平降低,肿瘤细胞的凋亡与细胞内的Caspase蛋白家族有关,Caspase蛋白家族是广泛存在于多种细胞中的凋亡调控因子,含有多个成员,在细胞凋亡中发挥促进作用,Caspase-3活化是细胞凋亡进入不可逆阶段的标志,Caspase-9活化增多是Caspase凋亡反应的标志[19-20]。Caspase-9参与细胞线粒体凋亡途径,可以被胞质中的Cytochrome C激活,正常情况下的Cytochrome C多存在于线粒体中,只有受到病理或生理因素刺激以后,线粒体膜电位降低,线粒体内的Cytochrome C才可以被释放到胞质中,发挥凋亡促进的作用[21-22]。本实验的结果显示,敲低EN2表达可以促进Caspase-3和Caspase-9活化,降低线粒体膜电位,提高胞质中Cytochrome C蛋白水平,敲低EN2可以通过线粒体途径诱导肝癌细胞凋亡。
本实验还显示,敲低EN2可以提高肝癌细胞中PTEN的表达水平,从而影响肝癌细胞的生物学特性。PTEN是一种抑癌基因,具有影响肿瘤浸润、生长、骨架蛋白组装等作用,参与细胞内多种信号的转导[23]。有研究报道显示,外源性的PTEN可以诱导肿瘤细胞的凋亡并阻滞细胞周期,并且对于不同的细胞周期阻滞作用不同,PTEN诱导细胞凋亡作用机制与影响线粒体中Cytochrome C的释放有关[24-26]。本实验提示敲低EN2能够通过PTEN介导的线粒体凋亡途径诱导肝癌细胞凋亡发生,为以后研究肝癌的发病机制提供了基础,而对于其具体的作用机制尚未验证,我们将会在以后的实验中进行探讨。
作者贡献
杨启:论文撰写和数据分析
万春:前期资料文献查询及整个实验思路设计
吕新远:实验基础操作和数据整理
| [1] | Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer[J]. Cancer, 2016, 122(9): 1312–37. DOI:10.1002/cncr.v122.9 |
| [2] | Mohankumar S, Patel T. Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer[J]. Brief Funct Genomics, 2016, 15(3): 249–56. DOI:10.1093/bfgp/elv058 |
| [3] | Li Q, Lu S, Li X, et al. Biological function and mechanism of miR-33a in prostate cancer survival and metastasis: via downregulating Engrailed-2[J]. Clin Transl Oncol, 2016, 19(5): 562–70. |
| [4] | Li Y, Liu H, Lai C, et al. Repression of engrailed 2 inhibits the proliferation and invasion of human bladder cancer in vitro and in vivo[J]. Oncol Rep, 2015, 33(5): 2319–30. DOI:10.3892/or.2015.3858 |
| [5] | Lavagnolli T, Gupta P, Mirabontenbal H, et al. Initiation and maintenance of pluripotency gene expression in the absence of cohesin[J]. Genes Dev, 2015, 29(1): 23–38. DOI:10.1101/gad.251835.114 |
| [6] | 苌新伟, 马秀现, 申红霞, 等. Engrailed-2基因在肝癌中的表达及其对肝癌细胞增殖和侵袭的影响[J]. 实用医学杂志, 2017, 33(4): 572–5. [ Chang XW, Ma XX, Shen HX, et al. Expression of Engrailed-2 in hepatocellular carcinoma and effect of silencing Engrailed-2 gene on the proliferation and invasion of hepatocellular carcinoma cells[J]. Shi Yong Yi Xue Za Zhi, 2017, 33(4): 572–5. DOI:10.3969/j.issn.1006-5725.2017.04.019 ] |
| [7] | Lai CY, Xu Y, Yu GS, et al. Engrailed-2 might play an anti-oncogenic role in clear-cell renal cell carcinoma[J]. J Mol Histol, 2016, 47(3): 229–37. DOI:10.1007/s10735-016-9665-4 |
| [8] | Majumdar D, Kanafi M, Bhonde R, et al. Differential neuronal plasticity of dental pulp stem cells from exfoliated deciduous and permanent teeth towards dopaminergic neurons[J]. J Cell Physiol, 2016, 231(9): 2048–63. DOI:10.1002/jcp.v231.9 |
| [9] | Kouwenhoven WM, Veenvliet JV, Van Hooft JA, et al. Engrailed 1 shapes the dopaminergic and serotonergic landscape through proper isthmic organizer maintenance and function[J]. Biol Open, 2016, 5(3): 279–88. DOI:10.1242/bio.015032 |
| [10] | Luo L, Siah CK, Cai Y. Engrailed acts with Nejire to control decapentaplegic expression in the Drosophila ovarian stem cell niche[J]. Development, 2017, 144(18): 3224–31. DOI:10.1242/dev.145474 |
| [11] | Sorolla A, Ho D, Wang E, et al. Sensitizing basal-like breast cancer to chemotherapy using nanoparticles conjugated with interference peptide[J]. Nanoscale, 2016, 8(17): 9343–53. DOI:10.1039/C5NR08331A |
| [12] | Morgan R, Bryan RT, Javed S, et al. Expression of Engrailed-2 (EN2) protein in bladder cancer and its potential utility as a urinary diagnostic biomarker[J]. Eur J Cancer, 2013, 49(9): 2214–22. DOI:10.1016/j.ejca.2013.01.019 |
| [13] | Bose SK, Bullard RS, Donald CD. Oncogenic role of engrailed-2 (en-2) in prostate cancercell growth and survival[J]. Transl Oncogenomics, 2008, 3(3): 37–43. |
| [14] | 李云飞, 赖彩永, 都兴华, 等. EN2在膀胱癌中的表达及其意义[J]. 实用医学杂志, 2013, 29(19): 3127–9. [ Li YF, Lai CY, Du XH, et al. Expression and significance of EN2 in bladder cancer[J]. Shi Yong Yi Xue Za Zhi, 2013, 29(19): 3127–9. DOI:10.3969/j.issn.1006-5725.2013.19.010 ] |
| [15] | Rajput S, Khera N, Guo Z, et al. Inhibition of cyclin dependent kinase 9 by dinaciclib suppresses cyclin B1 expression and tumor growth in triple negative breast cancer[J]. Oncotarget, 2016, 7(35): 56864–75. |
| [16] | Zhang J, Li H, Huang Z, et al. Hypoxia attenuates Hsp90 inhibitor 17-DMAG-induced cyclin B1 accumulation in hepatocellular carcinoma cells[J]. Cell Stress Chaperones, 2016, 21(2): 339–48. DOI:10.1007/s12192-015-0664-2 |
| [17] | Kaminski MM, Tosic J, Kresbach C, et al. Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors[J]. Nat Cell Biol, 2016, 145(12): 1269–80. |
| [18] | Martin NL, Saba-El-Leil MK, Sadekova S, et al. EN2 is a candidate oncogene in human breast cancer[J]. Oncogene, 2005, 24(46): 6890–901. DOI:10.1038/sj.onc.1208840 |
| [19] | Kong F, Wang H, Guo J, et al. Hsp70 suppresses apoptosis of BRL cells by regulating the expression of Bcl-2, cytochrome C, and caspase 8/3[J]. in Vitro Cell Dev Biol Anim, 2016, 52(5): 568–75. DOI:10.1007/s11626-016-0005-5 |
| [20] | Mohan V, Agarwal R, Singh RP. A novel alkaloid, evodiamine causes nuclear localization of cytochrome-c and induces apoptosis independent of p53 in human lung cancer cells[J]. Biochem Biophys Res Commun, 2016, 477(4): 1065–71. DOI:10.1016/j.bbrc.2016.07.037 |
| [21] | Shrivastava M, Subbiah V. Elevated caspase 3 activity and cytosolic cytochrome c in NT2 cybrids containing amyotrophic lateral sclerosis subject mtDNA[J]. Int J Neurosci, 2016, 126(9): 839–49. DOI:10.3109/00207454.2015.1074902 |
| [22] | Liu T, Wu X, Li Y, et al. RBFOX3 regulates the chemosensitivity of cancer cells to 5-fluorouracil via the pi3k/akt, emt and cytochrome-c/caspase pathways[J]. Cell Physiol Biochem, 2018, 46(4): 1365–80. DOI:10.1159/000489153 |
| [23] | 徐钢, 高轶, 闫茂生. miR-155/PTEN调控轴在肝癌中的表达及其机制[J]. 肿瘤防治研究, 2018, 45(2): 67–72. [ Xu G, Gao Y, Yan MS. Expression of miR-155/PTEN Axis in Hepatocellular Carcinoma and Its Mechanism[J]. Zhong Liu Fang Zhi Yan Jiu, 2018, 45(2): 67–72. DOI:10.3971/j.issn.1000-8578.2018.17.0931 ] |
| [24] | Yang TS, Yang XH, Chen X, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN[J]. FEBS Lett, 2014, 588(13): 2162–9. DOI:10.1016/j.febslet.2014.04.050 |
| [25] | Oliva CR, Zhang W, Langford C, et al. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit[J]. Oncotarget, 2017, 8(23): 37568–83. |
| [26] | Biswas R, Ahn JC, Kim JS. Sulforaphene synergistically sensitizes cisplatin via enhanced mitochondrial dysfunction and pi3k/pten modulation in ovarian cancer cells[J]. Anticancer Res, 2015, 35(7): 3901–8. |
 2019, Vol. 46
2019, Vol. 46


