文章信息
- 不同激素状态的HER2阳性晚期乳腺癌复发转移特征及生存分析
- Recurrent and Metastatic Characteristics and Prognosis of Advanced HER2-positive Breast Cancer Patients with Different HR Status
- 肿瘤防治研究, 2019, 46(1): 37-44
- Cancer Research on Prevention and Treatment, 2019, 46(1): 37-44
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.0962
- 收稿日期: 2018-07-16
- 修回日期: 2018-11-16
乳腺癌是一种高度异质性的恶性肿瘤,Perou等根据雌激素受体(estrogen receptor, ER)、孕激素受体(progesterone receptor, PR)及HER2等不同的分子特征将乳腺癌分为不同的亚型[1],这些亚型有着不同的生物学特性及治疗策略。其中15%~20%的乳腺癌患者存在HER2基因扩增或过表达[2],有研究表明HER2阳性乳腺癌患者具有恶性程度高、复发转移早、预后差等特点[3]。2005年圣加化国际乳腺癌会议(St.Gallen)国际专家共识将HER2阳性列为乳腺癌重要危险预后因素[4]。
ER和(或)PR阳性者为HR阳性,HER2阳性乳腺癌患者中有50%同时也为HR阳性[5]。与HER2基因过表达者预后相对较差不同,HR阳性(HR+)同时HER2阳性(HER2+)者预后相对较好。基因表达谱研究表明HR+/HER2+与HR-/HER2+是两种不同亚型[1],HR-/HER2+多高表达表皮生长因子受体(epidermal growth factor receptor, EGFR)、HER2和成纤维细胞生长因子型受体(fibroblast growth factor 4receptor, FGFR4)等酪氨酸激酶受体,而HR+/HER2+多表达GATA3、B细胞淋巴瘤/白血病-2(B cell lymphoma/leukemia2, Bcl2)以及雌激素受体α1基因(estrogen receptorα1 gene, ESR1)等Luminal亚群基因。国外有研究表明,与HR-/HER2+相比,HR+/HER2+组织学分级较低,多发生骨转移,较少发生脑转移,预后较好[6]。新辅助临床试验结果显示,不同HR状态的HER2阳性乳腺癌患者新辅助抗HER2靶向治疗疗效不同,HR阴性患者有更高的病理缓解率(pathologic complete response, pCR)[7-8];而不同HR状态在解救抗HER2靶向治疗疗效上是否存在差异目前还不明确,目前国内关于不同HR状态的HER2阳性乳腺癌生物学特性差异研究较少。本研究通过对237例HER2阳性晚期乳腺癌的临床病理学特征及预后进行回顾性分析,旨在探讨不同HR状态下的HER2阳性晚期乳腺癌患者复发转移特征及预后的关系。
1 资料与方法 1.1 病例资料收集2011年1月—2016年12月天津医科大学肿瘤医院收治的237名HER2阳性晚期乳腺癌患者相关资料。入选标准:HER2免疫组织化学染色+++或荧光原位杂交(fluorescence in situ hybridization, FISH)阳性的乳腺癌患者,女性原发单侧乳腺癌,经B型超声、CT、MRI、放射性核素扫描、PET/CT或病理活检证实出现复发和(或)转移,临床病理和随访资料完整。排除标准:HER2阴性患者、男性乳腺癌、原发性双乳癌、合并其他恶性肿瘤、临床病理或随访资料不完整者。
1.2 定义及观察指标采用免疫组织化学法对雌激素受体和孕激素受体进行检测,阳性细胞数所占比例≥1%定义为阳性。采用美国临床肿瘤学会(American Society of Clinical Oncology, ASCO)和美国病理学家协会(College of American Pathologists, CAP)指南推荐的评分系统对HER2进行评价,HER2免疫组织化学染色0分或(+)判定为阴性;(+++)判定为阳性;染色结果为(++)采用FISH进行验证,FISH结果阳性判定为阳性,反之则判定为阴性。采用美国癌症联合会(American Joint Committee on Cancer)2010年制定的第7版TNM分期对乳腺癌患者进行临床分期。采用2000年制定的实体瘤疗效评价标准进行疗效评价。本研究中内脏转移定义为出现肝、肺、脑、胸膜转移;淋巴结转移定义为出现同侧或对侧锁骨上、下淋巴结,纵隔淋巴结,腹腔淋巴结。
本研究根据HR状态将HER2阳性乳腺癌患者分为HR+/HER2+组和HR-/HER2+组。ER和(或)PR阳性归为HR+/HER2+组,ER和PR阴性归为HR-/HER2+组。
1.3 随访通过门诊、住院检查或电话询问的方法进行随访,随访日期截至2018年5月1日。预后评价指标为无进展生存时间(progression-free survival, PFS)和转移后总生存时间(metastases-overall survival, M-OS),PFS定义为从首次复发/转移之日起至疾病进展或死亡的时间。M-OS定义为从首次复发/转移之日起至任何原因所致死亡或末次随访的时间。
1.4 统计学方法采用SPSS22.0软件进行统计学分析。用百分比进行临床资料的描述,组间分类变量的比较采用χ2检验,Kaplan-Meier法进行生存分析,Log rank检验进行单因素分析,Cox比例风险回归模型进行多因素分析。P < 0.05为差异有统计学意义。
2 结果 2.1 HER2阳性患者的临床病理特征本研究共纳入了237名HER2阳性晚期乳腺癌患者,59.5%的患者确诊乳腺癌时的年龄≤50岁,81.9%的患者病理类型为浸润性导管癌,T1~2期者占71.3%,48.5%为组织学Ⅱ级,Ki-67指数>20%者占57.8%。除32名初诊Ⅳ期的患者外其余患者均进行了改良根治术或根治术,接受了术后辅助化疗者占81%,HR阳性患者中62.6%的患者接受了辅助内分泌治疗,19.2%的患者接受了辅助抗HER2靶向治疗。94.5%的患者在出现转移后接受了解救化疗。
2.2 HR+/HER2+与HR-/HER2+患者临床病理特征比较全组患者中,HR+/HER2+占48.5%(115例),其中ER+/PR+的患者占69.6%(80例)、ER+/PR-患者占20.9%(24例)、ER-/PR+患者占9.6%(11例);HR-/HER2+占51.5%(122例)。HR+/HER2+组中,58.3%的患者确诊乳腺癌时为绝经前状态,47.8%的患者临床分期为Ⅰ~Ⅱ期,T分期为T1~2者占80.0%,未发生腋窝淋巴结转移者占31.3%。HR-/HER2+组中,40.2%的患者确诊乳腺癌时为绝经前状态,27.1%的患者临床分期为Ⅰ~Ⅱ期,T分期为T1~2者占63.1%,未发生腋窝淋巴结转移者占13.1%。两组间上述差异均具有统计学意义(P < 0.05),在病理类型、组织学分级、Ki67指数上两组间差异无统计学意义,见表 1。
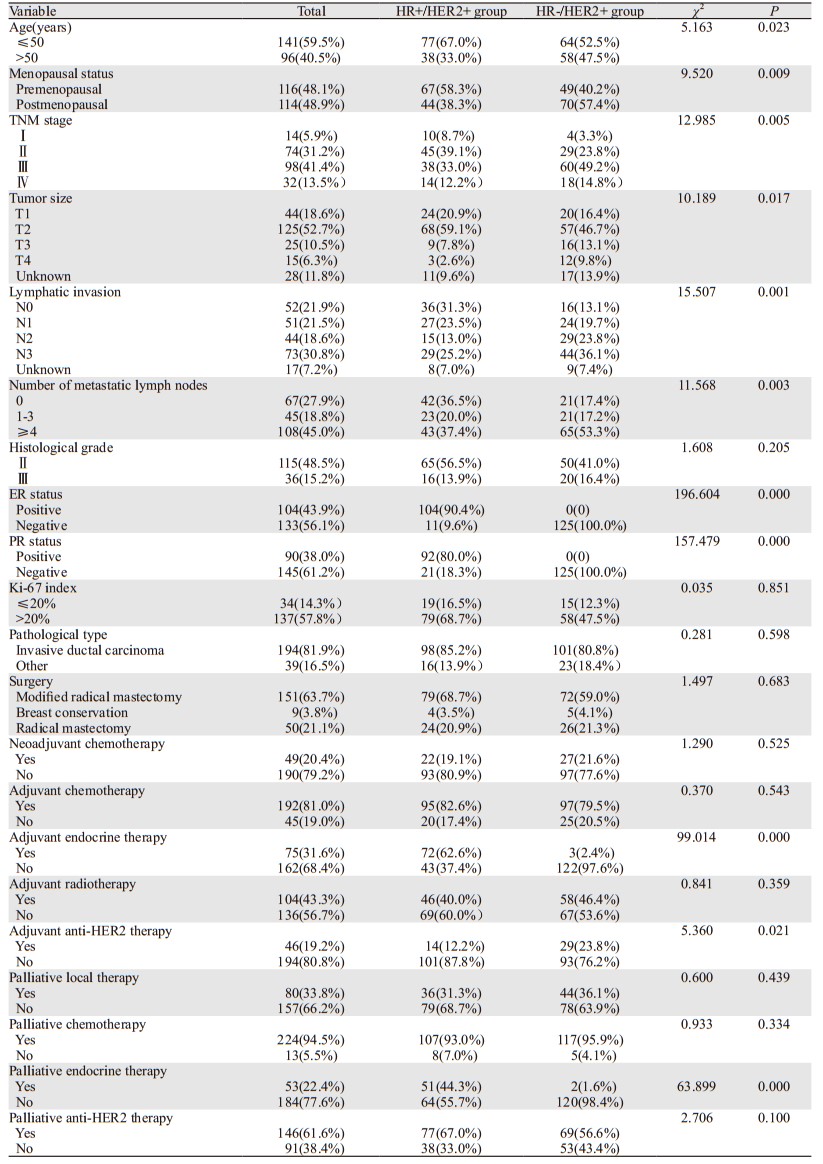 |
HR+/HER2+组中位DFS为21(0~115)月,首发转移时多发者占51.3%(59例),局部复发者占49.6%(57例),发生内脏转移者占56.5%(65例);HR-/HER2+组中位DFS为15(0~136)月,首发转移时多发者占54.1%(66例),局部复发者占41.8%(51例),发生内脏转移者占68.0%(83例)。与HR-/HER2+组相比,HR+/HER2+更多发生骨转移(分别为48.7%和32.0%, P=0.010),在肝、肺、脑的转移上两者差异无统计学意义,见表 2、图 1。
 |
|
| 图 1 不同HR状态HER2阳性晚期乳腺癌首发远处转移部位比例 Figure 1 Percentage of first distant metastasis sites in advanced HER2-positive breast cancer patients with different HR status |
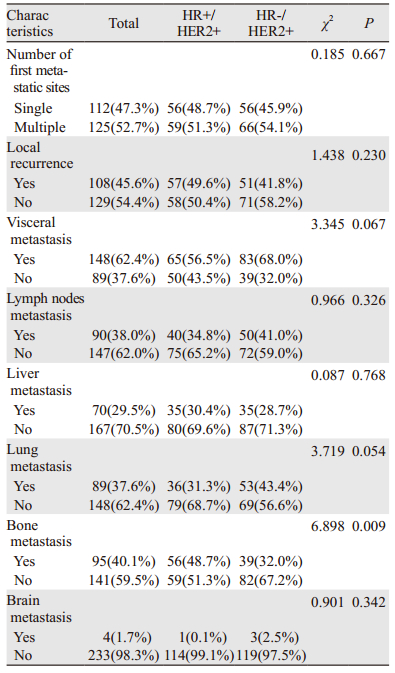
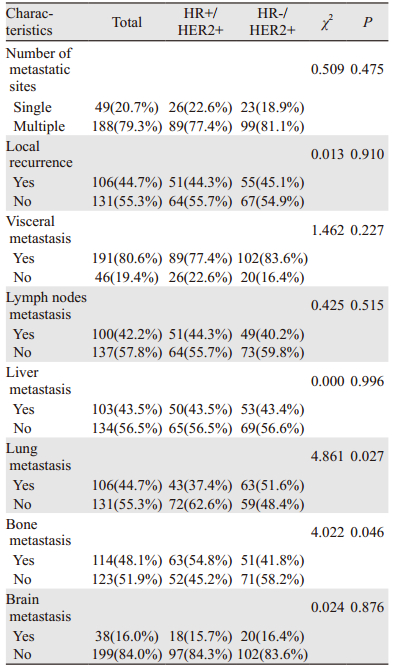
HR+/HER2+组发生骨转移者占54.8%(63例),肺转移者占37.4%(43例),肝转移者占43.5%(50例),脑转移者占15.7%(38例);HR-/HER2+组发生骨转移者占41.8%(51例),肺转移者占51.6%(63例),肝转移者占43.4%(53例),脑转移者占16.4%(20例),见图 2;与HR-/HER2+组相比,HR+/HER2+更多发生骨转移(P=0.046),较少发生肺转移(P=0.027);在肝、脑的转移上两者差异无统计学意义(P > 0.05),见表 3。
|
| 图 2 不同HR状态HER2阳性晚期乳腺癌总体转移部位比例 Figure 2 Percentage of first and subsequent distant metastasis sites based on different HR status in advanced HER2-positive breast cancer patients |
 |
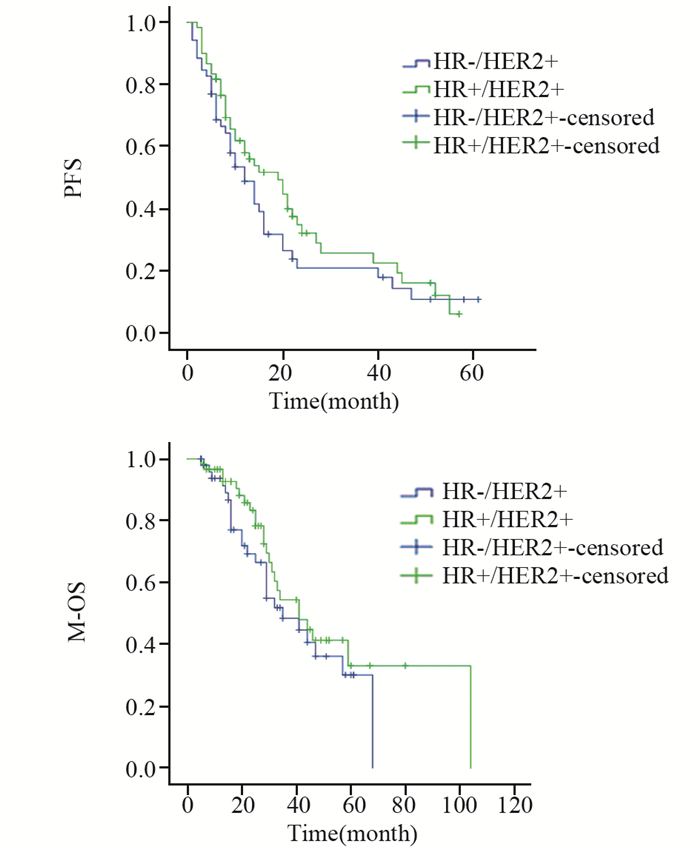
对112名接受解救曲妥珠单抗联合化疗治疗的HER2阳性晚期乳腺癌患者治疗疗效进行比较,HR+/HER2+组达到PR者占60.0%(36例),HR-/HER2+组为67.3%(35例),两组间差异无统计学意义(P=0.423)。HR+/HER2+组中位PFS为19(6~57)月,中位M-OS为41(6~80)月;HR-/HER2+组中位PFS为12(5~61)月,中位M-OS为35(5~61)月,两组间PFS及M-OS差异均无统计学意义(P=0.259, P=0.303),见图 3。
|
| PFS: progression-free survival; M-OS: metastases-overall survival 图 3 接受解救曲妥珠单抗联合化疗的HR+/HER2+与HR-/HER2+组乳腺癌患者PFS及M-OS比较 Figure 3 Comparison of PFS and M-OS between HR+/HER2+ group and HR-/HER2+ group treated with palliative trastuzumab plus chemotherapy |
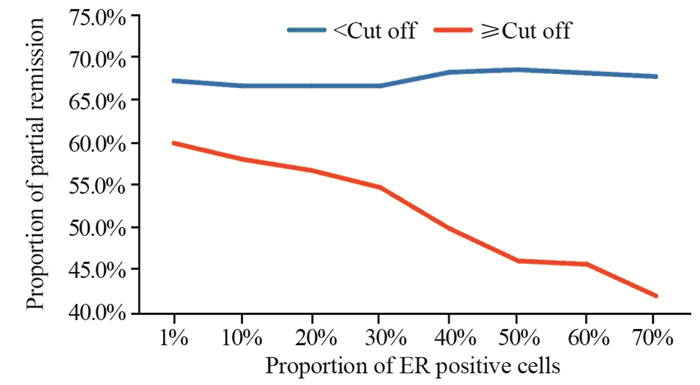
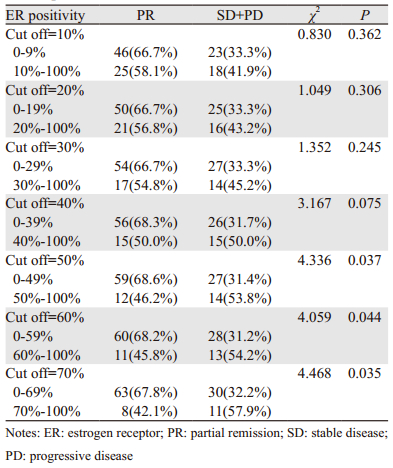
本研究中ER阳性定义为ER阳性细胞数所占比例≥1%,而将ER阳性细胞数所占比例采取不同截断值(截断值< 10%组与≥10%组;< 20%与≥20%组等),对这部分患者的疗效进行进一步比较结果显示,随着选取的截断值增加,ER阳性细胞数所占比例≥截断值的患者达到PR的比例逐渐降低,见图 4,当截断值取≥50%时,两组间差异有统计学意义(均P < 0.05),见表 4。
|
| 图 4 接受曲妥珠单抗联合化疗的不同ER阳性细胞数所占比例患者间达PR的比例比较 Figure 4 Comparison of PR proportion among patients with different proportions of estrogen receptor positive cells and treated with palliative trastuzumab plus chemotherapy |
 |
全组患者转移后中位随访时间49(5~122)月。HR+/HER2+组中位PFS 20(6~95)月,中位M-OS 34(5~102)月,1年、3年、5年M-OS分别为97.3%、48.6%、32.4%;HR-/HER2+组中位PFS 14(5~62)月,中位M-OS为29(3~70)月,1年、3年、5年M-OS分别为88.0%、36.6%、24.6%;两组间PFS及M-OS差异均有统计学意义(P=0.011, P=0.022),见图 5。
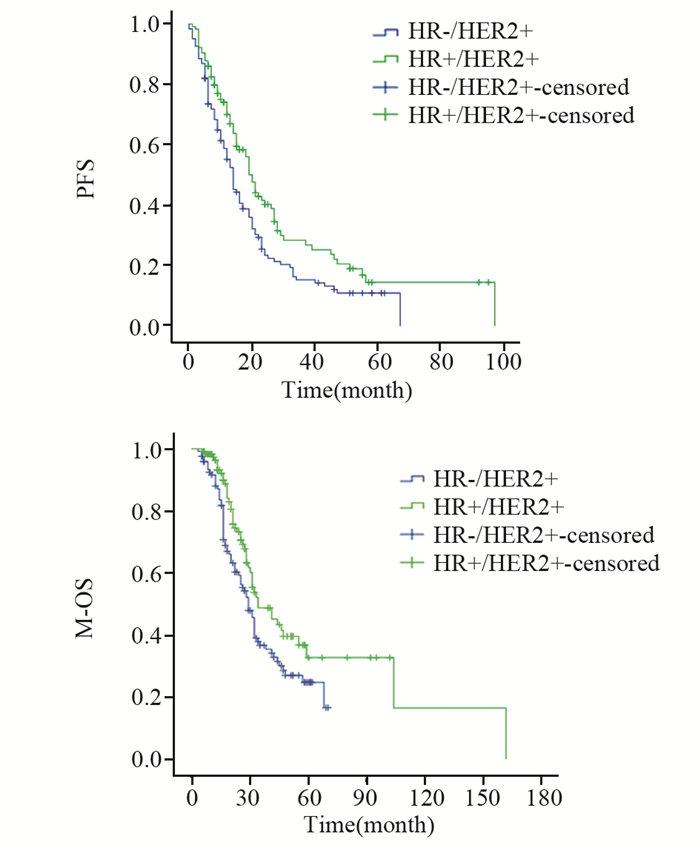
|
| 图 5 HR+/HER2+与HR-/HER2+组HER2+晚期乳腺癌PFS及M-OS比较 Figure 5 Comparison of PFS and M-OS between HR+/HER2+ group and HR-/HER2+ group |
在115名HR+/HER+晚期乳腺癌患者中,29.6%(34例)的患者接受了解救曲妥珠联合内分泌治疗,13.0%(15例)的患者接受了单内分泌治疗,接受解救曲妥珠联合内分泌治疗的患者与接受单内分泌治疗的患者中位M-OS分别为44(13~80)月、31(11~58)月,两组间差异有统计学意义(P=0.039),见图 6。
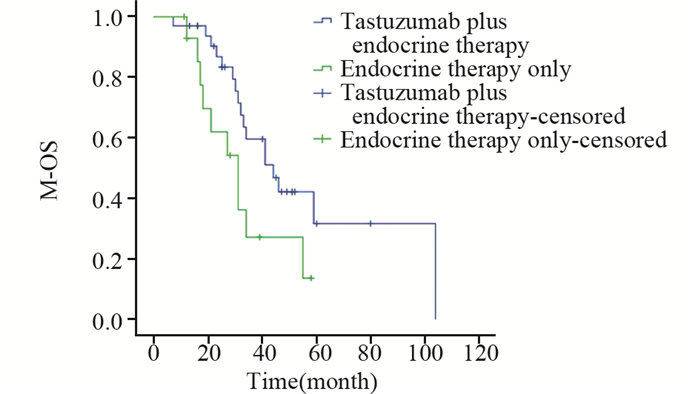
|
| 图 6 接受解救曲妥珠单抗联合内分泌治疗与单内分泌治疗的HR+/HER2+乳腺癌患者M-OS比较 Figure 6 Comparison of M-OS between palliative trastuzumab therapy plus endocrine therapy group and endocrine therapy alone group of advanced HR+/HER2+ breast cancer patients |
原癌基因HER2定位于17q12,是EGFR家族成员之一,其编码的跨膜糖蛋白具有酪氨酸激酶活性,在调节细胞的增殖、分化和生长上具有重要作用[9];HER2基因过度表达可促进乳腺癌细胞持续增殖,恶性度增高。HER2和ER介导的信号转导通路在多个环节相互交叉,互相影响,雌激素与ER结合激活ER后通过基因组学作用促进基因转录从而上调HER2的表达,通过非基因组学作用促进HER2磷酸化从而激活HER2;反之,HER2通过自身二聚化被激活后也会促进ER及ER共激活因子的磷酸化从而激活ER信号通路[10]。
国外研究结果显示HER2阳性乳腺癌患者中HR+/HER2+者占41.0%~62%[11-13],本研究中HR+/HER2+者占48.5%,HR-/HER2+者占51.5%,与上述结果相符。本研究结果显示HR-/HER2+与HR+/HER2+组T3~T4期患者分别为22.9%和10.4%、Ⅲ期患者分别为49.2%和33.0%、发生腋窝淋巴结转移者分别为70.5%和57.4%,差异均有统计学意义(P < 0.05)。总体而言,HR阳性的HER2阳性乳腺癌患者生物学行为更好,与前期研究结果相符。
HER2阳性乳腺癌患者易发生脑转移[14-16];然而当考虑到HR状态时HER2+乳腺癌复发转移模式发生了变化,HR+/HER2+者更易发生骨转移[12-13],HR-/HER2+者更易发生脑转移[13]。本研究结果显示,HER2阳性晚期乳腺癌患者发生骨、肺、肝、脑转移者分别占48.1%、44.7%、43.5%、16.0%;无论是首发转移部位还是总体转移部位,HR+/HER2+组都更易发生骨转移(P < 0.05),与上述结果一致;而在脑转移的发生上,HR-/HER2+组虽然多于HR+/HR2+组,但差异无统计学意义(分别为16.4%和15.7%,P=0.876),推测导致上述结果的原因为:(1)辅助抗HER2靶向治疗改变了HER2阳性乳腺癌患者的自然病程,使复发转移模式发生变化;(2)东西方人种遗传因素差异;(3)本研究中脑转移患者样本量较小,使差异的统计学意义未显示出来。
既往研究显示,HR阳性乳腺癌患者中靶向抗HR的内分泌治疗疗效在HER2+患者中较HER2-患者差[17-18],目前较为明确的机制之一为ER与HER2信号转导通路间的双向交叉对话所致。HER2+晚期乳腺癌首选抗HER2为基础的治疗,那么HR状态会影响抗HER2治疗疗效吗?在新辅助抗HER2临床试验中,抗HER2治疗的疗效在HR+/HER2+与HR-/HER2+患者间不同,HR-/HER2+患者获得更高的pCR[7-8]。而一项对805例HER2+晚期乳腺癌的回顾性研究结果显示,在解救治疗中无论是采用曲妥珠联合化疗方案还是曲妥珠单药治疗方案,HR+/HER2+与HR-/HER2+患者间临床获益无差异[11],与本研究结果一致。而当将ER阳性细胞数所占比例采取不同截断值进行疗效比较时,结果显示随着ER阳性细胞数所占比例增加,达PR的患者比例相应减少,当截断值定于50%时,两组间差异存在统计学意义(P=0.037)。提示HR状态同样影响解救曲妥珠单抗治疗疗效,HR阳性细胞所占比例低者曲妥珠单抗治疗疗效较好。该结果与Montemurro等[19]研究结果一致。上述结果提示在对HER2阳性乳腺癌患者进行靶向、内分泌、化疗最优化选择治疗时应同时兼顾HR状态。
不同HR状态的HER2阳性乳腺癌患者预后不同,本研究中HR+/HER2+组中位M-OS 34月(范围5~102月),HR-/HER2+组中位M-OS为29月(范围3~70月),HR+/HER2+乳腺癌预后好于HR-/HER2+(P=0.022),与以往研究结果一致[13]。
本研究也存在一定的局限性:所有入组患者均来自单中心且为回顾性分析,可能会造成选择偏倚,而研究的样本量也较小,有待以后更大样本的多中心前瞻性研究来进一步明确不同HR状态的HER2阳性晚期乳腺癌生物学特性的差异。
综上所述,不同HR状态的HER2阳性乳腺癌患者的临床病理特征、复发转移特点、预后及曲妥珠治疗疗效均不同,在对HER2阳性乳腺癌患者进行细化管理时应结合HR状态制定个性化的随访和治疗模式。
作者贡献
任玉琳:参与选题与设计,数据采集,分析、解释及论文撰写;张丽:对论文写作提出建议并参与修改;佟仲生:参与选题及设计,对论文提出建议并进行修改
| [1] | Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours[J]. Nature, 2000, 406(6797): 747–52. DOI:10.1038/35021093 |
| [2] | Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update[J]. Arch Pathol Lab Med, 2014, 138(2): 241–56. DOI:10.5858/arpa.2013-0953-SA |
| [3] | Bergen ES, Tichy C, Berghoff AS, et al. Prognostic impact of breast cancer subtypes in elderly patients[J]. Breast Cancer Res Treat, 2016, 157(1): 91–9. DOI:10.1007/s10549-016-3787-y |
| [4] | Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005[J]. Ann Oncol, 2005, 16(10): 1569–83. DOI:10.1093/annonc/mdi326 |
| [5] | Konecny G, Pauletti G, Pegram M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer[J]. J Natl Cancer Inst, 2003, 95(2): 142–53. DOI:10.1093/jnci/95.2.142 |
| [6] | Hess KR, Esteva FJ. Effect of HER2 Status on Distant Recurrence in Early-Stage Breast Cancer[J]. Breast Cancer Res Treat, 2013, 137(2): 449–55. DOI:10.1007/s10549-012-2366-0 |
| [7] | Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Oncol, 2018, 19(1): 115–26. DOI:10.1016/S1470-2045(17)30716-7 |
| [8] | de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response[J]. Lancet Oncol, 2014, 15(10): 1137–46. DOI:10.1016/S1470-2045(14)70320-1 |
| [9] | Klapper LN, Glathe S, Vaisman N, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors[J]. Proc Natl Acad Sci U S A, 1999, 96(9): 4995–5000. DOI:10.1073/pnas.96.9.4995 |
| [10] | Morrison G, Fu X, Shea M, et al. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance[J]. Breast Cancer Res Treat, 2014, 144(2): 263–72. DOI:10.1007/s10549-014-2878-x |
| [11] | Mohammed RAA, Radwan MEM, Alrufayi BM, et al. Does loss of hormonal receptors influence the pathophysiological characteristics of the HER-2 breast cancer phenotype?[J]. Pathophysiology, 2018, 25(4): 439–44. DOI:10.1016/j.pathophys.2018.09.004 |
| [12] | Lee HJ, Park IA, Park SY, et al. Two histopathologically different diseases: hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer[J]. Breast Cancer Res Treat, 2014, 145(3): 615–23. DOI:10.1007/s10549-014-2983-x |
| [13] | Paluch-Shimon S, Ben-Baruch N, Wolf I, et al. Hormone receptor expression is associated with a unique pattern of metastatic spread and increased survival among HER2-overexpressing breast cancer patients[J]. Am J Clin Oncol, 2009, 32(5): 504–8. DOI:10.1097/COC.0b013e3181967d72 |
| [14] | 郑红梅, 吴新红, 原俊, 等. HER2阳性乳腺癌脑转移3例并文献复习[J]. 肿瘤防治研究, 2015, 42(6): 637–40. [ Zheng HM, Wu XH, Yuan J, et al. Brain Metastases in Patients with HER2 Positive Breast Cancer: Three Cases Report and Literature Review[J]. Zhong Liu Fang Zhi Yan Jiu, 2015, 42(6): 637–40. DOI:10.3971/j.issn.1000-8578.2015.06.023 ] |
| [15] | Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases[J]. Breast, 2014, 23(5): 623–8. DOI:10.1016/j.breast.2014.06.009 |
| [16] | Hedayatizadeh-Omran A, Rafiei A, Alizadeh-Navaei R, et al. Role of HER2 in brain metastasis of breast cancer: a systematic review and meta-analysis[J]. Asian Pac J Cancer Prev, 2015, 16(4): 1431–4. DOI:10.7314/APJCP.2015.16.4.1431 |
| [17] | Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial[J]. J Clin Oncol, 2008, 26(7): 1059–65. DOI:10.1200/JCO.2007.12.9437 |
| [18] | Rasmussen BB, Regan MM, Lykkesfeldt AE, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrineresponsive early breast cancer: supplementary results from the BIG 1-98 randomised trial[J]. Lancet Oncol, 2008, 9(1): 23–8. DOI:10.1016/S1470-2045(07)70386-8 |
| [19] | Montemurro F, Rossi V, Cossu Rocca M, et al. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients[J]. Cancer, 2012, 118(1): 17–26. DOI:10.1002/cncr.26162 |
 2019, Vol. 46
2019, Vol. 46


