文章信息
- CDK4/6抑制剂的联合治疗模式
- CDK4/6 Inhibitors-based Combinational Therapy for Human Cancers
- 肿瘤防治研究, 2019, 46(1): 72-75
- Cancer Research on Prevention and Treatment, 2019, 46(1): 72-75
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.0906
- 收稿日期: 2018-07-02
- 修回日期: 2018-09-14
2. 730050 兰州,解放军940医院眼科
2. Department of Ophthalmology, People's Liberation Army 940 Hospital, Lanzhou 730050, China
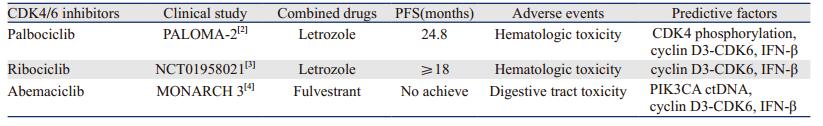
过去的20年里,细胞周期素依赖激酶(cyclin-dependent kinases, CDKs)抑制剂的发展取得了长足的进步,选择性CDK4/6抑制剂已经批准用于ER+/HER2-晚期乳腺癌的治疗[1]。选择性CDK4/6抑制剂包括瑞博西尼(ribociclib)、玻玛西尼(abemaciclib)和帕博西尼(palbociclib)阻滞细胞周期从G1期进入S期,Ⅲ期临床研究显示了良好的疗效并且不良反应较少,见表 1。获得性耐药是后CDK4/6抑制剂治疗时代必须面临的挑战[5],联合治疗模式克服了CDK4/6抑制剂的耐药并提高了临床疗效,开启了肿瘤精准治疗的一扇新窗口。本文系统综述CDK4/6抑制剂的综合治疗模式。
细胞周期治疗联合内分泌治疗是目前最主要的应用模式,CDK4/6抑制剂也已成为晚期乳腺癌治疗的新标准。选择性CDK4/6抑制剂明显提高了ER+/HER2-晚期乳腺癌的无进展生存时间(progression-free survival, PFS)[6]。对于HR+/HER2-的晚期乳腺癌患者,帕博西尼联合来曲唑较单药来曲唑有更长的PFS,但血液毒性增加[3]。另外一项Ⅱ期研究也显示瑞博西尼联合来曲唑较单药来曲唑有更长的PFS,同样骨髓抑制的发生率也增高[4]。玻玛西尼联合氟维司群组较安慰剂联合氟维司群组有更长的PFS(16.4月vs.9.3月,P < 0.001),两组的客观缓解率分别为48.1%和21.3%[7]。MONARCH 3研究发现,在ER+/HER2-的晚期乳腺癌患者中,玻玛西尼联合非甾体类芳香化酶抑制剂较单药非甾体类芳香化酶抑制剂有更长的PFS(0 vs. 14.7月,HR=0.54; 95%CI: 0.41~0.72; P=0.000021)和更高的客观缓解率(59% vs. 44%, P=0.004)[7]。O'Leary等[8]用PALOMA-3研究的乳腺癌患者血清样本发现,接受帕博西尼和氟维司群治疗患者15天后的磷脂酰肌醇-3激酶(phosphoinositide 3-kinase, PI3K)ctDNA水平可以预测PFS(HR=3.94, P=0.0013),为寻找CDK4/6抑制剂联合内分泌治疗的疗效预测因子进行了有益探索。
2 CDK4/6抑制剂联合EGFR抑制剂Goel等[9]研究显示CDK4/6抑制剂能抵抗HER2+乳腺癌靶向治疗的耐药。CDK4/6抑制剂不仅能抑制视网膜母细胞瘤(retinoblastoma, Rb)基因磷酸化,还能减少结节性硬化症(tuberous sclerosis complex, TSC)基因磷酸化和哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)活性,这解除了上游表皮生长因子(epidermal growth factor receptor, EGFR)家族激酶的抑制,使肿瘤重新对EGFR/HER2抑制剂敏感。因此,抗EGFR/HER2和CDK4/6抑制剂双靶向治疗可能是HER2+乳腺癌的新治疗选择。CDK4/6抑制剂PD0332991可以逆转人肺癌细胞对EGFR酪氨酸激酶抑制剂的耐药,并提高人肺癌细胞对EGFR酪氨酸激酶抑制剂的敏感度[10]。Zhou等[11]发现CDK4/6抑制剂可以提高EGFR抑制剂对食管鳞状细胞癌的疗效并逆转耐药。
3 CDK4/6抑制剂联合PI3K/mTOR抑制剂PI3K-AKT-mTOR信号通路是肿瘤中最常见的失调通路之一,超过40个靶向此信号通路的分子正在进行各期临床试验。CDK4/6抑制剂能够延缓ER+乳腺癌患者内分泌治疗耐药,联合内分泌治疗可以明显延长PFS,但最终耐药仍然会发生。CDK4/6抑制剂联合PI3K或mTOR抑制剂和内分泌治疗可能提高CDK4/6抑制剂敏感度,早期临床试验也已经显示三药联合的良好效果[12]。mTOR抑制剂西罗莫司和依维莫司以及PI3K抑制剂idelalisib和copanlisib已经被批准用于多种肿瘤的治疗。考虑到PI3K-AKT-mTOR信号通路癌基因激活常常伴有其他信号通路原癌基因突变,联合治疗模式无疑可以提高治疗效果[13]。Teo等[14]研究发现,CDK4/6抑制剂联合PI3K抑制剂可以增加人三阴性乳腺癌细胞株的凋亡、细胞周期阻滞、肿瘤免疫原性和免疫原性细胞死亡,联合治疗也能够诱导体内三阴性乳腺癌的完全缓解和持续消退。最新研究显示,CDK-RB-E2F通路在控制ER+乳腺癌细胞周期中发挥重要作用。ER+乳腺癌生长抑制需要多途径阻断E2F转录因子释放,mTORC1/2和CDK4/6双抑制可以从根本上阻断E2F,导致肿瘤生长阻滞和耐药延迟[15]。联合模式的作用机制也包括影响乳腺癌的糖代谢[16]。
4 CDK4/6抑制剂联合化疗化疗药物的剂量限制性毒性可能导致长期的血液毒性(骨髓衰竭),严重影响了化疗应用和治疗效果。He等[17]研究显示,CDK4/6抑制剂trilaciclib联合化疗可以保护造血干细胞,避免化疗导致的骨髓衰竭。Iyengar等[18]评估了CDK4/6抑制剂瑞博西尼单药、联合化疗和维持治疗对高级别浆液性卵巢癌的治疗效果。令人惊讶的是,瑞博西尼联合顺铂后序贯瑞博西尼维持治疗有协同效应。瑞博西尼也通过Rad-3相关蛋白和检查点激酶1脱磷酸作用发挥G2/M检查点作用。靶向细胞周期蛋白的小分子抑制剂可以诱导肿瘤细胞周期阻滞和凋亡,Ⅰ期临床研究显示对成人和儿童肿瘤患者安全性良好,但单药应用临床获益有限。CDK4/6抑制剂联合化疗药物是合理的联合策略,因为化疗药物导致的肿瘤DNA损伤可能使肿瘤对细胞周期治疗更敏感[19]。
5 CDK4/6抑制剂联合免疫治疗CDK4/6抑制剂通过多种机制促进抗肿瘤免疫。Goel等[20]应用乳腺癌和其他实体瘤小鼠模型研究证实,CDK4/6抑制剂不仅能诱导肿瘤细胞周期阻滞,还能够促进抗肿瘤免疫。抗肿瘤免疫反应有两个基础:首先,CDK4/6抑制剂激活肿瘤细胞内源性反转录病毒成分表达,增加细胞内双链RNA水平,进而刺激产生Ⅲ型干扰素并增加肿瘤抗原递呈; 其次,CDK4/6抑制剂显著抑制调节性T细胞的增殖,促进细胞毒性T细胞对肿瘤细胞的清除。此研究为CDK4/6抑制剂联合肿瘤免疫治疗提供了理论基础。Cor1家族成员联会复合体蛋白(synaptonemal complex protein 3, SCP3)在免疫编辑细胞中过表达,并且通过超活化Cyclin D1-CDK4/6轴上调NANOG转录因子。SCP3-Cyclin D1-CDK4/6轴广泛存在于各类人体肿瘤中并伴有较差预后,CDK4/6抑制剂帕博西尼通过阻断SCP3-NANOG轴增加免疫抵抗肿瘤的敏感度,从而控制肿瘤[21]。另外,CDK4/6抑制剂可以增加程序化死亡配体1(programmed cell death ligand 1, PD-L1)表达、激活T细胞、诱导炎性反应微环境和提高肿瘤免疫原性[22-24]。Zhang等[25]证实细胞周期激酶可以调节PD-L1稳定性,提示CDK4/6抑制剂联合程序性死亡受体1(programmed death 1, PD-1)或PD-L1免疫检查点抑制剂可以提高肿瘤治疗效果。
6 CDK4/6抑制剂联合分子靶向治疗除了联合EGFR抑制剂和PI3K/mTOR抑制剂外,CDK4/6抑制剂还和其他分子靶向治疗药物联合,提高抗肿瘤治疗效果。CDK4/6抑制剂联合BRAF抑制剂或MEK抑制剂是黑色素瘤的有效治疗模式,但不良反应增加。Teh等[26]研究显示,持续MEK抑制剂联合间歇CDK4/6抑制剂较其他联合模式有更高的肿瘤缓解率。然而,一些肿瘤在治疗过程中因核糖体S6蛋白磷酸化而获得耐药。进一步研究发现,CDK4/6抑制剂联合BRAF抑制剂或MEK抑制剂获得性耐药的机制是激活了mTOR通路,mTORC1/2抑制剂AZD2014可以克服联合治疗模式的耐药。临床前研究显示,帕博西尼联合MEK抑制剂PD0325901是KRAS依赖性和BRAF突变转移性结直肠癌的有效治疗模式[27]。Wood等[28]研究显示,CDK4/6抑制剂瑞博西尼联合ALK抑制剂色瑞替尼(ceritinib)治疗ALK突变儿童神经母细胞瘤有更高的不良反应(P=0.008)和更佳的治疗效果(P=0.006),联合治疗提高了肿瘤生长抑制率、细胞周期阻滞和Caspase依赖的细胞死亡。RAF抑制剂联合CDK4/6抑制剂可以提高RAS或BRAF突变肿瘤的疗效[29]。Small等[30]开展的肾癌临床前研究证实,玻玛西尼联合舒尼替尼可以缩小肿瘤,而且无明显不良反应。
7 CDK4/6抑制剂联合其他治疗CDK4/6抑制剂联合自噬抑制剂能够维护G1/S检查点完整,可能成为包括乳腺癌在内的多种实体瘤的新治疗策略[31]。Francis等[32]研究显示,CDK4/6抑制剂使Rb阳性肉瘤细胞对Wee1激酶抑制剂AZD1775敏感,这为开展CDK4/6抑制剂联合Wee1激酶抑制剂治疗平滑肌肉瘤的临床试验打下了基础。
8 未来方向以细胞周期蛋白为靶标的肿瘤细胞周期治疗前景广阔,也为肿瘤精准治疗增添了新内容。筛选肿瘤驱动基因是实现精准肿瘤治疗的关键[33]。Bailey等[34]报告了最全面的肿瘤驱动基因和突变图谱,为实现包括CDK4/6抑制剂在内的肿瘤精准治疗奠定了基础。寻找针对细胞周期的新调控靶标是细胞周期治疗的未来方向。生长信号激发cyclin D1 mRNA在母细胞中累积,DNA损伤则导致母细胞中活化p53升高。遗传了更多细胞周期蛋白D1的子细胞继续进入下一个细胞周期,遗传更多活化p53的子细胞则进入静止状态,因此cyclin D1或p53可能成为细胞周期治疗的新靶标[35]。CDK8抑制剂也已成为肿瘤靶向治疗的新希望。
在联合治疗的基础上,寻找CDK4/6抑制剂的疗效预测因子是未来发展方向。Cyclin D3-CDK6激酶可以磷酸化糖代谢通路中的两个关键酶(6-磷酸果糖激酶和丙酮酸激酶M2)并抑制它们的代谢活性,这直接激活糖代谢的磷酸戊糖通路和丝氨酸通路,因此cyclin D3-CDK6可能成为人类肿瘤亚型的分层因子和CDK4/6抑制剂的预测因子[36]。CDK激活需要干扰素β表达,提示干扰素β可能成为CDK4/6抑制剂的预测因子[37]。其他的疗效预测因子包括CDK4磷酸化、D型细胞周期素和肿瘤克隆动力学等[38-40]。CDK4/6抑制剂为基础的联合治疗模式将成为肿瘤精准治疗的新策略。
作者贡献
张百红:文章书写和通信; 岳红云:文章审校
| [1] | Ingham M, Schwartz GK. Cell-Cycle Therapeutics Come of Age[J]. J Clin Oncol, 2017, 35(25): 2949–59. DOI:10.1200/JCO.2016.69.0032 |
| [2] | Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer[J]. N Engl J Med, 2016, 375(20): 1925–36. DOI:10.1056/NEJMoa1607303 |
| [3] | Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer[J]. N Engl J Med, 2016, 375(18): 1738–48. DOI:10.1056/NEJMoa1609709 |
| [4] | Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer[J]. J Clin Oncol, 2017, 35(32): 3638–46. DOI:10.1200/JCO.2017.75.6155 |
| [5] | O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors[J]. Nat Rev Clin Oncol, 2016, 13(7): 417–30. DOI:10.1038/nrclinonc.2016.26 |
| [6] | Turner NC, Neven P, Loibl S, et al. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer[J]. Lancet, 2017, 389(10087): 2403–14. DOI:10.1016/S0140-6736(16)32419-9 |
| [7] | Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy[J]. J Clin Oncol, 2017, 35(25): 2875–84. DOI:10.1200/JCO.2017.73.7585 |
| [8] | O'Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer[J]. Nat Commun, 2018, 9(1): 896. DOI:10.1038/s41467-018-03215-x |
| [9] | Goel S, Wang Q, Watt AC, et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors[J]. Cancer Cell, 2016, 29(3): 255–69. |
| [10] | Liu M, Xu S, Wang Y, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, sensitizes lung cancer cells to treatment with epidermal growth factor receptor tyrosine kinase inhibitors[J]. Oncotarget, 2016, 7(51): 84951–64. |
| [11] | Zhou J, Wu Z, Wong G, et al. CDK4/6 or MAPK blockade enhances efficacy of EGFR inhibition in oesophageal squamous cell carcinoma[J]. Nat Commun, 2017, 8: 13897. DOI:10.1038/ncomms13897 |
| [12] | Cortés J, Im SA, Holgado E, et al. The next era of treatment for hormone receptor-positive, HER2-negative advanced breast cancer: Triplet combination-based endocrine therapies[J]. Cancer Treat Rev, 2017, 61: 53–60. DOI:10.1016/j.ctrv.2017.09.011 |
| [13] | Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway?[J]. Nat Rev Clin Oncol, 2018, 15(5): 273–91. DOI:10.1038/nrclinonc.2018.28 |
| [14] | Teo ZL, Versaci S, Dushyanthen S, et al. Combined CDK4/6 and PI3Kα inhibition is synergistic and immunogenic in triple negative breast cancer[J]. Cancer Res, 2017, 77(22): 6340–52. DOI:10.1158/0008-5472.CAN-17-2210 |
| [15] | Michaloglou C, Crafter C, Siersbaek R, et al. Combined Inhibition of mTOR and CDK4/6 Is Required for Optimal Blockade of E2F Function and Long-term Growth Inhibition in Estrogen Receptor-positive Breast Cancer[J]. Mol Cancer Ther, 2018, 17(5): 908–20. DOI:10.1158/1535-7163.MCT-17-0537 |
| [16] | Cretella D, Ravelli A, Fumarola C, et al. The anti-tumor efficacy of CDK4/6 inhibition is enhanced by the combination with PI3K/AKT/mTOR inhibitors through impairment of glucose metabolism in TNBC cells[J]. J Exp Clin Cancer Res, 2018, 37(1): 72. DOI:10.1186/s13046-018-0741-3 |
| [17] | He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-inducedexhaustion[J]. Sci Transl Med, 2017, 9(387): pii: eaal3986. DOI:10.1126/scitranslmed.aal3986 |
| [18] | Iyengar M, O'Hayer P, Cole A, et al. CDK4/6 inhibition as maintenance and combination therapy for high grade serous ovarian cancer[J]. Oncotarget, 2018, 9(21): 15658–72. |
| [19] | Mills CC, Kolb EA, Sampson VB. Development of Chemotherapy with Cell-Cycle Inhibitors for Adult and Pediatric CancerTherapy[J]. Cancer Res, 2018, 78(2): 320–5. DOI:10.1158/0008-5472.CAN-17-2782 |
| [20] | Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity[J]. Nature, 2017, 548(7668): 471–5. DOI:10.1038/nature23465 |
| [21] | Oh SJ, Cho H, Kim S, et al. Targeting Cyclin D-CDK4/6 sensitizes immune-refractory cancer by blocking the SCP3-NANOG axis[J]. Cancer Res, 2018, 78(10): 2638–53. DOI:10.1158/0008-5472.CAN-17-2325 |
| [22] | Deng J, Wang ES, Jenkins RW, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation[J]. Cancer Discov, 2018, 8(2): 216–33. DOI:10.1158/2159-8290.CD-17-0915 |
| [23] | Schaer DA1, Beckmann RP, Dempsey JA, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade[J]. Cell Rep, 2018, 22(11): 2978–94. DOI:10.1016/j.celrep.2018.02.053 |
| [24] | Minton K. Tumour immunology: Cell cycle inhibitors boost tumour immunogenicity[J]. Nat Rev Immunol, 2017, 17(9): 529. DOI:10.1038/nri.2017.104 |
| [25] | Zhang J, Bu X, Wang H, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immunesurveillance[J]. Nature, 2018, 553(7686): 91–5. |
| [26] | Teh JLF, Cheng PF, Purwin TJ, et al. In Vivo E2F Reporting Reveals Efficacious Schedules of MEK1/2-CDK4/6 Targeting and mTOR-S6 Resistance Mechanisms[J]. Cancer Discov, 2018, 8(5): 568–81. DOI:10.1158/2159-8290.CD-17-0699 |
| [27] | Pek M, Yatim SMJM, Chen Y, et al. Oncogenic KRAS-associated gene signature defines co-targeting of CDK4/6 and MEK as a viable therapeutic strategy in colorectal cancer[J]. Oncogene, 2017, 36(35): 4975–86. DOI:10.1038/onc.2017.120 |
| [28] | Wood AC, Krytska K, Ryles HT, et al. Dual ALK and CDK4/6 Inhibition Demonstrates Synergy against Neuroblastoma[J]. Clin Cancer Res, 2017, 23(11): 2856–68. DOI:10.1158/1078-0432.CCR-16-1114 |
| [29] | Chen SH, Gong X, Zhang Y, et al. RAF inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1[J]. Oncogene, 2018, 37(6): 821–32. DOI:10.1038/onc.2017.384 |
| [30] | Small J, Washburn E, Millington K, et al. The addition of abemaciclib to sunitinib induces regression of renal cell carcinoma xenograft tumors[J]. Oncotarget, 2017, 8(56): 95116–34. |
| [31] | Vijayaraghavan S, Karakas C, Doostan I, et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmiccyclin E negative cancers[J]. Nat Commun, 2017, 8: 15916. DOI:10.1038/ncomms15916 |
| [32] | Francis AM, Alexander A, Liu Y, et al. CDK4/6 Inhibitors Sensitize Rb-positive Sarcoma Cells to Wee1 Kinase Inhibition through Reversible Cell-Cycle Arrest[J]. Mol Cancer Ther, 2017, 16(9): 1751–64. DOI:10.1158/1535-7163.MCT-17-0040 |
| [33] | 张百红, 岳红云. 肿瘤的细胞周期治疗[J]. 中国肿瘤临床, 2018, 45(6): 18–21. [ Zhang BH, Yue HY. Cancer cell cycle therapeutics[J]. Zhongguo Zhong Liu Lin Chuang, 2018, 45(6): 18–21. ] |
| [34] | Bailey MH, Tokheim C, Porta-Pardo E, et al. Comprehensive Characterization of Cancer Driver Genes and Mutations[J]. Cell, 2018, 173(2): 371–85. |
| [35] | Yang HW, Chung M, Kudo T, et al. Competing memories of mitogen and p53 signalling control cell-cycle entry[J]. Nature, 2017, 549(7672): 404–8. DOI:10.1038/nature23880 |
| [36] | Wang H, Nicolay BN, Chick JM, et al. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival[J]. Nature, 2017, 546(7658): 426–30. DOI:10.1038/nature22797 |
| [37] | Cingöz O, Goff SP. Cyclin-dependent kinase activity is required for type Ⅰ interferon production[J]. Proc Natl Acad Sci U S A, 2018, 115(13): E2950–9. DOI:10.1073/pnas.1720431115 |
| [38] | Raspé E, Coulonval K, Pita JM, et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib[J]. EMBO Mol Med, 2017, 9(8): 1052–66. DOI:10.15252/emmm.201607084 |
| [39] | Gong X, Litchfield LM, Webster Y, et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib[J]. Cancer Cell, 2017, 32(6): 761–76, e6. DOI:10.1016/j.ccell.2017.11.006 |
| [40] | Sidaway P. Breast cancer: Changes in clonal dynamics predict response to palbociclib[J]. Nat Rev Clin Oncol, 2018, 15(5): 264–5. |
 2019, Vol. 46
2019, Vol. 46